Anti-Inflammatory Activity of Kurarinone Involves Induction of HO-1 via the KEAP1/Nrf2 Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Plasmids, and RNA Interference
2.2. RNA Extraction, Reverse Transcription (RT), and Quantitative PCR (qPCR)
2.3. Immunochemical Methods and Antibodies
2.4. Chromatin Immunoprecipitation (ChIP) Assay
2.5. Cell Viability Assay
2.6. Chemicals
2.7. Statistical Tests
3. Results
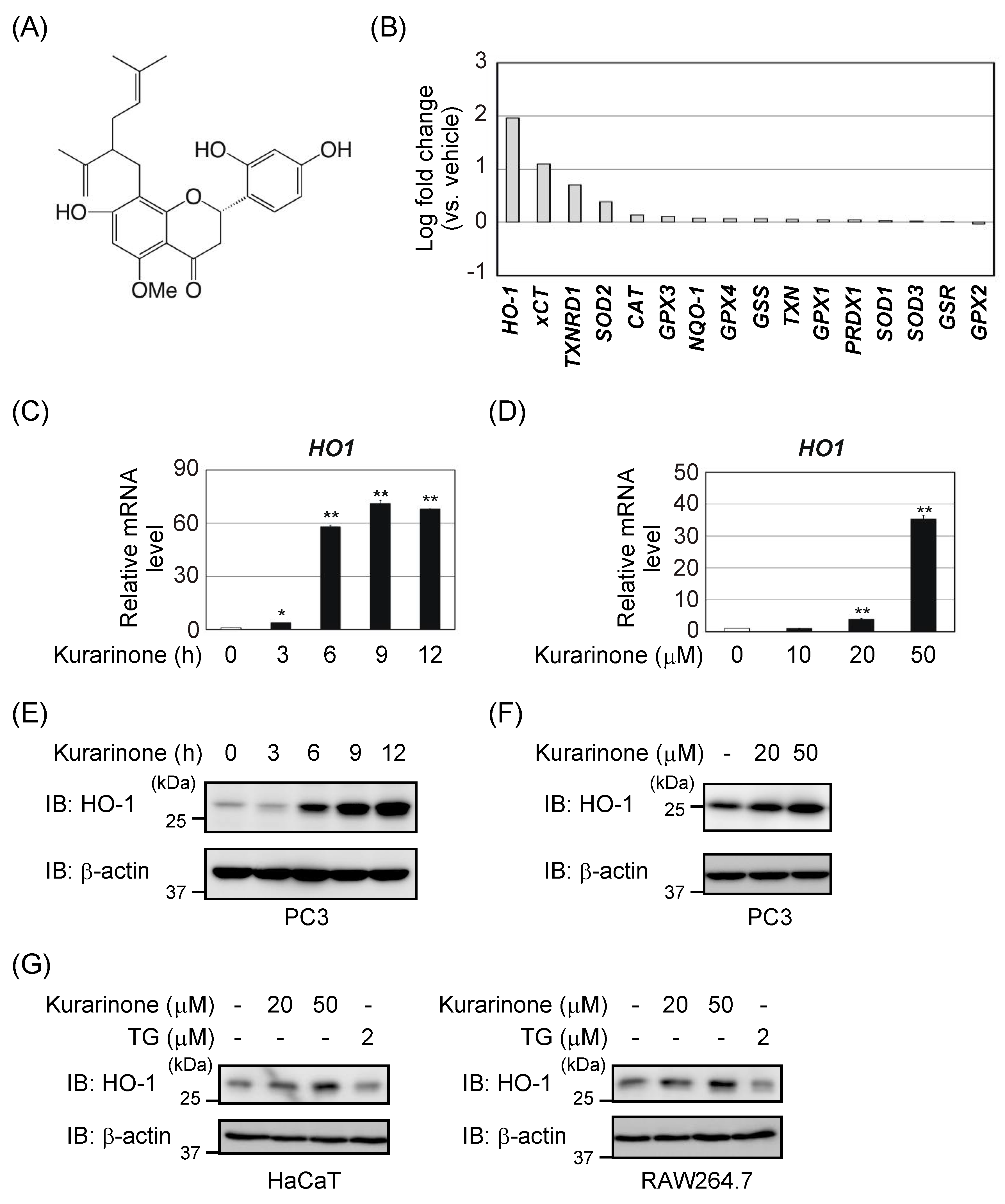
3.1. Kurarinone Induces Several Antioxidant Enzymes, Including HO-1
3.2. Kurarinone Induces HO-1 Expression in an Nrf2-Dependent Manner
3.3. Kurarinone-Induced KEAP1 Downregulation Contributes to the Activation of Nrf2
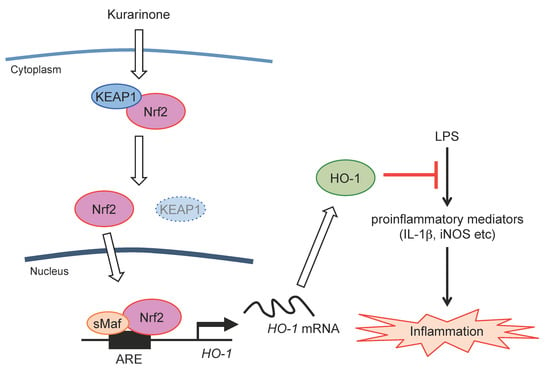
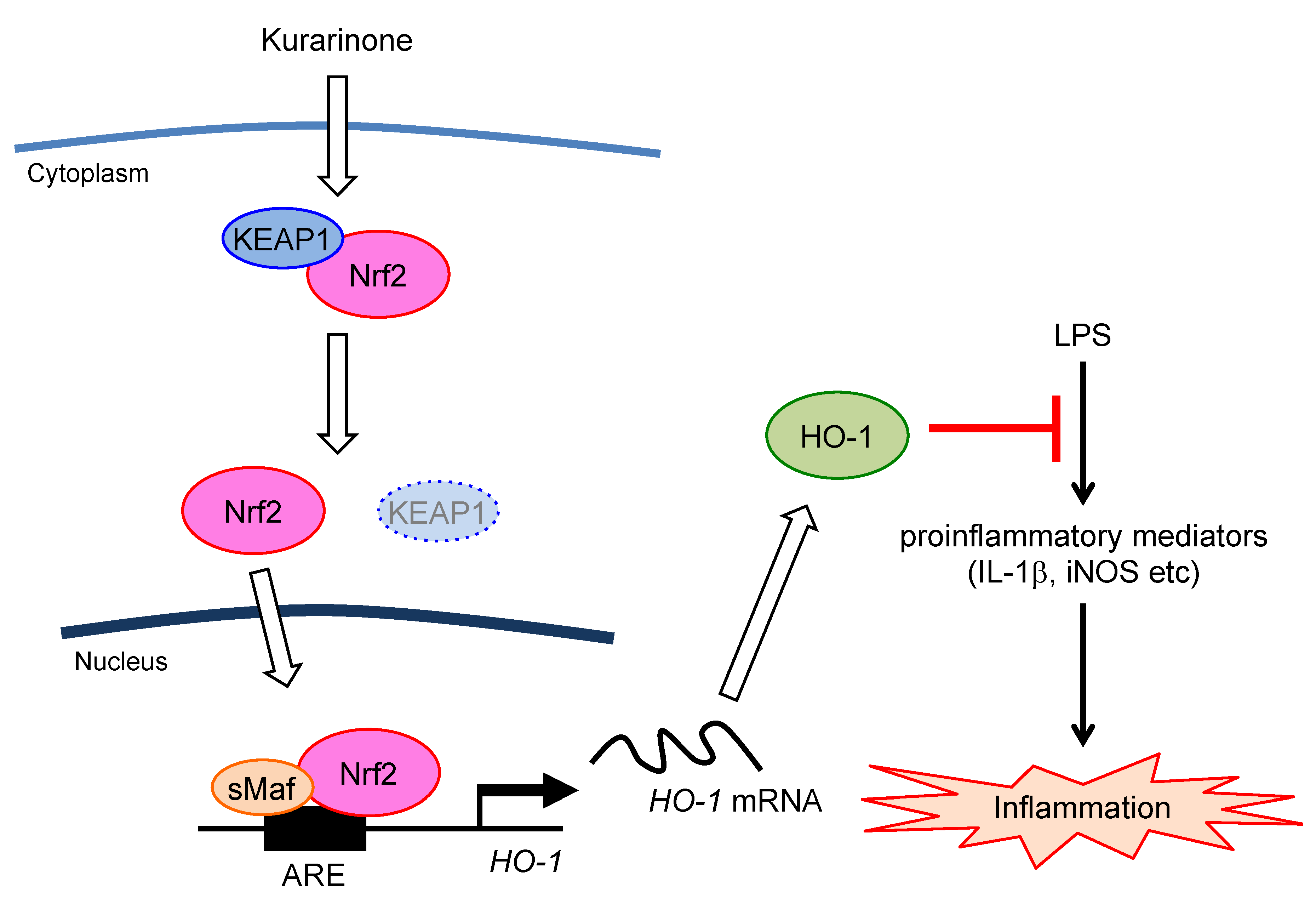
3.4. Kurarinone Suppressed the Expression of Il-1β and iNos in LPS-Stimulated RAW264.7 Macrophages by Upregulating the Expression of HO-1
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef]
- Jung, H.A.; Jeong, D.M.; Chung, H.Y.; Lim, H.A.; Kim, J.Y.; Yoon, N.Y.; Choi, J.S. Re-evaluation of the antioxidant prenylated flavonoids from the roots of Sophora flavescens. Biol. Pharm. Bull. 2008, 31, 908–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, S.Y.; Lee, H.S.; Kim, Y.K.; Kim, S.H. Determination of isoprenyl and lavandulyl positions of flavonoids from Sophora flavescens by NMR experiment. Arch. Pharm. Res. 1997, 20, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent., Sophora flavescens Ait. and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Kim, J.S.; Kang, S.S.; Son., K.H.; Chang, H.W.; Kim, H.P. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J. Ethnopharmacol. 2010, 127, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Jin, Y.Y.; Kim, H.Y.; Park, K.H.; Lee, W.S.; Jeong, T.S. Lavandulyl flavonoids from Sophora flavescens suppress lipopolysaccharide-induced activation of nuclear factor-κB and mitogen-activated protein kinases in RAW264.7 cells. Biol. Pharm. Bull. 2010, 33, 1019–1023. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Ryter, S.W.; Otterbein, L.E.; Morse, D.; Choi, A.M.K. Heme oxygenase/carbon monoxide signaling pathways: Regulation and functional significance. Mol. Cell. Biochem. 2002, 234–235, 249–263. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [Green Version]
- Pae, H.O.; Chung, H.T. Heme oxygenase-1: Its therapeutic roles in inflammatory diseases. Immune Netw. 2009, 9, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Immenschuh, S.; Ramadori, G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem. Pharmacol. 2000, 60, 1121–1128. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid. Med. Cell Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Soin, D.; Ito, K.; Dhib-Jalbut, S. Insight into the mechanism of action of dimethyl fumarate in multiple sclerosis. J. Mol. Med. 2019, 97, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Kawarada, Y.; Inoue, Y.; Kawasaki, F.; Fukuura, K.; Sato, K.; Tanaka, T.; Itoh, Y.; Hayashi, H. TGF-β induces p53/Smads complex formation in the PAI-1 promoter to active transcription. Sci. Rep. 2016, 6, 35483. [Google Scholar] [CrossRef]
- Nishikawa, S.; Itoh, Y.; Tokugawa, M.; Inoue, Y.; Nakashima, K.I.; Hori, Y.; Miyajima, C.; Yoshida, K.; Morishita, D.; Ohoka, N.; et al. Kurarinone from Sophora flavescens roots triggers ATF4 activation and cytostatic effects through PERK phosphorylation. Molecules 2019, 24, 3110. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, H.; Blomer, U.; Takahashi, M.; Gage, F.H.; Verma, I.M. Development of a self-inactivating lentivirus vector. J. Virol. 1998, 72, 8150–8157. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, M.; Hashimoto, R.; Inoue, Y.; Ishiuchi, K.; Matsuno, M.; Itoh, Y.; Tokugawa, M.; Ohoka, N.; Morishita, D.; Mizukami, H.; et al. Anti-tumorigenic activity of chrysin from Oroxylum indicum via non-genotoxic p53 activation through the ATM-Chk2 pathway. Molecules 2018, 23, 1394. [Google Scholar] [CrossRef] [Green Version]
- Miyajima, C.; Kawarada, Y.; Inoue, Y.; Suzuki, C.; Mitamura, K.; Morishita, D.; Ohoka, N.; Imamura, T.; Hayashi, H. Transcriptional coactivator TAZ negatively regulates tumor suppressor p53 activity and cellular senescence. Cells 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuura, K.; Inoue, Y.; Miyajima, C.; Watanabe, S.; Tokugawa, M.; Morishita, D.; Ohoka, N.; Komada, M.; Hayashi, H. The ubiquitin-specific protease USP17 prevents cellular senescence by stabilizing the methyltransferase SET8 and transcriptionally repressing p21. J. Biol. Chem. 2019, 294, 16429–16439. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kawachi, S.; Ohkubo, T.; Nagasaka, M.; Ito, S.; Fukuura, K.; Itoh, Y.; Ohoka, N.; Morishita, D.; Hayashi, H. The CDK inhibitor p21 is a novel target gene of ATF4 and contributes to cell survival under ER stress. FEBS Lett. 2017, 591, 3682–3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasaka, M.; Tsuzuki, K.; Ozeki, Y.; Tokugawa, M.; Ohoka, N.; Inoue, Y.; Hayashi, H. Lysine-specific demethylase 1 (LSD1/KDM1A) is a novel target gene of c-Myc. Biol. Pharm. Bull. 2019, 42, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S.; et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef] [Green Version]
- Richard, J.F.; Motz, G.T.; Puga, A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acid Res. 2007, 35, 7074–7086. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Lamark, T.; Sjøttem, E.; Larsen, K.B.; Awuh, J.A.; Øvervatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Alam, J.; Cook, J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007, 36, 166–174. [Google Scholar] [CrossRef]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.I.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep. 2019, 28, 746–758. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio, F.P.; Sparaneo, A.; Trombetta, D.; Muscarella, L.A. Epigenetic versus genetic deregulation of the KEAP1/NRF2 axis in solid tumors: Focus on methylation and noncoding RNAs. Oxid. Med. Cell. Longev. 2018, 2018, 2492063. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Fujikawa, N.; Komatsu, M.; Ishii, T.; Unno, M.; Akaike, T.; Motohashi, H.; Yamamoto, M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 13561–13566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keleku-Lukwete, N.; Suzuki, M.; Yamamoto, M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid. Redox Signal. 2018, 29, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Yamazaki, H.; Tanji, K.; Engler, M.J.; Matsumiya, T.; Itoh, K. Role of the ISR-ATF4 pathway and its cross talk with Nrf2 in mitochondrial quality control. J. Clin. Biochem. Nutr. 2019, 64, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Sayers, C.M.; Verginadis, I.I.; Lehman, S.L.; Cheng, Y.; Cerniglia, G.J.; Tuttle, S.W.; Feldman, M.D.; Zhang, P.J.; Fuchs, S.Y.; et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J. Clin. Investig. 2015, 125, 2592–2608. [Google Scholar] [CrossRef] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Chanas, S.A.; Henderson, C.J.; McLellan, L.I.; Wolf, C.R.; Cavin, C.; Hayes, J.D. The cap‘n’collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001, 61, 3299–3307. [Google Scholar]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar]
- Yang, L.; Palliyaguru, D.L.; Kensler, T.W. Frugal chemoprevention: Targeting Nrf2 with foods rich in sulforaphane. Semin. Oncol. 2016, 43, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W.; et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, M.S.; Tauchi, M.; Katsuoka, F.; Flanders, K.C.; Liby, K.T.; Honda, T.; Gribble, G.W.; Johnson, D.A.; Johnson, J.A.; Burton, N.C.; et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol. Cancer Ther. 2007, 6, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Zeeuw, D.; Akizawa, T.; Agarwal, R.; Audhya, P.; Bakris, G.L.; Chin, M.; Krauth, M.; Lambers Heerspink, H.J.; Meyer, C.J.; McMurray, J.J.; et al. Rationale and trial design of bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes: The occurrence of renal events (BEACON). Am. J. Nephrol. 2013, 37, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [Green Version]
- Takaya, K.; Suzuki, T.; Motohashi, H.; Onodera, K.; Satomi, S.; Kensler, T.W.; Yamamoto, M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2012, 53, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Fujiki, T.; Ando, F.; Murakami, K.; Isobe, K.; Mori, T.; Susa, K.; Nomura, N.; Sohara, E.; Rai, T.; Uchida, S. Tolvaptan activates the Nrf2/HO-1 antioxidant pathway through PERK phosphorylation. Sci. Rep. 2019, 9, 9245. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Zhang, X.; Huang, Y.; Cheng, N.; Ma, Y. Hepatotoxicity induced by Sophora flavescens and hepatic accumulation of kurarinone, a major hepatotoxic constituent of Sophora flavescens in rats. Molecules 2017, 22, 1809. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Cheng, N.; Ni, X. Identifying 2 prenylflavanones as potential hepatotoxic compounds in the ethanol extract of Sophora flavescens. J. Food Sci. 2013, 78, T1830–T1834. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Wang, Q.; Deng, S.; Huang, M.; Ma, X.; Song, P.; Du, J.; Huang, Y.; Wen, Y.; et al. Inhibitory effect of kurarinone on growth of human non-small cell lung cancer: An experimental study both in vitro and in vivo studies. Front. Pharmacol. 2018, 9, 252. [Google Scholar] [CrossRef]
- Lv, H.; Qi, Z.; Wang, S.; Feng, H.; Deng, X.; Ci, X. Asiatic acid exhibits anti-inflammatory and antioxidant activities against lipopolysaccharide and D-galactosamine-induced fulminant hepatic failure. Front. Immunol. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.N.; Lu, H.P.; Peng, Y.L.; Zhang, B.S.; Gong, X.B.; Su, J.; Zhou, Y.; Pan, M.H.; Xu, L. Oxyresveratrol prevents lipopolysaccharide/D-galactosamine-induced acute liver injury in mice. Int. Immunopharmacol. 2018, 56, 105–112. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Sequences | NCBI Accession Number |
|---|---|---|
| human ACTB | 5′-TGGCACCCAGCACAATGAA-3′ 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ | NM_001101.4 |
| human CAT | 5′-CCATTATAAGACTGACCAGGGC-3′ 5′-AGTCCAGGAGGGGTACTTTCC-3′ | NM_001752.3 |
| human GPX1 | 5′-CAGTCGGTGTATGCCTTCTCG-3′ 5′-GAGGGACGCCACATTCTCG-3′ | NM_000581.3 |
| human GPX2 | 5′-CCCCTACCCTTATGATGACC-3′ 5′-GTTGATGGTTGGGAAGGTG-3′ | NM_002083.3 |
| human GPX3 | 5′-CGGGGACAAGAGAAGTCG-3′ 5′-CCCAGAATGACCAGACCG-3′ | NM_002084.4 |
| human GPX4 | 5′-GAGTTTTCCGCCAAGGACATCGA-3′ 5′-GGTCGACGAGCTGAGTGTAGTTT-3′ | NM_002085.4 |
| human GSR | 5′-ATGATCAGCACCAACTGCAC-3′ 5′-CGACAAAGTCTTTTTAACCTCCTT-3′ | NM_000637.4 |
| human GSS | 5′-AAGACACTCGTGATGAACAAG-3′ 5′-AGAGGAATGACAAATACAGAGGAT-3′ | NM_000178.3 |
| human HO-1 | 5′-ATGGCCTCCCTGTACCACATC-3′ 5′-TGTTGCGCTCAATCTCCTCCT-3′ | NM_002133.2 |
| human KEAP1 | 5′-ATTGGCTGTGTGGAGTTGC-3′ 5′-CAGGTTGAAGAACTCCTCTTGC-3′ | NM_012289.3 |
| human NQO-1 | 5′-ATCCTGCCGAGTCTGTTCTG-3′ 5′-AGGGACTCCAAACCACTGC-3′ | NM_000903.2 |
| human Nrf2 | 5′-CTTTTGGCGCAGACATTCC-3′ 5′-AAGACTGGGCTCTCGATGTG-3′ | NM_006164.4 |
| human PRDX1 | 5′-AGGCCTTCCAGTTCACTGAC-3′ 5′-CAGGCTTGATGGTATCACTGC-3′ | NM_002574.3 |
| human SOD1 | 5′-TGGTTTGCGTCGTAGTCTCC-3′ 5′-CTTCGTCGCCATAACTCGCT-3′ | NM_000454.4 |
| human SOD2 | 5′-GGAAGCCATCAAACGTGACTT-3′ 5′-CCCGTTCCTTATTGAAACCAAGC-3′ | NM_000636.3 |
| human SOD3 | 5′-GGTGCAGCTCTCTTTTCAGG-3′ 5′-AACACAGTAGCGCCAGCAT-3′ | NM_003102.2 |
| human TXN | 5′-GCCTTTCTTTCATTCCCTCTC-3′ 5′-GCTTTTCCTTATTGGCTCCAG-3′ | NM_003329.3 |
| human TXNRD1 | 5′-TGTTGGAGCATCCTATGTCG-3′ 5′-TCAAATCCTCTAAGAAGAATGGACC-3′ | NM_182729.2 |
| human xCT | 5′-TCCTGCTTTGGCTCCATGAACG-3′ 5′-AGAGGAGTGTGCTTGCGGACAT-3′ | NM_014331.3 |
| mouse Actb | 5′-GGCTGTATTCCCCTCCATCG-3′ 5′-CCAGTTGGTAACAATGCCATGT-3′ | NM_007393.5 |
| mouse Ho-1 | 5′-ACAGAGGAACACAAAGACCAG-3′ 5′-GTGTCTGGGATGAGCTAGTG-3′ | NM_010442.2 |
| mouse Il-1β | 5′-GCAACTGTTCCTGAACTCAACT-3′ 5′-ATCTTTTGGGGTCCGTCAACT-3′ | NM_008361.4 |
| mouse iNos | 5′-GGCAGCCTGTGAGACCTTTG-3′ 5′-TGCATTGGAAGTGAAGCGTTT-3′ | NM_010927.4 |
| mouse Nrf2 | 5′-CCCAGCAGGACATGGATTTGA-3′ 5′-AGCTCATAGTCCTTCTGTCGC-3′ | NM_010902.4 |
| Gene | Primer Sequences | NCBI Accession Number |
|---|---|---|
| human HO-1 p1 | 5′-GCTGAGTCGCGATTTCCTCAT-3′ 5′-GAGGCTTCTGCCGTTTTCTA-3′ | NC_000022.11 |
| human HO-1 p2 | 5′-CCCTGCTGAGTAATCCTTTCC-3′ 5′-TTAAACCTGGAGCAGCTGGA-3′ | NC_000022.11 |
| human HPRT1-int1 | 5′-TGTTTGGGCTATTTACTAGTTG-3′ 5′-ATAAAATGACTTAAGCCCAGAG-3′ | NC_000023.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, S.; Inoue, Y.; Hori, Y.; Miyajima, C.; Morishita, D.; Ohoka, N.; Hida, S.; Makino, T.; Hayashi, H. Anti-Inflammatory Activity of Kurarinone Involves Induction of HO-1 via the KEAP1/Nrf2 Pathway. Antioxidants 2020, 9, 842. https://doi.org/10.3390/antiox9090842
Nishikawa S, Inoue Y, Hori Y, Miyajima C, Morishita D, Ohoka N, Hida S, Makino T, Hayashi H. Anti-Inflammatory Activity of Kurarinone Involves Induction of HO-1 via the KEAP1/Nrf2 Pathway. Antioxidants. 2020; 9(9):842. https://doi.org/10.3390/antiox9090842
Chicago/Turabian StyleNishikawa, Sakiko, Yasumichi Inoue, Yuka Hori, Chiharu Miyajima, Daisuke Morishita, Nobumichi Ohoka, Shigeaki Hida, Toshiaki Makino, and Hidetoshi Hayashi. 2020. "Anti-Inflammatory Activity of Kurarinone Involves Induction of HO-1 via the KEAP1/Nrf2 Pathway" Antioxidants 9, no. 9: 842. https://doi.org/10.3390/antiox9090842
APA StyleNishikawa, S., Inoue, Y., Hori, Y., Miyajima, C., Morishita, D., Ohoka, N., Hida, S., Makino, T., & Hayashi, H. (2020). Anti-Inflammatory Activity of Kurarinone Involves Induction of HO-1 via the KEAP1/Nrf2 Pathway. Antioxidants, 9(9), 842. https://doi.org/10.3390/antiox9090842