Influence of Oxidative Stress Biomarkers and Genetic Polymorphisms on the Clinical Severity of Hydroxyurea-Free Senegalese Children with Sickle Cell Anemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment of the Cohort and Clinical Data Recording
2.2. Biochemical and Hematological Parameters
2.3. Measurement of Plasma Oxidative Stress Markers
2.4. Genotyping of SCA Modifiers and SNPs of Anti/Pro-Oxidant Enzymes Genes
2.5. Statistical Analyses
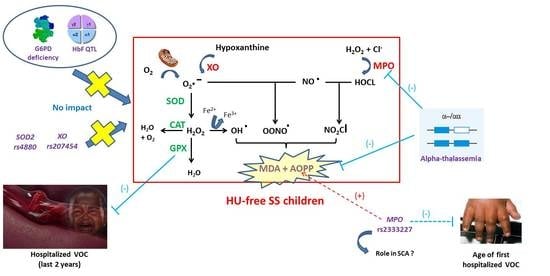
3. Results
3.1. Description of the SCA Population
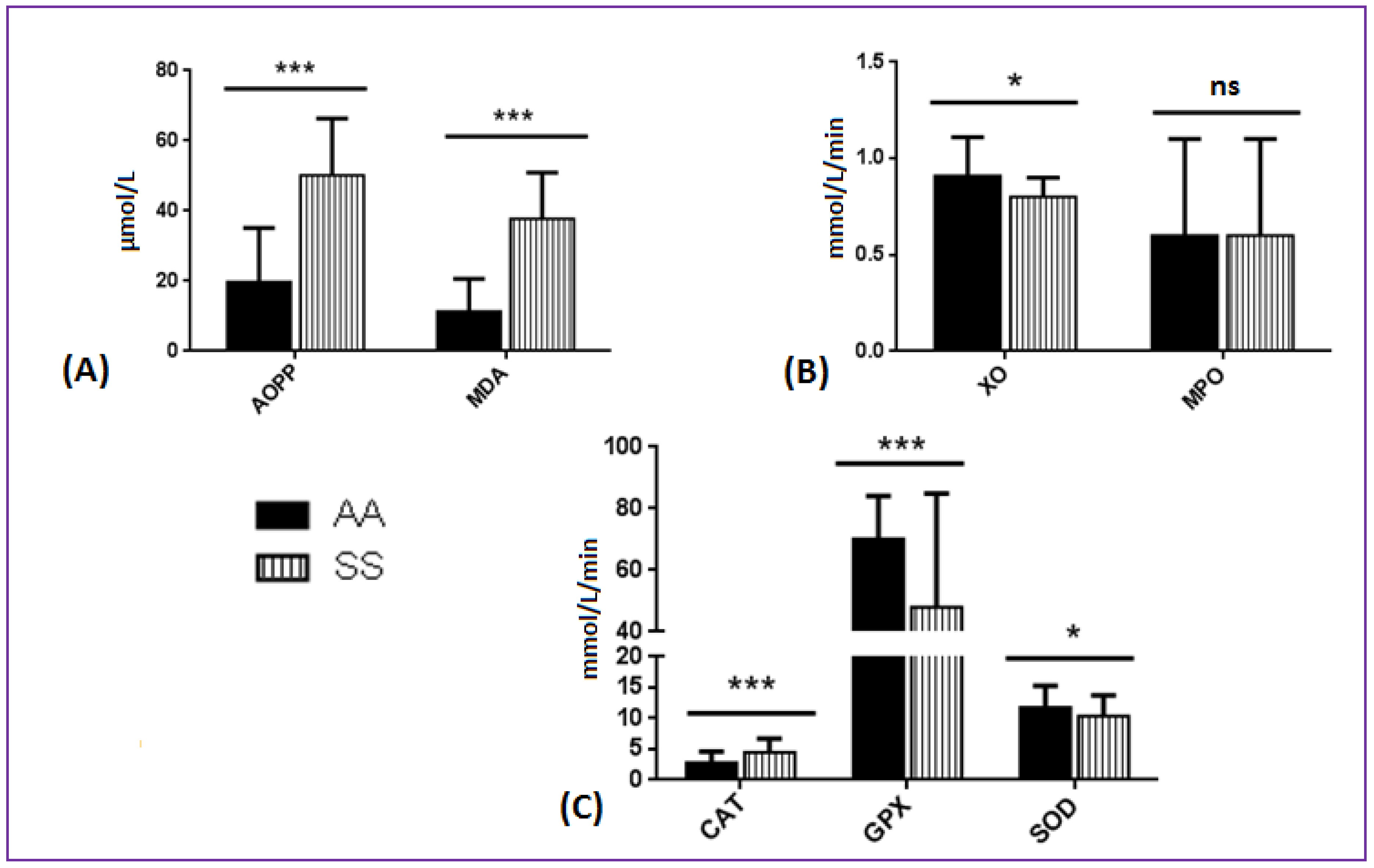
3.2. Comparison of Oxidative Stress Biomarkers between SCA and AA Patients
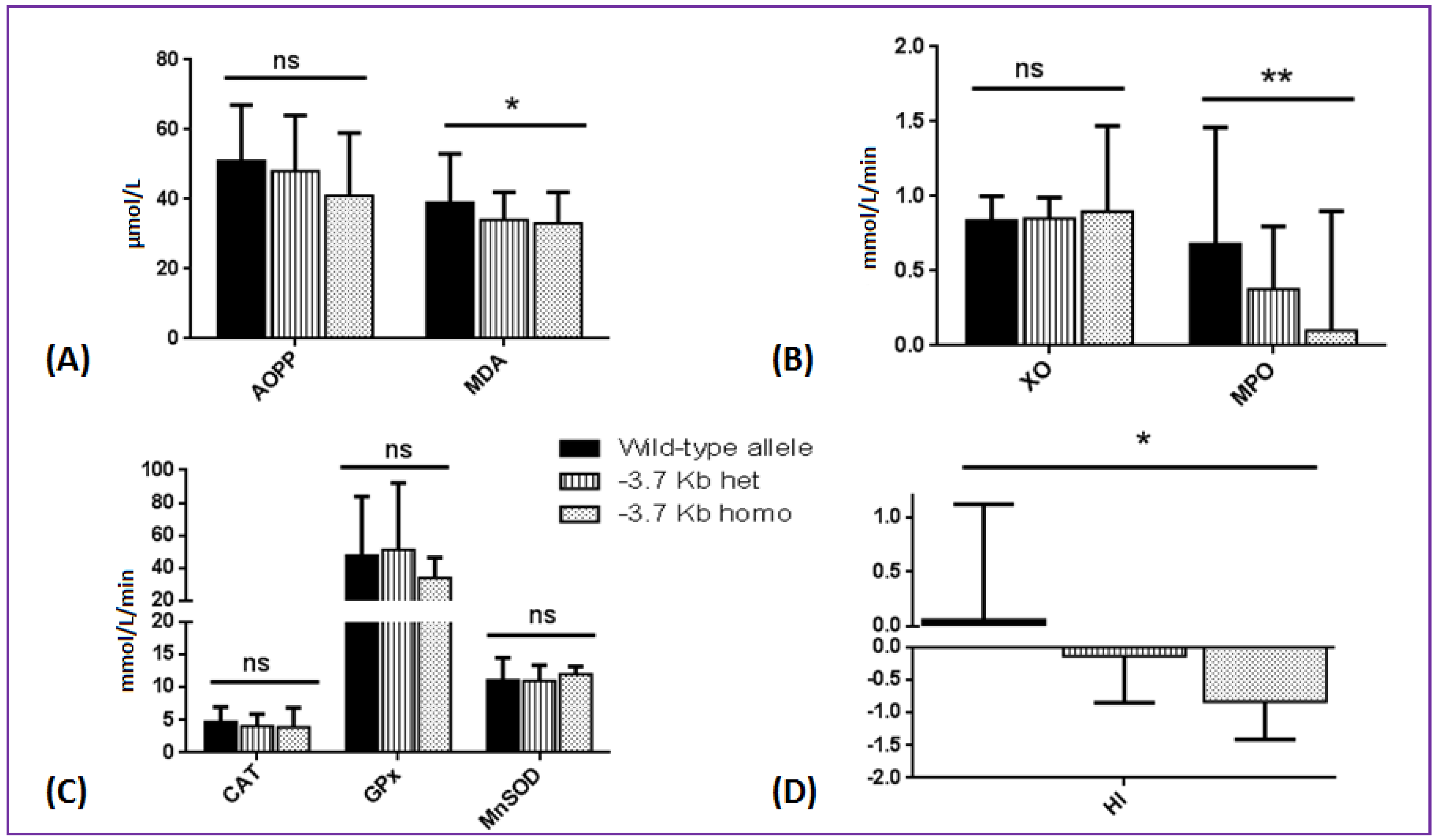
3.3. Effects of SCA Genetic Modifiers on Oxidative Stress Parameters and Hemolytic Index
3.4. Associations between Oxidative Stress Biomarkers and Indicators of Clinical Severity
3.5. Influence of SOD2, MPO, and XO Genetic Polymorphisms on SCA Complications and Oxidative Stress Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Steinberg, M.H.; Adewoye, A.H. Modifier genes and sickle cell anemia. Curr. Opin. Hematol. 2006, 13, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hierso, R.; Waltz, X.; Mora, P.; Romana, M.; Lemonne, N.; Connes, P.; Hardy-Dessources, M.-D. Effects of oxidative stress on red blood cell rheology in sickle cell patients. Br. J. Haematol. 2014, 166, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 1, 127, 750–760. [Google Scholar] [CrossRef] [PubMed]
- van Beers, E.J.; van Wijk, R. Oxidative stress in sickle cell disease; more than a DAMP squib. Clin. Hemorheol. Microcirc. 2018, 68, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Jocelyne, A.M.V.; Pieme, C.A.; Chemegne, B.C.; Manonji, H.; Nono, B.L.N.; Mamiafo, C.T.; Moukette, B.M.; Nzufo, F.T.; Tazoacha, A. Oxidative profile of sickle cell patients in a Cameroonian urban hospital. BMC Clin. Pathol. 2016, 16, 15. [Google Scholar]
- Castilhos, L.G.; De Oliveira, J.S.; Adefegha, S.A.; Magni, L.P.; Doleski, P.H.; Abdalla, F.H.; De Andrade, C.M.; Leal, D.B.R. Increased oxidative stress alters nucleosides metabolite levels in sickle cell anemia. Redox Rep. 2017, 22, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Möckesch, B.; Connes, P.; Charlot, K.; Skinner, S.; Hardy-Dessources, M.-D.; Romana, M.; Jumet, S.; Petras, M.; Divialle-Doumdo, L.; Martin, C.; et al. Association between oxidative stress and vascular reactivity in children with sickle cell anaemia and sickle haemoglobin C disease. Br. J. Haematol. 2017, 178, 468–475. [Google Scholar] [CrossRef]
- Elias, D.B.D.; De Freitas, R.M.; Gonçalves, R.P.; Magalhães, H.Y.F.; De Sousa, J.H.; Magalhães, S.M.M. Evaluation of the concentration of malondialdehyde and nitrite in patients with sickle cell anemia treated or not with hydroxyurea. Einstein (Sao Paulo) 2010, 8, 414–418. [Google Scholar] [CrossRef]
- Emokpae, M.A.; Uadia, P.O. Association of oxidative stress markers with atherogenic index of plasma in adult sickle cell nephropathy. Anemia 2012, 2012, 767501. [Google Scholar] [CrossRef] [Green Version]
- Lazzaretti, L.L.; Griebeler, I.H.; Manfredini, V.; Brandão, V.D.M.; Benfato, M.; Santin, A.P.; Wagner, S.; Castro, S.M.; Peralba, M.D.C.R. Blood antioxidant parameters in sickle cell anemia patients in steady state. J. Natl. Med. Assoc. 2008, 100, 897–902. [Google Scholar] [CrossRef]
- Biswal, S.; Rizwan, H.; Pal, S.; Sabnam, S.; Parida, P.; Pal, A. Oxidative stress, antioxidant capacity, biomolecule damage, and inflammation symptoms of sickle cell disease in children. Hematology 2018, 24, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renoux, C.; Joly, P.; Faës, C.; Mury, P.; Eglenen, B.; Turkay, M.; Yavas, G.; Yalcin, O.; Bertrand, Y.; Garnier, N.; et al. Association between oxidative stress, genetic factors, and clinical severity in children with sickle cell anemia. J. Pediatr. 2018, 195, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Faës, C.; Balayssac-Siransy, E.; Connes, P.; Hivert, L.; Danho, C.; Bogui, P.; Martin, C.; Pialoux, V. Moderate endurance exercise in patients with sickle cell anaemia: Effects on oxidative stress and endothelial activation. Br. J. Haematol. 2014, 164, 124–130. [Google Scholar] [CrossRef]
- Nader, E.; Grau, M.; Fort, R.; Collins, B.; Cannas, G.; Gauthier, A.; Walpurgis, K.; Martin, C.; Bloch, W.; Poutrel, S.; et al. Hydroxyurea therapy modulates sickle cell anemia red blood cell physiology: Impact on RBC deformability, oxidative stress, nitrite levels and nitric oxide synthase signalling pathway. Nitric Oxide 2018, 81, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, P.E.; Lu, H.; Mann, E.H.; Chen, Y.-H.; Ho, T.-R.; Cousins, D.J.; Corrigan, C.; Kelly, F.J.; Mudway, I.S.; Hawrylowicz, C. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS ONE 2018, 13, e0200040. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, S.; Ganguly, U.; Khemka, V.K.; Banerjee, A. Decreased glucose-6-phosphate dehydrogenase activity along with oxidative stress affects visual contrast sensitivity in alcoholics. Alcohol 2018, 73, 17–24. [Google Scholar] [CrossRef]
- Crawford, A.; Fassett, R.G.; Geraghty, D.P.; Kunde, D.; Ball, M.; Robertson, I.K.; Coombes, J.S. Relationships between single nucleotide polymorphisms of antioxidant enzymes and disease. Gene 2012, 501, 89–103. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Khoa, T.N.; Jungers, P.; Drüeke, T.; Descamps-Latscha, B. Advanced oxidation protein products: Oxidative stress markers and mediators of inflammation in uremia. Adv. Nephrol. Necker Hosp. 1998, 28, 321–341. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Oberley, L.W.; Spitz, D.R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984, 105, 457–464. [Google Scholar] [PubMed]
- Laouafa, S.; Ribon-Demars, A.; Marcouiller, F.; Roussel, D.; Bairam, A.; Pialoux, V.; Joseph, A. Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep 2017, 40, zsx104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.C.; Wojtkiewicz, G.R.; Linnoila, J.J.; Chen, J.W. Measuring myeloperoxidase activity in biological samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [Green Version]
- Chong, S.S.; Boehm, C.D.; Higgs, D.R.; Cutting, G.R. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood 2000, 95, 360–362. [Google Scholar] [CrossRef]
- Tall, F.G.; Martin, C.; Ndour, E.H.M.; Ly, I.D.; Renoux, C.; Chillotti, L.; Veyrenche, N.; Connes, P.; Gueye, P.M.; Diallo, R.N.; et al. Genetic background of the sickle cell disease pediatric population of dakar, senegal, and characterization of a novel frameshift beta-thalassemia mutation [HBB: C.265_266del; p.Leu89Glufs*2]. Hemoglobin 2017, 41, 89–95. [Google Scholar] [CrossRef]
- Joly, P.; Lacan, P.; Garcia, C.; Martin, C.; Francina, A. Rapid genotyping of two common G6PD variants, African (A-) and Mediterranean, by high-resolution melting analysis. Clin. Biochem. 2009, 43, 193–197. [Google Scholar] [CrossRef]
- Tall, F.G.; Martin, C.; Ndour, E.H.M.; Renoux, C.; Ly, I.D.; Connes, P.; Gueye, P.M.; Diallo, R.N.; Diagne, I.; Diop, P.A.; et al. Combined and differential effects of alpha-thalassemia and HbF-quantitative trait loci in Senegalese hydroxyurea-free children with sickle cell anemia. Pediatr. Blood Cancer 2019, 66, e27934. [Google Scholar]
- Farias, I.C.C.; Mendonça-Belmont, T.F.; Da Silva, A.S.; Ó, K.P.D.; Ferreira, F.B.; Medeiros, F.S.; Vasconcelos, L.R.D.S.; Rego, M.J.B.D.M.; Bezerra, M.A.C.; Araújo, A.D.S.; et al. Association of the SOD2 polymorphism (Val16Ala) and SOD activity with vaso-occlusive crisis and acute splenic sequestration in children with sickle cell anemia. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018012. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, P.; De Marco, G.; Furriol, J.; Mansego, M.L.; Pineda-Alonso, M.; González-Neira, A.; Martín-Escudero, J.C.; Benitez, J.; Lluch, A.; Chaves, F.J.; et al. Oxidative stress in susceptibility to breast cancer: Study in Spanish population. BMC Cancer 2014, 14, 861. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, L.C.P.; Miranda-Vilela, A.L.; Hiragi, C.D.O.; Ribeiro, I.F.; Daldegan, M.B.; Grisolia, C.K.; Dos Santos-Neto, L.L. Haptoglobin and myeloperoxidase (-G463A) gene polymorphisms in Brazilian sickle cell patients with and without secondary iron overload. Blood Cells Mol. Dis. 2014, 52, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.N.P.; Conran, N.; Albuquerque, D.M.; Soares, P.H.; O Saad, S.T.; Costa, F.F. Association of the G-463A myeloperoxidase polymorphism with infection in sickle cell anemia. Haematologica 2005, 90, 977–979. [Google Scholar]
- Yahouedehou, S.C.M.A.; Carvalho, M.O.S.; Oliveira, R.M.; Santiago, R.P.; Da Guarda, C.C.; Carvalho, S.P.; Ferreira, J.R.D.; Aleluia, M.M.; Adorno, E.V.; Gonçalves, M.S. Sickle cell anemia patients in use of hydroxyurea: Association between polymorphisms in genes encoding metabolizing drug enzymes and laboratory parameters. Dis. Markers 2018, 2018, 6105691. [Google Scholar] [CrossRef]
- Connes, P.; Lamarre, Y.; Waltz, X.; Ballas, S.K.; Lemonne, N.; Etienne-Julan, M.; Hue, O.; Hardy-Dessources, M.-D.; Romana, M. Haemolysis and abnormal haemorheology in sickle cell anaemia. Br. J. Haematol. 2014, 165, 564–572. [Google Scholar] [CrossRef]
- Nouraie, M.; Lee, J.S.; Zhang, Y.; Kanias, T.; Zhao, X.; Xiong, Z.; Oriss, T.B.; Zeng, Q.; Kato, G.J.; Gibbs, J.S.R.; et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2012, 98, 464–472. [Google Scholar] [CrossRef]
- Gizi, A.; Papassotiriou, I.; Apostolakou, F.; Lazaropoulou, C.; Papastamataki, M.; Kanavaki, I.; Kalotychou, V.; Goussetis, E.; Kattamis, A.; Rombos, I.; et al. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant-antioxidant status. Blood Cells Mol. Dis. 2011, 46, 220–225. [Google Scholar] [CrossRef]
- Al-Naama, L.M.; Hassan, M.K.; Mehdi, J.K. Association of erythrocytes antioxidant enzymes and their cofactors with markers of oxidative stress in patients with sickle cell anemia. Qatar Med. J. 2015, 2015, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubong, L.N.; Nya Biapa, P.C.; Chetcha, B.; Yanou-Njintang, N.; Moor Ama, V.J.; Pieme, C.A. Relationship between higher atherogenic index of plasma and oxidative stress of a group of patients living with sickle cell anemia in Cameroon. Adv. Hematol. 2020, 2020, 9864371. [Google Scholar] [CrossRef] [Green Version]
- Adelakun, A.; Ajani, O.; Ogunleye, T.; Disu, E.; Kosoko, A.; Arinola, G. Respiratory burst enzymes and oxidantantioxidant status in nigerian children with sickle cell disease. Br. Biotechnol. J. 2014, 4, 270–278. [Google Scholar] [CrossRef]
- Schmidt, H.M.; Kelley, E.E.; Straub, A.C. The impact of xanthine oxidase (XO) on hemolytic diseases. Redox Biol. 2019, 21, 101072. [Google Scholar] [CrossRef] [PubMed]
- Hierso, R.; Lemonne, N.; Villaescusa, R.; Lalanne-Mistrih, M.-L.; Charlot, K.; Etienne-Julan, M.; Tressières, B.; Lamarre, Y.; Tarer, V.; Garnier, Y.; et al. Exacerbation of oxidative stress during sickle vaso-occlusive crisis is associated with decreased anti-band 3 autoantibodies rate and increased red blood cell-derived microparticle level: A prospective study. Br. J. Haematol. 2016, 176, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Osarogiagbon, U.R.; Choong, S.; Belcher, J.D.; Vercellotti, G.M.; Paller, M.S.; Hebbel, R.P. Reperfusion injury pathophysiology in sickle transgenic mice. Blood 2000, 96, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Detterich, J.A.; Liu, H.; Suriany, S.; Kato, R.M.; Chalacheva, P.; Tedla, B.; Shah, P.M.; Khoo, M.C.; Wood, J.C.; Coates, T.D.; et al. Erythrocyte and plasma oxidative stress appears to be compensated in patients with sickle cell disease during a period of relative health, despite the presence of known oxidative agents. Free Radic. Biol. Med. 2019, 141, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Woo, H.A.; Kang, D. The role of peroxiredoxins in the transduction of H2O2 signals. Antioxid. Redox Signal. 2018, 28, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Tshilolo, M.; Tomlinson, G.; Williams, T.N.; Santos, B.; Olupot-Olupot, P.; Lane, A.; Aygun, B.; Stuber, S.E.; Latham, T.; McGann, P.T.; et al. Hydroxyurea for children with sickle cell anemia in sub-saharan Africa. N. Engl. J. Med. 2019, 380, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.C.; Santos, T.E.D.J.D.; Dos Santos, T.N.; Pedrosa, A.M.; Elias, D.B.D.; Leal, L.K.A.M.; Lopes, A.D.A.; Sasahara, G.L.; Lemes, R.P.G. The Effect of a selective inhibitor of phosphodiesterase-9 on oxidative stress, inflammation and cytotoxicity in neutrophils from patients with sickle cell anaemia. Basic Clin. Pharmacol. Toxicol. 2016, 118, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Belisário, A.R.; Sales, R.R.; Toledo, N.E.; Velloso-Rodrigues, C.V.R.; Silva, C.M.; Viana, M.B. Glucose-6-phosphate dehydrogenase deficiency in brazilian children with sickle cell anemia is not associated with clinical ischemic stroke or high-risk transcranial doppler. Pediatr. Blood Cancer. 2016, 63, 1046–1049. [Google Scholar] [CrossRef]
- Bernaudin, F.; Verlhac, S.; Chevret, S.; Torres, M.; Coic, L.; Arnaud, C.; Kamdem, A.; Hau, I.; Neonato, M.G.; Delacourt, C. G6PD deficiency, absence of alpha-thalassemia, and hemolytic rate at baseline are significant independent risk factors for abnormally high cerebral velocities in patients with sickle cell anemia. Blood 2008, 112, 4314–4317. [Google Scholar] [CrossRef]
- Connes, P.; Verlhac, S.; Bernaudin, F. Advances in understanding the pathogenesis of cerebrovascular vasculopathy in sickle cell anaemia. Br. J. Haematol. 2013, 161, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, I.; Escames, G.; Cossio, G.; de Borace, R.G.; Moreno, B.; Chahboune, M.; López, M.C.; Díez, T.; Acuña-Castroviejo, D. Oxidative stress status, clinical outcome, and beta-globin gene cluster haplotypes in pediatric patients with sickle cell disease. Eur. J. Haematol. 2010, 85, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Nur, E.; Brandjes, D.P.; Schnog, J.-J.B.; Otten, H.-M.; Fijnvandraat, K.; Schalkwijk, C.G.; Biemond, B.J. Plasma levels of advanced glycation end products are associated with haemolysis-related organ complications in sickle cell patients. Br. J. Haematol. 2010, 151, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antwi-Boasiako, C.; Dankwah, G.B.; Aryee, R.; Hayfron-Benjamin, C.; Aboagye, G.; Campbell, A.D. Correlation of lipid peroxidation and nitric oxide metabolites, trace elements, and antioxidant enzymes in patients with sickle cell disease. J. Clin. Lab. Anal. 2020, 34, e23294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hundekar, P.S.; Suryakar, A.N.; Karnik, A.C.; Katkam, R.V.; Joshi, N.G.; Ghone, R.A. Level of nitric oxide and antioxidant vitamins in sickle cell anaemia patients. Indian J. Physiol. Pharmacol. 2012, 56, 125–129. [Google Scholar]
- Itokua, K.E.; Makulo, J.R.; Lepira, F.B.; Aloni, M.N.; Ekulu, P.M.; Sumaili, E.; Bukabau, J.B.; Mokoli, V.M.; Longo, A.; Kajingulu, F.; et al. Albuminuria, serum antioxidant enzyme levels and markers of hemolysis and inflammation in steady state children with sickle cell anemia. BMC Nephrol. 2016, 17, 178. [Google Scholar] [CrossRef] [Green Version]
- Nacoulma, E.W.C.; Sawadogo, D.; Sakande, J.; Mansour, A.; Hien, F.H.; Sangaré, A.; Sess, E.D. Influence of fetal haemoglobin rate (FHb) on the oxidizing stress in homozygote sickle cell patient living in Abidjan, Cote-d’Ivoire]. Bull. Soc. Pathol. Exot. 2006, 99, 241–244. [Google Scholar]
- Oztas, Y.; Durukan, I.; Unal, S.; Ozgunes, N. Plasma protein oxidation is correlated positively with plasma iron levels and negatively with hemolysate zinc levels in sickle-cell anemia patients. Int. J. Lab. Hematol. 2012, 34, 129–135. [Google Scholar] [CrossRef]
- Sess, D.; A Carbonneau, M.; Thomas, M.J.; Dumon, M.F.; Peuchant, E.; Perromat, A.; Le Bras, M.; Clerc, M. First observations on the main plasma parameters of oxidative stress in homozygous sickle cell disease. Bull. Soc. Pathol. Exot. 1992, 85, 174–179. [Google Scholar]
| Assays | Intra-Assay CV | Limit of Detection |
|---|---|---|
| AOPP | 5.20% | 2 µmol/L |
| MDA | 4.23% | 1 µmol/L |
| GPX | 6.30% | 2 mmol/L/min |
| Catalase | 5.49% | 0.1 mmol/L/min |
| SOD | 6.35% | 0.2 mmol/L/min |
| MPO | 8.12% | 0.02 mmol/L/min |
| XO | 3.41% | 0.01 mmol/L/min |
| Clinical and Biological Parameters | Mean (±SD) or N | Range (min–max) |
|---|---|---|
| Epidemiological parameters | ||
| Inclusion age (years) | 9.7 ± 4.6 | 2.0–22.9 |
| Sex ratio (M/F) | 170/131 | / |
| Age of first SCA complication | ||
| <3 years | 146 | / |
| >3 years | 155 | / |
| Hospitalized VOC in last 2 years | ||
| None | 176 | / |
| At least one | 125 | / |
| Other complications in last 2 years (No/Yes) | ||
| Osteomyelitis | 286/15 | / |
| Osteonecrosis | 292/9 | / |
| Stroke | 289/12 | / |
| Acute splenic sequestration | 293/8 | / |
| Sepsis | 295/6 | / |
| Acute chest syndrome | 290/11 | / |
| Biological parameters | ||
| WBC count (103/L) | 14.3 ± 4.3 | 4.2–28.5 |
| Hb (g/dL) | 7.8 ± 1.1 | 5.5–12.0 |
| Reticulocytes count (103/L) | 330 ± 166 | 28–1117 |
| Reticulocytes (%) | 12.0 ± 5.6 | 0.6–36.5 |
| Platelets count (103/L) | 449 ± 136 | 135–945 |
| HbF (%) | 9.5 ± 5.1 | 1.1–26.8 |
| ASAT (UI/L) | 61 ± 34 | 20–341 |
| Total bilirubin (mg/dL) | 44 ± 24 | 7–107 |
| Direct bilirubin (mg/dL) | 23 ± 15 | 2–68 |
| LDH (UI/ L) | 940 ± 499 | 148–3318 |
| CRP (mg/L) | 4.6 ± 5.4 | 0.1–34.6 |
| HbF QTLs (0–6) | p * | HbF Level (%) | p | G6PD Genotype | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (0–1) n = 51 | (2–3) n = 195 | (4–6) n = 55 | <5 n = 68 | 5–15 n = 185 | ≥15 n = 48 | Wild n = 249 | Het n = 25 | Mute n = 27 | ||||
| Oxidative stress products | ||||||||||||
| AOPP (µmol/L) | 48 ± 16 | 51 ± 17 | 49 ± 14 | 0.58 | 51 ± 17 | 50 ± 16 | 48 ± 15 | 0.68 | 50 ± 16 | 49 ± 17 | 50 ± 16 | 0.89 |
| MDA (µmol/L) | 37 ± 8 | 38 ± 10 | 38 ± 23 | 0.82 | 37 ± 11 | 38 ± 14 | 39 ± 13 | 0.63 | 38 ± 14 | 37 ± 7 | 37 ± 7 | 0.90 |
| Pro-oxidant enzymes | ||||||||||||
| XO (mmol/L/min) | 0.84 ± 0.16 | 0.86 ± 0.16 | 0.84 ± 0.16 | 0.87 | 0.85 ± 0.18 | 0.84 ± 0.15 | 0.87 ± 0.17 | 0.56 | 0.84 ± 0.16 | 0.86 ± 0.16 | 0.84 ± 0.10 | 0.83 |
| MPO (mmol/L/min) | 0.6 ± 0.7 | 0.6 ± 0.9 | 0.5 ± 0.5 | 0.76 | 0.62 ± 0.86 | 0.64 ± 0.73 | 0.50 ± 0.50 | 0.47 | 0.6 ± 0.7 | 0.5 ± 0.5 | 0.6 ± 0.5 | 0.50 |
| Antioxidant enzymes | ||||||||||||
| Catalase (mmol/L/min) | 4.2 ± 1.2 | 4.7 ± 2.3 | 4.3 ± 2.0 | 0.22 | 4.7 ± 1.8 | 4.6 ± 2.4 | 4.2 ± 1.7 | 0.51 | 4.6 ± 2.2 | 3.8 ± 1.7 | 4.6 ± 1.8 | 0.24 |
| GPX (mmol/L/min) | 46.6 ± 34.4 | 49.3 ± 38.0 | 45.5 ± 35.5 | 0.75 | 49.4 ± 41.4 | 47.5 ± 36.0 | 48.8 ± 33.2 | 0.93 | 48.3 ± 37.0 | 53.9 ± 38.3 | 40.8 ± 32.0 | 0.42 |
| SOD (mmol/L/min) | 11.1 ± 2.6 | 11.1 ± 3.3 | 11.3 ± 3.4 | 0.73 | 10.9 ± 2.7 | 11.5 ± 3.4 | 10.4 ± 2.8 | 0.07 | 10.3 ± 3.26 | 10.8 ± 3.1 | 11.5 ± 3.4 | 0.31 |
| Hemolytic index | 0.07 ± 1.06 | −0.02 ± 0.97 | 0.01 ± 1.05 | 0.84 | 0.03 ± 0.86 | 0.05 ± 1.09 | −0.24 ± 0.81 | 0.19 | −0.01 ± 1.02 | 0.10 ± 0.90 | 0.02 ± 0.92 | 0.87 |
| Age of First Complication | Hospitalized VOC in Last 2 Years | Acute Chest Syndrome in Last 2 Years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <3 years n = 146 | >3 years n = 155 | p | None n = 176 | At Least 1 n = 125 | p | None n = 290 | At Least 1 n = 11 | p | |
| Oxidative stress products | |||||||||
| AOPP (µmol/L) | 49 ± 16 | 51 ± 16 | 0.15 | 50 ± 16 | 51 ± 16 | 0.48 | 50 ± 16 | 54 ± 20 | 0.46 |
| MDA (µmol/L) | 38 ± 16 | 37 ± 10 | 0.53 | 38 ± 14 | 37 ± 11 | 0.51 | 38 ± 13 | 42 ± 10 | 0.28 |
| Pro-oxidant enzymes | |||||||||
| XO (mmol/L/min) | 0.84 ± 0.15 | 0.85 ± 0.16 | 0.88 | 0.84 ± 0.16 | 0.84 ± 0.16 | 0.85 | 0.85 ± 0.16 | 0.85 ± 0.18 | 0.89 |
| MPO (mmol/L/min) | 0.6 ± 0.7 | 0.6 ± 0.7 | 0.98 | 0.6 ± 0.7 | 0.6 ± 0.7 | 0.75 | 0.62 ± 0.74 | 0.50 ± 0.51 | 0.60 |
| Antioxidant enzymes | |||||||||
| MnSOD (mmol/L/min) | 11.0 ± 3.2 | 9.7 ± 3.3 | 0.001 | 10.6 ± 3.3 | 10.0 ± 3.2 | 0.19 | 11.1 ± 3.2 | 12.5 ± 1.8 | 0.14 |
| CAT (mmol/L/min) | 5.0 ± 2.5 | 4.1 ± 1.8 | 0.002 | 4.6 ± 2.4 | 4.4 ± 1.7 | 0.46 | 4.5 ± 2.2 | 5.2 ± 2.4 | 0.36 |
| GPX (mmol/L/min) | 49.0 ± 38.9 | 47.3 ± 34.7 | 0.67 | 52.4 ± 35.2 | 42.0 ± 38.1 | 0.01 | 48.1 ± 37.0 | 47.7 ± 32.5 | 0.97 |
| SOD2 (rs4880) | MPO (rs2333227) | XO (rs207454) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild n = 132 | Het + Mute n = 169 | p | Wild n = 163 | Het + Mute n = 138 | p | Wild n = 142 | Het + Mute n = 159 | p | |
| Quantitative clinical parameters * | |||||||||
| Age first complication (years) | 4.4 ± 3.4 | 3.8 ± 3.2 | 0.12 | 3.7 ± 3.0 | 4.5 ± 3.5 | 0.04 | 4.0 ± 3.2 | 4.0 ± 3.2 | 0.86 |
| Number of hospitalized VOC (last 2 years) | 0.80 ± 1.3 | 0.69 ± 1.1 | 0.43 | 0.77 ± 1.3 | 0.71 ± 1.1 | 0.69 | 0.85 ± 1.4 | 0.65 ± 1.1 | 0.16 |
| Other clinical parameters (No/Yes) ** | |||||||||
| Osteomyelitis | 124/8 | 162/7 | 0.75 | 153/10 | 133/5 | 0.31 | 133/9 | 153/6 | 0.30 |
| Osteonecrosis | 129/3 | 163/6 | 0.51 | 157/6 | 135/3 | 0.44 | 137/5 | 158/1 | 0.07 |
| Stroke | 124/8 | 165/4 | 0.10 | 156/7 | 133/5 | 0.76 | 137/5 | 152/7 | 0.42 |
| Acute splenic sequestration | 128/4 | 165/4 | 0.72 | 157/6 | 136/2 | 0.23 | 137/5 | 156/3 | 0.37 |
| Sepsis | 129/3 | 166/3 | 0.75 | 160/3 | 135/3 | 0.83 | 137/5 | 158/1 | 0.07 |
| Acute chest syndrome | 126/6 | 164/5 | 0.53 | 156/7 | 134/4 | 0.52 | 134/8 | 156/3 | 0.08 |
| Oxidative stress parameters | |||||||||
| AOPP (µmol/L) | 51.2 ± 16.4 | 49.4 ± 16.1 | 0.33 | 48.1 ± 15.5 | 52.6 ± 16.8 | 0.02 | 49.9 ± 15.1 | 50.3 ± 17.2 | 0.83 |
| MDA (µmol/L) | 37.6 ± 10.7 | 37.9 ± 14.7 | 0.87 | 38.1 ± 14.9 | 37.2 ± 10.5 | 0.54 | 37.9 ± 9.8 | 37.6 ± 15.4 | 0.85 |
| XO (mmol/L/min) | Not calculated | Not calculated | 0.57 ± 0.65 | 0.67 ± 0.79 | 0.38 | ||||
| MPO (mmol/L/min) | Not calculated | 0.84 ± 0.15 | 0.84 ± 0.16 | 0.93 | Not calculated | ||||
| MnSOD (mmol/L/min) | 10.9 ± 2.9 | 11.1 ± 3.4 | 0.22 | Not calculated | Not calculated | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gueye Tall, F.; Martin, C.; Ndour, E.h.M.; Faes, C.; Déme Ly, I.; Pialoux, V.; Connes, P.; Gueye, P.M.; Ndiaye Diallo, R.; Renoux, C.; et al. Influence of Oxidative Stress Biomarkers and Genetic Polymorphisms on the Clinical Severity of Hydroxyurea-Free Senegalese Children with Sickle Cell Anemia. Antioxidants 2020, 9, 863. https://doi.org/10.3390/antiox9090863
Gueye Tall F, Martin C, Ndour EhM, Faes C, Déme Ly I, Pialoux V, Connes P, Gueye PM, Ndiaye Diallo R, Renoux C, et al. Influence of Oxidative Stress Biomarkers and Genetic Polymorphisms on the Clinical Severity of Hydroxyurea-Free Senegalese Children with Sickle Cell Anemia. Antioxidants. 2020; 9(9):863. https://doi.org/10.3390/antiox9090863
Chicago/Turabian StyleGueye Tall, Fatou, Cyril Martin, El hadji Malick Ndour, Camille Faes, Indou Déme Ly, Vincent Pialoux, Philippe Connes, Papa Madieye Gueye, Rokhaya Ndiaye Diallo, Céline Renoux, and et al. 2020. "Influence of Oxidative Stress Biomarkers and Genetic Polymorphisms on the Clinical Severity of Hydroxyurea-Free Senegalese Children with Sickle Cell Anemia" Antioxidants 9, no. 9: 863. https://doi.org/10.3390/antiox9090863
APA StyleGueye Tall, F., Martin, C., Ndour, E. h. M., Faes, C., Déme Ly, I., Pialoux, V., Connes, P., Gueye, P. M., Ndiaye Diallo, R., Renoux, C., Diagne, I., Diop, P. A., Cissé, A., Sall, P. L., & Joly, P. (2020). Influence of Oxidative Stress Biomarkers and Genetic Polymorphisms on the Clinical Severity of Hydroxyurea-Free Senegalese Children with Sickle Cell Anemia. Antioxidants, 9(9), 863. https://doi.org/10.3390/antiox9090863