Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections
Abstract
1. Introduction
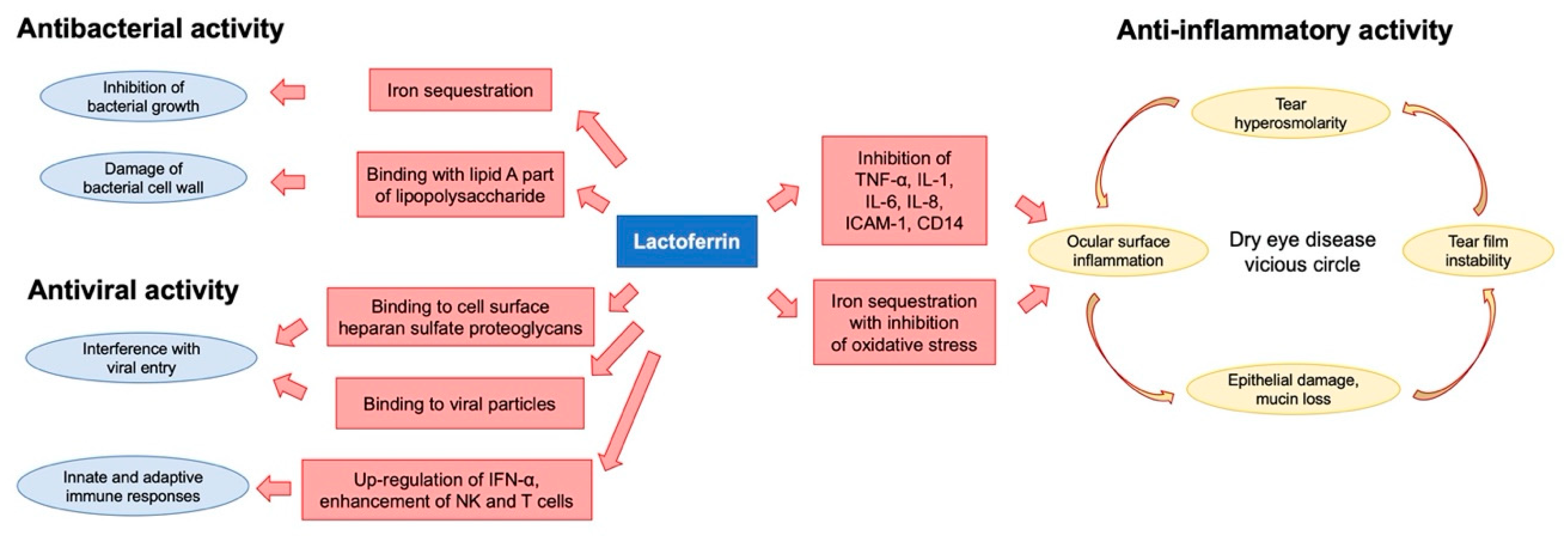
2. Lactoferrin
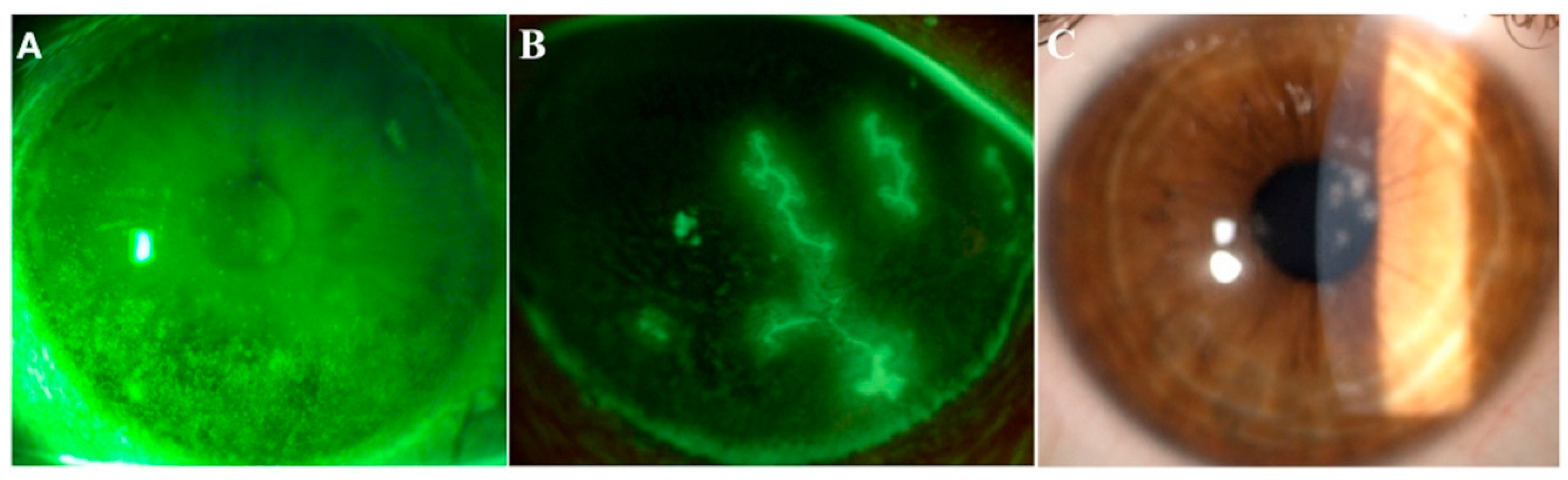
3. Lactoferrin and Dry Eye
4. Lactoferrin and Infections
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gipson, I.K. The ocular surface: The challenge to enable and protect vision: The Friedenwald lecture. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4391–4398. [Google Scholar] [CrossRef]
- Muller, L.J.; Pels, L.; Vrensen, G.F. Ultrastructural organization of human corneal nerves. Investig. Ophthalmol. Vis. Sci. 1996, 37, 476–488. [Google Scholar]
- Tran, M.T.; Ritchie, M.H.; Lausch, R.N.; Oakes, J.E. Calcitonin gene-related peptide induces IL-8 synthesis in human corneal epithelial cells. J. Immunol. 2000, 164, 4307–4312. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T.; Lausch, R.N.; Oakes, J.E. Substance P differentially stimulates IL-8 synthesis in human cornea epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3871–3877. [Google Scholar]
- Burg, N.D.; Pillinger, M.H. The neutrophil: Function and regulation in innate and humoral immunity. Clin. Immunol. 2001, 99, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Ozinsky, A. Phagocytosis of microbes: Complexity in action. Annu. Rev. Immunol. 2002, 20, 825–852. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Garreis, F.; Gottschalt, M.; Paulsen, F.P. Antimicrobial peptides as a major part of the innate immune defense at the ocular surface. Dev. Ophthalmol. 2010, 45, 16–22. [Google Scholar] [CrossRef]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef]
- Teng, C.T.; Pentecost, B.T.; Chen, Y.H.; Newbold, R.R.; Eddy, E.M.; McLachlan, J.A. Lactotransferrin gene expression in the mouse uterus and mammary gland. Endocrinology 1989, 124, 992–999. [Google Scholar] [CrossRef]
- Teng, C.T.; Beard, C.; Gladwell, W. Differential expression and estrogen response of lactoferrin gene in the female reproductive tract of mouse, rat, and hamster. Biol. Reprod. 2002, 67, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Abrink, M.; Larsson, E.; Gobl, A.; Hellman, L. Expression of lactoferrin in the kidney: Implications for innate immunity and iron metabolism. Kidney Int. 2000, 57, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Mitchell, V.; Dexter, D.; Benaissa, M.; Beauvillain, J.C.; Spik, G.; Pierce, A. Lactoferrin is synthesized by mouse brain tissue and its expression is enhanced after MPTP treatment. Brain Res. Mol. Brain Res. 1999, 72, 183–194. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. Comptes Rendus Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Rusciano, D.; Pezzino, S.; Olivieri, M.; Cristaldi, M.; Gagliano, C.; Lupo, G.; Anfuso, C.D. Age-Related Dry Eye Lactoferrin and Lactobionic Acid. Ophthalmic Res 2018, 60, 94–99. [Google Scholar] [CrossRef]
- Fujihara, T.; Nagano, T.; Endo, K.; Nakamura, M.; Nakata, K. Lactoferrin protects against UV-B irradiation-induced corneal epithelial damage in rats. Cornea 2000, 19, 207–211. [Google Scholar] [CrossRef]
- Pattamatta, U.; Willcox, M.; Stapelton, F.; Garrett, Q. Bovine lactoferrin promotes corneal wound healing and supresses IL-1 expression in alkali wounded mouse cornea. Curr. Eye Res. 2013, 38, 1110–1117. [Google Scholar] [CrossRef]
- Danjo, Y.; Lee, M.; Horimoto, K.; Hamano, T. Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol. 1994, 72, 433–437. [Google Scholar] [CrossRef]
- Sonobe, H.; Ogawa, Y.; Yamada, K.; Shimizu, E.; Uchino, Y.; Kamoi, M.; Saijo, Y.; Yamane, M.; Citterio, D.; Suzuki, K.; et al. A novel and innovative paper-based analytical device for assessing tear lactoferrin of dry eye patients. Ocul. Surf. 2019, 17, 160–166. [Google Scholar] [CrossRef]
- Kiratli, H.; Irkeç, M.; Orhan, M. Tear lactoferrin levels in chronic meibomitis associated with acne rosacea. Eur. J. Ophthalmol. 2000, 10, 11–14. [Google Scholar]
- Roda, M.; Corazza, I.; Bacchi Reggiani, M.L.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry Eye Disease and Tear Cytokine Levels-A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Moscardelli, F.; Versura, P.; Campos, E.C. In vivo confocal microscopy morphometric analysis of corneal subbasal nerve plexus in dry eye disease using newly developed fully automated system. Graefes. Arch. Clin. Exp. Ophthalmol. 2019, 257, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.L.; Willcox, M.D. Role of lactoferrin in the tear film. Biochimie 2009, 91, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Matsumoto, Y.; Yamamoto, Y.; Goto, E.; Saiki, M.; Shimazaki, J.; Takebayashi, T.; Tsubota, K. Lactoferrin in Sjögren’s syndrome. Ophthalmology 2007, 114, 2366–2367. [Google Scholar] [CrossRef] [PubMed]
- Devendra, J.; Singh, S. Effect of Oral Lactoferrin on Cataract Surgery Induced Dry Eye: A Randomised Controlled Trial. J. Clin. Diagn. Res. 2015, 9, NC06–NC09. [Google Scholar] [CrossRef]
- Fujihara, T.; Nagano, T.; Nakamura, M.; Shirasawa, E. Lactoferrin suppresses loss of corneal epithelial integrity in a rabbit short-term dry eye model. J. Ocul. Pharmacol. Ther. 1998, 14, 99–107. [Google Scholar] [CrossRef]
- Bullen, J.J. The significance of iron in infection. Rev. Infect. Dis. 1981, 3, 1127–1138. [Google Scholar] [CrossRef]
- Braun, V.; Braun, M. Active transport of iron and siderophore antibiotics. Curr. Opin. Microbiol. 2002, 5, 194–201. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Cohen, M.S.; Mao, J.; Rasmussen, G.T.; Serody, J.S.; Britigan, B.E. Interaction of lactoferrin and lipopolysacharide (LPS): Effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J. Infect. Dis. 1992, 166, 1375–1378. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Mazurier, J.; Legrand, D. Lactoferrin inhibits the binding of lipopolysaccharides to L-se- lectin and subsequent production of reactive oxygen species by neutrophils. FEBS Lett. 2000, 469, 5–8. [Google Scholar] [CrossRef]
- Bullen, J.J.; Leigh, L.C.; Rogers, H.J. The effect of iron compounds on the virulence of Escherichia coli for guinea-pigs. Immunology 1968, 15, 581–588. [Google Scholar]
- Schryvers, A.B. Characterization of the human transferrin and lactoferrin receptors in Haemophilus influenzae. Mol. Microbiol. 1988, 2, 467e472. [Google Scholar]
- Oram, J.D.; Reiter, B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim. Biophys. Acta 1968, 170, 351–365. [Google Scholar] [CrossRef]
- Leitch, E.C.; Willcox, M.D. Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Curr. Eye Res. 1999, 19, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Vorland, L.H. Lactoferrin: A multifunctional glycoprotein. APMIS 1999, 107, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef]
- Williams, T.J.; Schneider, R.P.; Willcox, M.D. The effect of protein-coated contact lenses on the adhesion and viability of gram negative bacteria. Curr. Eye Res. 2003, 27, 227–235. [Google Scholar] [CrossRef]
- van der Strate, B.W.; Beljaars, L.; Molema, G.; Harmsen, M.C.; Meijer, D.K. Antiviral activities of lactoferrin. Antiviral. Res. 2001, 52, 225–239. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef]
- Peroni, D.G. Viral infections: Lactoferrin, a further arrow in the quiver of prevention. J. Pediatr. Neonat. Individual. Med. 2020, 9, e090142. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Sato, K.; Shinmoto, H.; Dosako, S. Role of basic residues of human lactoferrin in the interaction with B lymphocytes. Biosci. Biotechnol. Biochem. 2000, 64, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Campos, E.C. Neurotrophic keratitis: Current challenges and future prospects. Eye Brain 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Kurokawa, M.; Shin, K.; Teraguchi, S.; Tamura, Y.; Shiraki, K. Oral lactoferrin prevents body weight loss and increases cytokine responses during herpes simplex virus type 1 infection of mice. Biosci. Biotechnol. Biochem. 2004, 68, 537e44. [Google Scholar] [CrossRef]
- Hasegawa, K.; Motsuchi, W.; Tanaka, S.; Dosako, S. Inhibition with lactoferrin of in vitro infection with human herpes virus. Jpn. J. Med. Sci. Biol. 1994, 47, 73e85. [Google Scholar] [CrossRef]
- Marchetti, M.; Longhi, C.; Conte, M.P.; Pisani, S.; Valenti, P.; Seganti, L. Lactoferrin inhibits herpes simplex virus type 1 adsorption to vero cells. Antivir. Res. 1996, 29, 221e31. [Google Scholar] [CrossRef]
- Marchetti, M.; Pisani, S.; Antonini, G.; Valenti, P.; Seganti, L.; Orsi, N. Metal complexes of bovine lactoferrin inhibit in vitro replication of herpes simplex virus type 1 and 2. Biometals 1998, 11, 89e94. [Google Scholar] [CrossRef]
- Siciliano, R.; Rega, B.; Marchetti, M.; Seganti, L.; Antonini, G.; Valenti, P. Bovine lactoferrin peptidic fragments involved in inhibition of herpes simplex virus type 1 infection. Biochem. Biophys. Res. Commun. 1999, 264, 19e23. [Google Scholar] [CrossRef]
- Seganti, L.; Di Biase, A.M.; Rega, B.; De Giulio, B.; Nicoletti, M.; Antonini, G.; Valenti, P. Involvement of bovine lactoferrin moieties in the inhibition of herpes simplex virus type 1 infection. Int. J. Immunopathol. Pharmacol. 2001, 14, 71e9. [Google Scholar]
- Marr, A.K.; Jenssen, H.; Moniri, M.R.; Hancock, R.E.W.; Panté, N. Bovine lactoferrin and lactoferricin interfere with intracellular trafficking of Herpes simplex virus-1. Biochemistry 2009, 91, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, T.; Hayashi, K. Lactoferrin inhibits herpes simplex virus type-1 (HSV-1) infection to mouse cornea. Arch. Virol. 1995, 140, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Keijser, S.; Jager, M.J.; Dogterom-Ballering, H.C.; Schoonderwoerd, D.T.; de Keizer, R.J.; Krose, C.J.; Houwing-Duistermaat, J.J.; van der Plas, M.J.; van Dissel, J.T.; Nibbering, P.H. Lactoferrin Glu561Asp polymorphism is associated with susceptibility to herpes simplex keratitis. Exp. Eye. Res. 2008, 86, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE 2011, 6, 23710. [Google Scholar] [CrossRef] [PubMed]
- Marques de Carvalho, C.A.; Matos, A.D.R.; Caetano., B.C.; Pedro de Sousa Junior, I.; Pereira da Costa Campos, S.; Geraldino, B.R.; Barros, C.A.; Patricio de Almeida, M.A.; Rocha, V.P.; Vieira da Silva, A.M.; et al. In vitro inhibition of SARS-CoV-2 infection by bovine lactoferrin. BioRxiv 2020. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagge, A.; Senni, C.; Bernabei, F.; Pellegrini, M.; Scorcia, V.; Traverso, C.E.; Giannaccare, G. Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections. Int. J. Mol. Sci. 2020, 21, 6668. https://doi.org/10.3390/ijms21186668
Vagge A, Senni C, Bernabei F, Pellegrini M, Scorcia V, Traverso CE, Giannaccare G. Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections. International Journal of Molecular Sciences. 2020; 21(18):6668. https://doi.org/10.3390/ijms21186668
Chicago/Turabian StyleVagge, Aldo, Carlotta Senni, Federico Bernabei, Marco Pellegrini, Vincenzo Scorcia, Carlo E Traverso, and Giuseppe Giannaccare. 2020. "Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections" International Journal of Molecular Sciences 21, no. 18: 6668. https://doi.org/10.3390/ijms21186668
APA StyleVagge, A., Senni, C., Bernabei, F., Pellegrini, M., Scorcia, V., Traverso, C. E., & Giannaccare, G. (2020). Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections. International Journal of Molecular Sciences, 21(18), 6668. https://doi.org/10.3390/ijms21186668






