Effect of High Hydrostatic Pressure Extraction on Biological Activities and Phenolics Composition of Winter Savory Leaf Extracts
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemical Materials
2.2. Biological Samples and Extraction Conditions
2.3. Total Phenolic Compounds
2.4. LC-MS/MS Analysis of Phenolic Compounds
2.5. Determination of Antioxidant Activity
2.6. Determination of Antimicrobial and Antibiofilm Activities
2.7. Determination of Extracts Capacity to Prevent/Cause DNA Oxidative Damage
2.7.1. Prevention of DNA Oxidation
2.7.2. DNA Degradation Assessment (Pro-Oxidant Assays)
2.7.3. Electrophoresis
2.8. Determination of Extracts Cytotoxicity and Anticancer Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Individual Compounds
3.2. Antioxidant Activity
3.3. Antimicrobial and Antibiofilm Activity
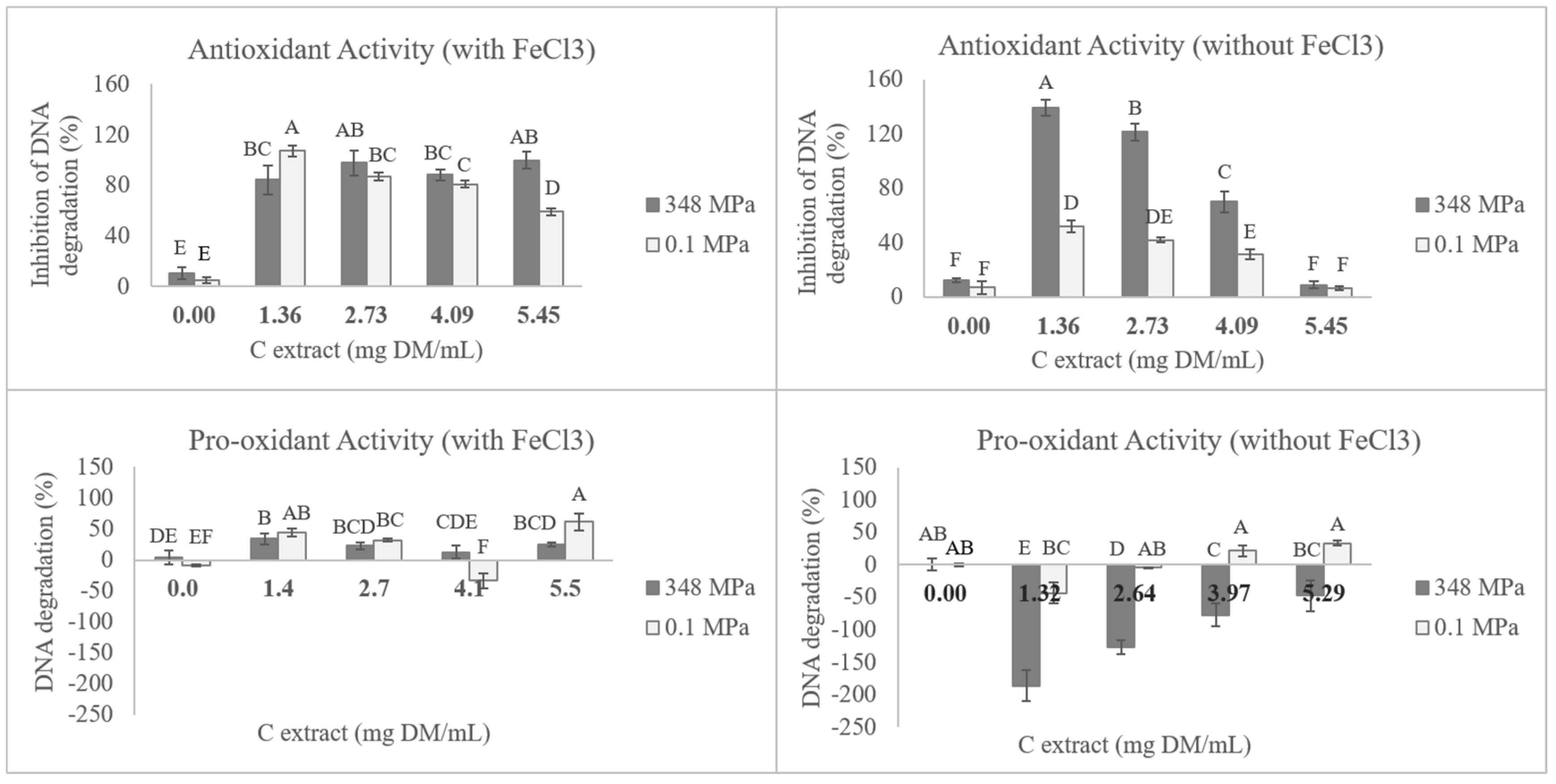
3.4. DNA Antioxidant Protection and Pro-Oxidant Activity
3.5. Cytotoxicity
3.6. Correlation between Variables
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Zeljković, S.Ć.; Topčagić, A.; Požgan, F.; Štefane, B.; Tarkowski, P.; Maksimović, M. Antioxidant activity of natural and modified phenolic extracts from Satureja montana L. Ind.Crop. Prod. 2015, 76, 1094–1099. [Google Scholar] [CrossRef]
- Coutinho de Oliveira, T.L.; Malfitano de Carvalho, S.; de Araújo Soares, R.; Andrade, M.A.; Cardoso, M.d.G.; Ramos, E.M.; Piccoli, R.H. Antioxidant effects of Satureja montana L. essential oil on TBARS and color of mortadella-type sausages formulated with different levels of sodium nitrite. Lwt—Food Sci. Technol. 2012, 45, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Giao, M.S.; Gonzalez-Sanjose, M.L.; Rivero-Perez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Foodagric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Hajdari, A.; Mustafa, B.; Kaciku, A.; Mala, X.; Lukas, B.; Ibraliu, A.; Stefkov, G.; Novak, J. Chemical composition of the essential oil, total phenolics, total flavonoids and antioxidant activity of methanolic extracts of Satureja montana L. Rec. Nat. Prod. 2016, 10, 750–760. [Google Scholar]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Švarc-Gajić, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds and antioxidant activity of a Mediterranean plant: The case of Satureja montana subsp. kitaibelii. J. Funct. Foods 2015, 18, 1167–1178. [Google Scholar] [CrossRef]
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Ricci, D.; Epifano, F.; Genovese, S.; Curini, M. Chemical composition and antifungal activity of the essential oil of Satureja montana from central Italy. Chem. Nat. Compd. 2007, 43, 622–624. [Google Scholar] [CrossRef]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M.F. Enrichment of the thymoquinone content in volatile oil from Satureja montana using supercritical fluid extraction. J. Sep. Sci. 2009, 32, 328–334. [Google Scholar] [CrossRef]
- Ćetković, S.G.; Čanadanović-Brunet, M.J.; Djilas, M.S.; Tumbas, T.V.; Markov, L.S.; Cvetković, D.D. Antioxidant potential, lipid peroxidation inhibition and antimicrobial activities of Satureja montana L. subsp. kitaibelii extracts. Int. J. Mol. Sci. 2007, 8, 1013–1027. [Google Scholar] [CrossRef] [Green Version]
- Elgndi, M.A.; Filip, S.; Pavlić, B.; Vladić, J.; Stanojković, T.; Žižak, Ž.; Zeković, Z. Antioxidative and cytotoxic activity of essential oils and extracts of Satureja montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 128–137. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Martins, A.; Salta, J.; Neng, N.R.; Nogueira, J.M.F.; Mira, D.; Gaspar, N.; Justino, J.; Grosso, C.; Urieta, J.S.; et al. Phytochemical profile and anticholinesterase and antimicrobial activities of supercritical versus conventional extracts of Satureja montana. J. Agric. Food Chem. 2009, 57, 11557–11563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, E.; Kelly, A.L.; Sun, D.-W. High pressure processing of foods: An overview. In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Academic Press: London, UK, 2005; pp. 3–32. [Google Scholar] [CrossRef]
- US-FDA. Kinetics of microbial inactivation for alternative food processing technologies—high pressure processing. Available online: http://www.fda.gov/Food/FoodScienceResearch/SafePracticesforFoodProcesses/ucm101456.htm (accessed on 12 November 2018).
- Huang, H.-W.; Hsu, C.-P.; Yang, B.B.; Wang, C.-Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.C.; Pintado, M.; Saraiva, J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. (Ott. Ont.) 2019, 115, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, S.A.; Pintado, M.; Saraiva, J.A. Optimization of antioxidant activity and bioactive compounds extraction of winter savory leaves by high hydrostatic pressure. High Press. Res. 2020, in press. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: London, UK, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Amorim, A.G.N.; Souza, J.M.T.; Santos, R.C.; Gullón, B.; Oliveira, A.; Santos, L.F.A.; Virgino, A.L.E.; Mafud, A.C.; Petrilli, H.M.; Mascarenhas, Y.P.; et al. HPLC-DAD, ESI–MS/MS, and NMR of lycopene isolated from P. guajava L. and its biotechnological applications. Eur. J. Lipid Sci. Technol. 2018, 120, 1700330. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Costa, E.M.; Pereira, M.F.; Costa, M.R.; Pintado, M.E. Evaluation of the antimicrobial activity of aqueous extracts from dry Vaccinium corymbosum extracts upon food microorganism. Food Control 2013, 34, 645–650. [Google Scholar] [CrossRef]
- M07-A8. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In Approved standard, 8th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; p. 0273-3099. [Google Scholar]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.M.; Calhau, C.; Pintado, M.M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Vicente, S.; Veiga, M.; Calhau, C.; Morais, R.M.; Pintado, M.E. DNA agarose gel electrophoresis for antioxidant analysis: Development of a quantitative approach for phenolic extracts. Food Chem. 2017, 233, 45–51. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef]
- Stinco, C.M.; Szczepanska, J.; Marszalek, K.; Pinto, C.A.; Inacio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Melendez-Martinez, A.J. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, G.S.; Mandić, A.I.; Čanadanović-Brunet, J.M.; Djilas, S.M.; Tumbas, V.T. HPLC screening of phenolic compounds in winter savory (Satureja montana L.) extracts. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 293–306. [Google Scholar] [CrossRef]
- Rangel, J.C.; Benavides Lozano, J.; Heredia, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990. [Google Scholar] [CrossRef]
- Palavra, A.M.F.; Coelho, J.P.; Barroso, J.G.; Rauter, A.P.; Fareleira, J.M.N.A.; Mainar, A.; Urieta, J.S.; Nobre, B.P.; Gouveia, L.; Mendes, R.L.; et al. Supercritical carbon dioxide extraction of bioactive compounds from microalgae and volatile oils from aromatic plants. J. Supercrit. Fluids 2011, 60, 21–27. [Google Scholar] [CrossRef]
- Coelho, J.P.; Cristino, A.F.; Matos, P.G.; Rauter, A.P.; Nobre, B.P.; Mendes, R.L.; Barroso, J.G.; Mainar, A.; Urieta, J.S.; Fareleira, J.M.N.A.; et al. Extraction of volatile oil from aromatic plants with supercritical carbon dioxide: Experiments and modeling. Molecules (Basel, Switz.) 2012, 17, 10550–10573. [Google Scholar] [CrossRef]
- Vladić, J.; Zeković, Z.; Jokić, S.; Svilović, S.; Kovačević, S.; Vidović, S. Winter savory: Supercritical carbon dioxide extraction and mathematical modeling of extraction process. J. Supercrit. Fluids 2016, 117, 89–97. [Google Scholar] [CrossRef]
- Serrano, C.; Matos, O.; Teixeira, B.; Ramos, C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and antimicrobial activity of Satureja montana L. extracts. J. Sci. Food Agric. 2011, 91, 1554–1560. [Google Scholar] [CrossRef]
- Damjanovic-Vratnica, B.; Perovic, S.; Lu, T.J.; Santos, R. Effect of matrix pretreatment on the supercritical CO2 extraction of Satureja montana essential oil. Chem. Ind. Chem. Eng. Q. 2016, 22, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhu, H.; Wang, J.; Liu, Z.; Bi, J. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J. Chromatogr. B 2009, 877, 733–737. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef]
- González de Llano, D.; Esteban-Fernández, A.; Sánchez-Patán, F.; Martínlvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Anti-adhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic Escherichia coli in bladder epithelial cell cultures. Int. J. Mol. Sci. 2015, 16, 12119–12130. [Google Scholar]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Horta, B.; Calhau, C.; Morais, R.M.; Pintado, M.M. Anti-biofilm potential of phenolic acids: The influence of environmental pH and intrinsic physico-chemical properties. Biofouling 2016, 32, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Jagani, S.; Chelikani, R.; Kim, D.-S. Effects of phenol and natural phenolic compounds on biofilm formation by Pseudomonas aeruginosa. Biofouling 2009, 25, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.A.; dos Santos Rodrigues, J.B.; Magnani, M.; de Souza, E.L.; de Siqueira-Júnior, J.P. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb. Pathog. 2017, 107, 193–197. [Google Scholar] [CrossRef]
- Oliveira, B.D.A.; Rodrigues, A.C.; Bertoldi, M.C.; Taylor, J.G.; Pinto, U.M. Microbial control and quorum sensing inhibition by phenolic compounds of acerola (Malpighia emarginata). Int. Food Res. J. 2017, 24, 2228–2237. [Google Scholar]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Diamantoglou, S.; Tajmir-Riahi, H.A. DNA interaction with naturally occurring antioxidant flavonoids quercetin, kaempferol, and delphinidin. J. Biomol. Struct. Dyn. 2005, 22, 719–724. [Google Scholar] [CrossRef]
- ISO10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Četojević-Simin, D.D.; Čanadanović-Brunet, J.M.; Bogdanović, G.M.; Ćetković, G.S.; Tumbas, V.T.; Djilas, S.M. Antioxidative and antiproliferative effects of Satureja montana L. extracts. J. BUON 2004, 9, 443–449. [Google Scholar]
- Koparal, A.T.; Zeytinoglu, M. Effects of carvacrol on a human non-small cell lung cancer (NSCLC) cell line, A549. Cytotechnology 2003, 43, 149–154. [Google Scholar] [CrossRef]
- Arunasree, K.M. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 581–588. [Google Scholar] [CrossRef]
- Fitsiou, E.; Anestopoulos, I.; Chlichlia, K.; Galanis, A.; Kourkoutas, I.; Panayiotidis, M.I.; Pappa, A. Antioxidant and antiproliferative properties of the essential oils of Satureja thymbra and Satureja parnassica and their major constituents. Anticancer Res. 2016, 36, 5757–5763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellion, P.; Olk, M.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Formation of hydrogen peroxide in cell culture media by apple polyphenols and its effect on antioxidant biomarkers in the colon cell line HT-29. Mol. Nutr. Food Res. 2009, 53, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic acids: Potential source of natural drugs for the treatment of fibrosis disease and cancer. Front Pharm. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.-L.; Li, Y.-H.; Lin, Y.-H.; Wang, R.; Li, Y.-B.; Tie, L.; Song, Q.-L.; Guo, D.-A.; Yu, H.-M.; Li, X. Salvianolic acid B protects human endothelial cells from oxidative stress damage: A possible protective role of glucose-regulated protein 78 induction. Cardiovasc. Res. 2008, 81, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Mohiseni, M. Medicinal herbs, strong source of antioxidant in aquaculture: A mini review. Mod. Appl. Pharm. Pharmacol. 2017, 1. [Google Scholar] [CrossRef]


| Pressure Level | Extraction Time | Solvent | Nomenclature | |
|---|---|---|---|---|
| Extract 1 | 500 MPa | 20 min | 0% (v/v) Ethanol | S500/20/0 |
| Extract 2 | 0.1 MPa | 20 min | 0% (v/v) Ethanol | S0.1/20/0 |
| Extract 3 | 348 MPa | 20 min | 35% (v/v) Ethanol | S348/20/35 |
| Extract 4 | 0.1 MPa | 20 min | 35% (v/v) Ethanol | S0.1/20/35 |
| Extract 5 | 500 MPa | 1 min | 70% (v/v) Ethanol | S500/1/70 |
| Extract 6 | 0.1 MPa | 1 min | 70% (v/v) Ethanol | S0.1/1/70 |
| Identification of Extracts | Total Phenolic Compounds (g/100 g DW) | Caffeic Acid | Tuberonic Acid Glucoside | 4-Butoxyphenol | Rosmarinic Acid | Sagerinic Acid | Salvianolic Acid A | Salvianolic Acid B Isomer | Antioxidant Activity (mgTrolox/g DW) |
|---|---|---|---|---|---|---|---|---|---|
| S500/20/0 | 2.4 ± 0.2 a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | (4.1 ± 0.5) × 102 c |
| S0.1/20/0 | 1.2 ± 0.9 c | ✓ | n.d. | ✓ | ✓ | n.d. | ✓ | ✓ | (4.1 ± 0.8) × 102 c |
| S348/20/35 | 2.3 ± 0.4 ab | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | (5.4 ± 0.6) × 102 b |
| S0.1/20/35 | 2.0 ± 0.4 ab | ✓ | ✓ | ✓ | ✓ | n.d. | ✓ | ✓ | (3.9 ± 0.2) × 102 c |
| S500/1/70 | 1.8 ± 0.2 bc | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | (6.8 ± 0.7) × 102 a |
| S0.1/1/70 | 1.2 ± 0.3 c | ✓ | n.d. | n.d. | ✓ | n.d. | ✓ | ✓ | (3.9 ± 0.4) × 102 c |
| Identification of Extracts | E. coli | S. enteritidis | S. aureus | B. cereus | L. monocytogenes | |
|---|---|---|---|---|---|---|
| MIC (mg/mL) | S500/20/0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| S0.1/20/0 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| S348/20/35 | n.d. | n.d. | 20 | 10 | 20 | |
| S0.1/20/35 | n.d. | n.d. | 20 | 10 | n.d. | |
| S500/1/70 | n.d. | n.d. | n.d. | 10 | n.d. | |
| S0.1/1/70 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| MBC (mg/mL) | S500/20/0 | >20 | >20 | >20 | >20 | >20 |
| S0.1/20/0 | >20 | >20 | >20 | >20 | >20 | |
| S348/20/35 | >20 | >20 | >20 | >20 | 20 | |
| S0.1/20/35 | >20 | >20 | >20 | >20 | >20 | |
| S500/1/70 | >20 | >20 | >20 | >20 | >20 | |
| S0.1/1/70 | >20 | >20 | >20 | >20 | >20 | |
| Biofilm formation inhibition (%) | E. coli | S. enteritidis | S. aureus | B. cereus | L. monocytogenes | |
| S500/20/0 | 89.05 ± 0.11 b | 81.37 ± 5.05 a | 11.75 ± 2.39 d | 98.42 ± 1.43 a | 89.60 ± 2.68 a | |
| S0.1/20/0 | 81.75 ± 1.20 c | 56.98 ± 10.26 b | −3.82 ± 1.66 e | 96.64 ± 2.35 a | 3.76 ± 1.04 c | |
| S348/20/35 | 91.51 ± 0.61 b | 42.02 ± 9.80 bc | 98.04 ± 1.41 a | 90.47 ± 1.06 a | 96.18 ± 0.67 a | |
| S0.1/20/35 | 69.02 ± 3.23 d | 12.44 ± 3.22 de | 93.51 ± 1.55 ab | 89.92 ± 6.73 a | 93.05 ± 6.02 a | |
| S500/1/70 | 97.62 ± 0.60 a | 35.19 ± 6.85 cd | 91.03 ± 1.63 b | 90.04 ± 1.46 a | 93.01 ± 7.05 a | |
| S0.1/1/70 | 81.68 ± 1.42 c | 5.00 ± 0.51 e | 41.16 ± 3.49 c | 58.97 ± 8.26 b | 74.20 ± 8.31 b |
| Antioxidant Activity (%) * | Pro-Oxidant Activity (%) * | Cytotoxicity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| With FeCl3 | Without FeCl3 | With FeCl3 | Without FeCl3 | HT29-MTX | Caco-2 | TR146 | HeLa | ||
| (%cell Metabolism Inhibition) | (IC50 (mg/mL)) | ||||||||
| 0.5 mg/mL | 1.0 mg/mL | ||||||||
| S500/20/0 | 73 ± 17 bc | 65 ± 11 a | −16 ± 14 c | −14 ± 7 d | −25 ± 2 c | 24 ± 3 d | 1.47 | 1.50 | 0.63 |
| S0.1/20/0 | 68 ± 5 c | 36 ± 7 b | 31 ± 6 b | 14 ± 4 c | 14 ± 7 a | 38 ± 0 a | 1.59 | 1.61 | 0.67 |
| S348/20/35 | 100 ± 7 ab | 9 ± 3 cd | 25 ± 6 b | −46 ± 23 e | −22 ± 5 c | 30 ± 2 bc | 1.64 | 0.67 | 0.85 |
| S0.1/20/35 | 59 ± 3 c | 6 ± 2 d | 62 ± 1 a | 34 ± 4 bc | −8 ± 2 b | 38 ± 2 a | 0.76 | 0.71 | 1.51 |
| S500/1/70 | 124 ± 13 a | 22 ± 3 c | 28 ± 4 b | 46 ± 6 ab | 13 ± 3 a | 25 ± 1 cd | 1.93 | 1.80 | 1.72 |
| S0.1/1/70 | 73 ± 17 c | 20 ± 5 cd | 76 ± 11 a | 63 ± 12 a | 22 ± 2 a | 32 ± 1 b | 1.49 | 1.57 | 1.53 |
| DNA Degradation (%) | Inhibition of DNA Degradation (%) | Biofilm Formation Inhibition (%) | Cytotoxicity (% Cell Viability) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORAC (mg/g) | Without Fe3+ | With Fe3+ | Without Fe3+ | With Fe3+ | E. coli | S. enteritidis | S. aureus | B. cereus | L. monocytogens | HT29-MTX | Caco−2 | TR146 | HeLa | ||
| Pearson Correlation (R) | Phenolics | 0.376 (0.206) | −0.638 (0.010) | −0.524 (0.045) | −0.431 (0.084) | 0.044 (0.862) | 0.273 (0.273) | 0.307 (0.248) | 0.331 (0.179) | 0.458 (0.056) | 0.656 (0.004) | −0.876 (0.000) | −0.837 (0.022) | −0.806 (0.013) | 0.693 (0.016) |
| ORAC | n.d. | −0.381 (0.145) | −0.064 (0.813) | −0.267 (0.285) | 0.776 (0.000) | 0.421 (0.225) | −0.045 (0.908) | 0.538 (0.108) | −0.048 (0.896) | 0.474 (0.197) | 0.382 (0.198) | −0.006 (0.978) | 0.117 (0.596) | 0.154 (0.433) | |
| Spearman correlation (rho) | Phenolics | 0.264 (0.384) | −0.579 (0.024) | −0.568 (0.027) | −0.348 (0.171) | 0.848 (0.049) | 0.375 (0.125) | 0.312 (0.239) | 0.325 (0.188) | 0.349 (0.156) | 0.551 (0.022) | −0.862 (0.000) | −0.296 (0.266) | −0.529 (0.043) | 0.499 (0.069) |
| ORAC | n.d. | −0.412 (0.113) | −0.168 (0.535) | −0.168 (0.505) | 0.717 (0.001) | 0.491 (0.150) | 0.000 (1.000) | 0.285 (0.425) | −0.467 (0.174) | 0.267 (0.488) | −0.341 (0.255) | 0.019 (0.926) | −0.150 (0.494) | 0.234 (0.230) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, S.A.; Silva, S.; Costa, E.; Pinto, S.; Sarmento, B.; Saraiva, J.A.; Pintado, M. Effect of High Hydrostatic Pressure Extraction on Biological Activities and Phenolics Composition of Winter Savory Leaf Extracts. Antioxidants 2020, 9, 841. https://doi.org/10.3390/antiox9090841
Moreira SA, Silva S, Costa E, Pinto S, Sarmento B, Saraiva JA, Pintado M. Effect of High Hydrostatic Pressure Extraction on Biological Activities and Phenolics Composition of Winter Savory Leaf Extracts. Antioxidants. 2020; 9(9):841. https://doi.org/10.3390/antiox9090841
Chicago/Turabian StyleMoreira, Sílvia A., Sara Silva, Eduardo Costa, Soraia Pinto, Bruno Sarmento, Jorge A. Saraiva, and Manuela Pintado. 2020. "Effect of High Hydrostatic Pressure Extraction on Biological Activities and Phenolics Composition of Winter Savory Leaf Extracts" Antioxidants 9, no. 9: 841. https://doi.org/10.3390/antiox9090841
APA StyleMoreira, S. A., Silva, S., Costa, E., Pinto, S., Sarmento, B., Saraiva, J. A., & Pintado, M. (2020). Effect of High Hydrostatic Pressure Extraction on Biological Activities and Phenolics Composition of Winter Savory Leaf Extracts. Antioxidants, 9(9), 841. https://doi.org/10.3390/antiox9090841









