Yuzu and Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Transient Brain Ischemia Model
2.3. Measurement of Brain Water Content
2.4. Measurement of Evans Blue Extravasation
2.5. Immunohistochemistry
2.6. bEnd.3 Cell Culture
2.7. Permeability Assay In Vitro
2.8. Immunocytochemistry
2.9. Western Blot Analysis
2.10. Subcellular Preparation for Membrane and Cytoskeleton Fraction
2.11. Reverse Transcription Polymerase Chain Reaction
2.12. siRNA Transfection
2.13. 2,2-Diphenyl-1-Picrylhydrazyl Assay
2.14. 2’7’-Dichlorofluorescein Diacetate Fluorescence Assay
2.15. Statistical Analysis
3. Results
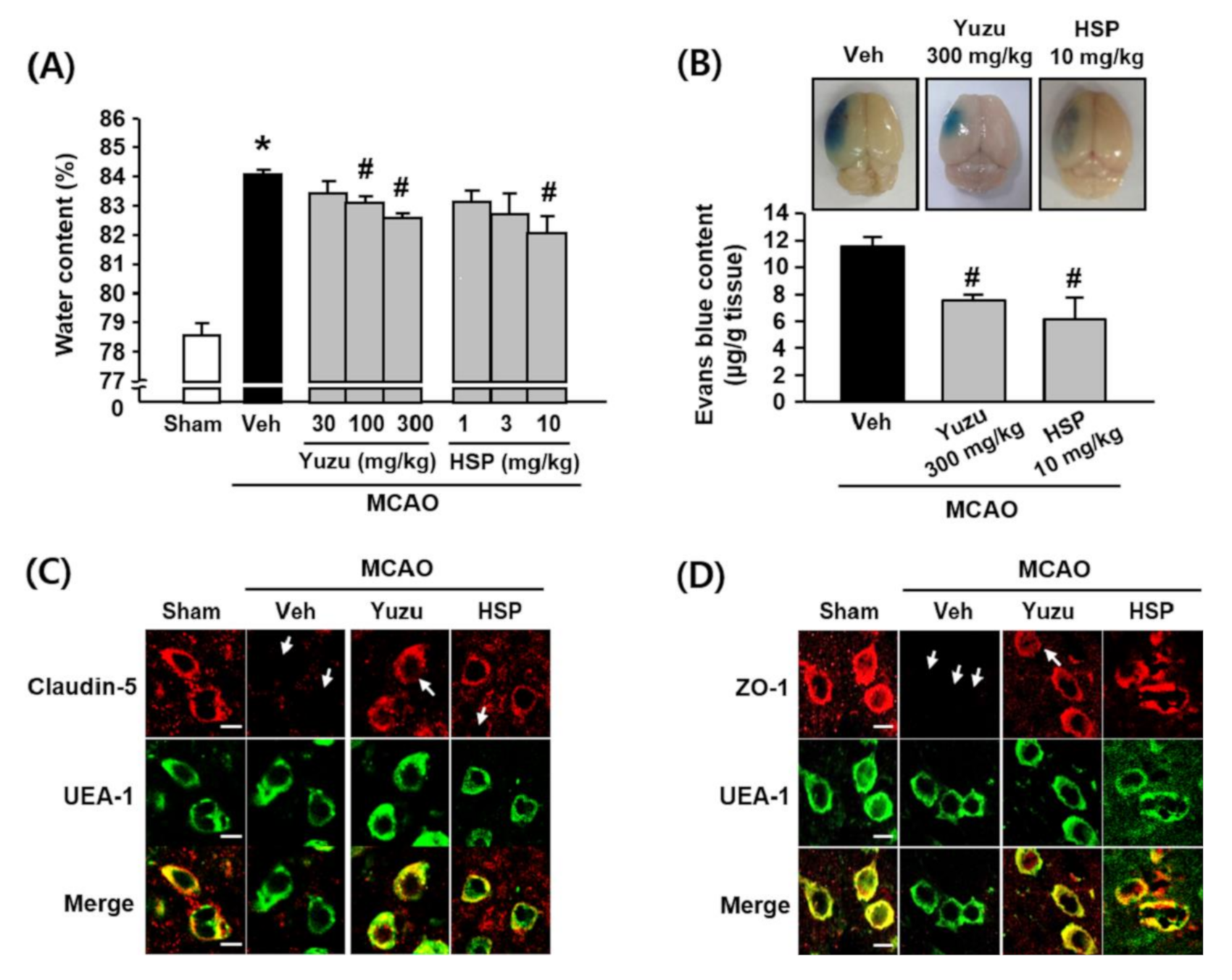
3.1. Effects of Yuzu and HSP on Brain Edema and BBB Dysfunction in a Mouse MCAO Model
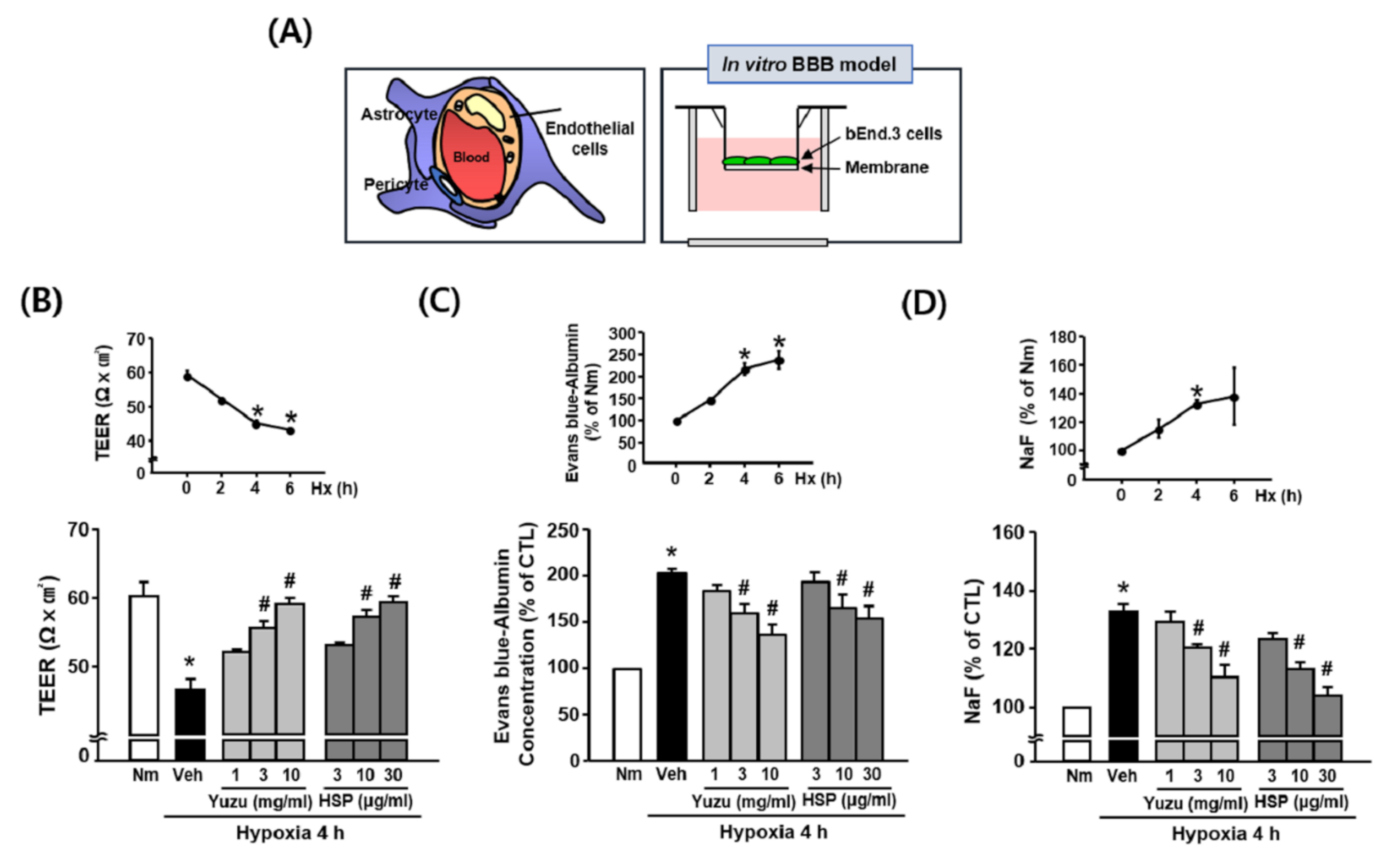
3.2. Effects of Yuzu and HSP on BBB Permeability during Hypoxia in bEnd.3 Cells
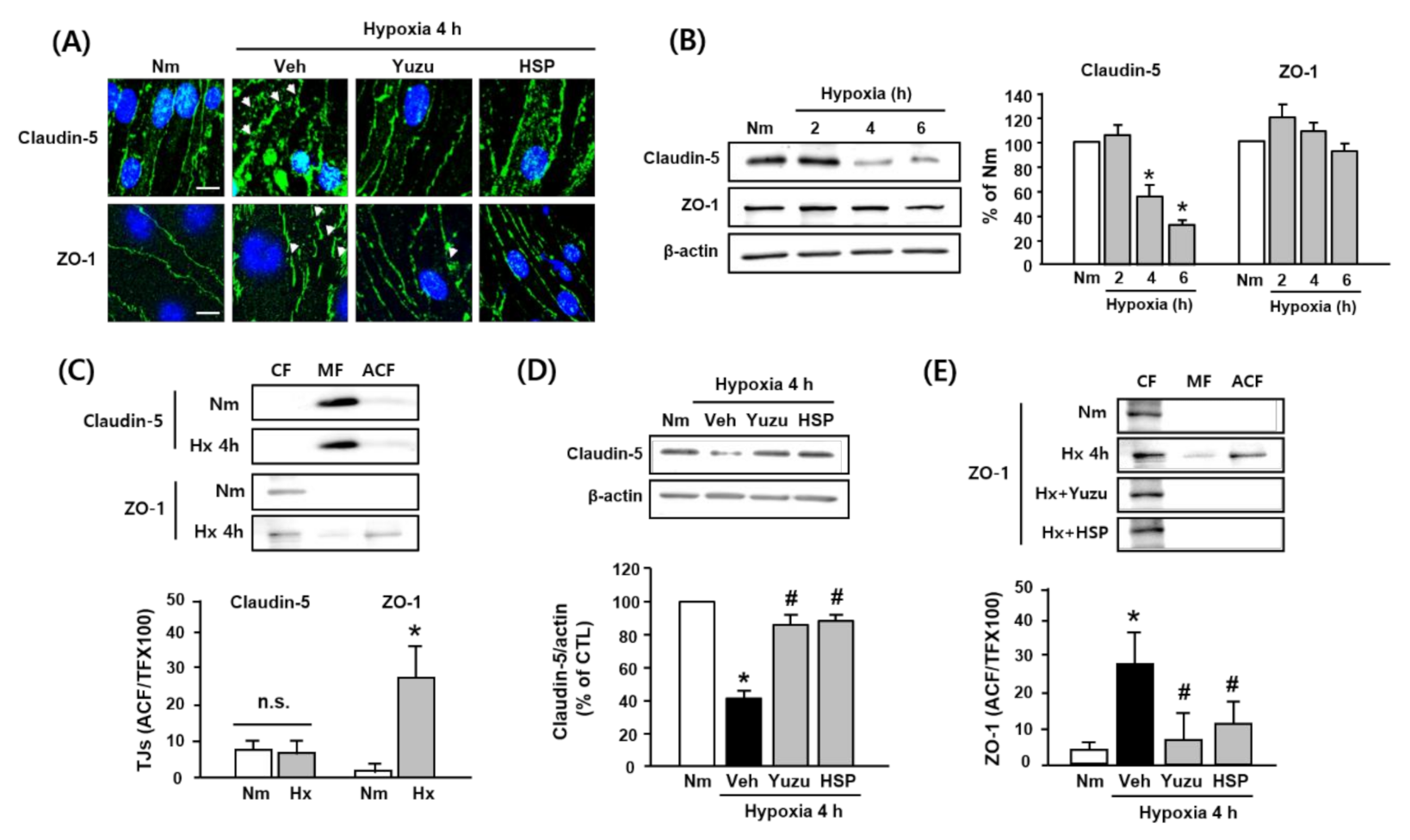
3.3. Effects of Yuzu and HSP on Disruption of Claudin-5 and ZO-1 during Hypoxia in bEnd.3 Cells
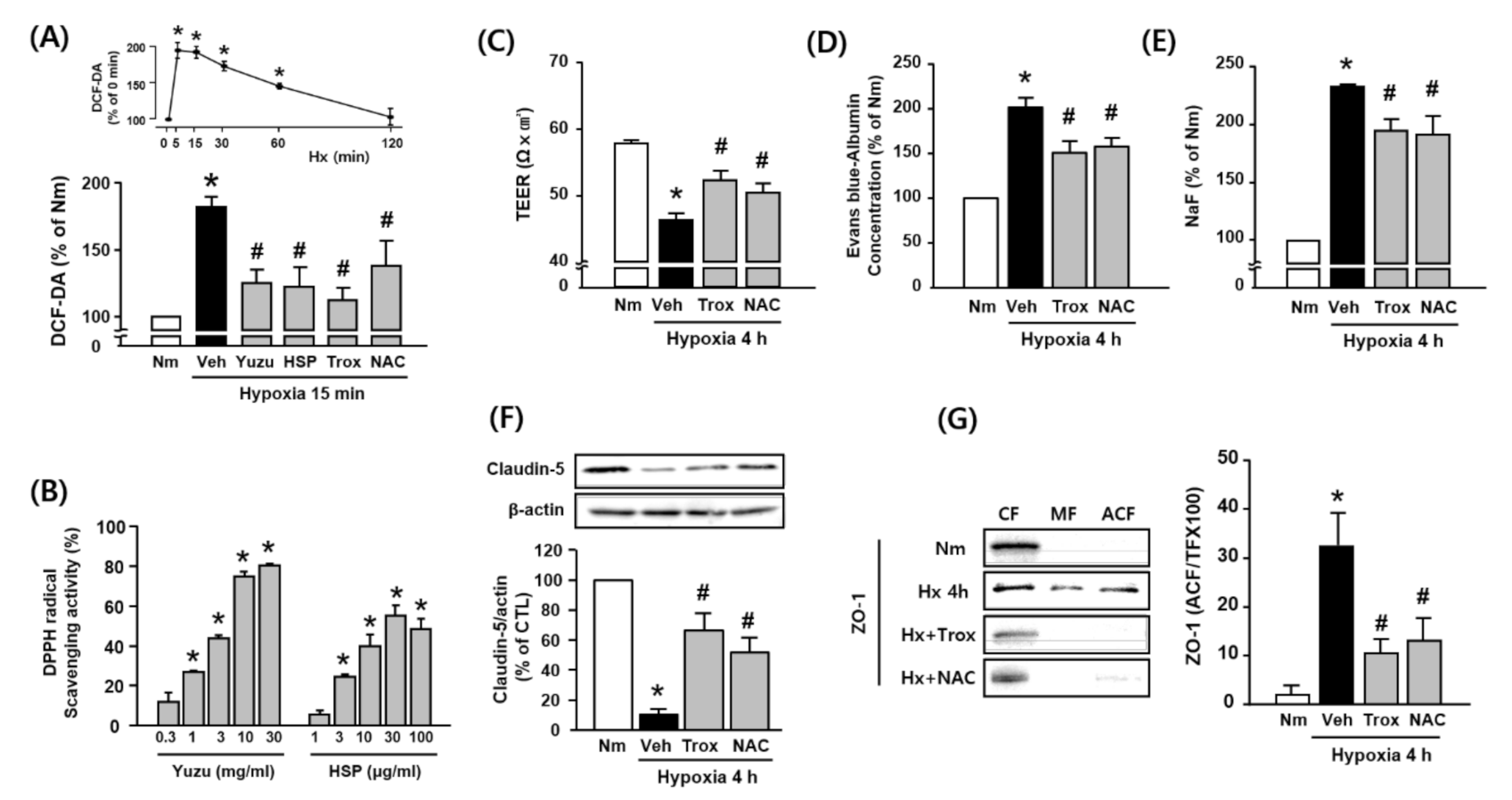
3.4. Protective Effects of Yuzu and HSP on BBB are Associated with Antioxidant Activity in bEnd.3 Cells
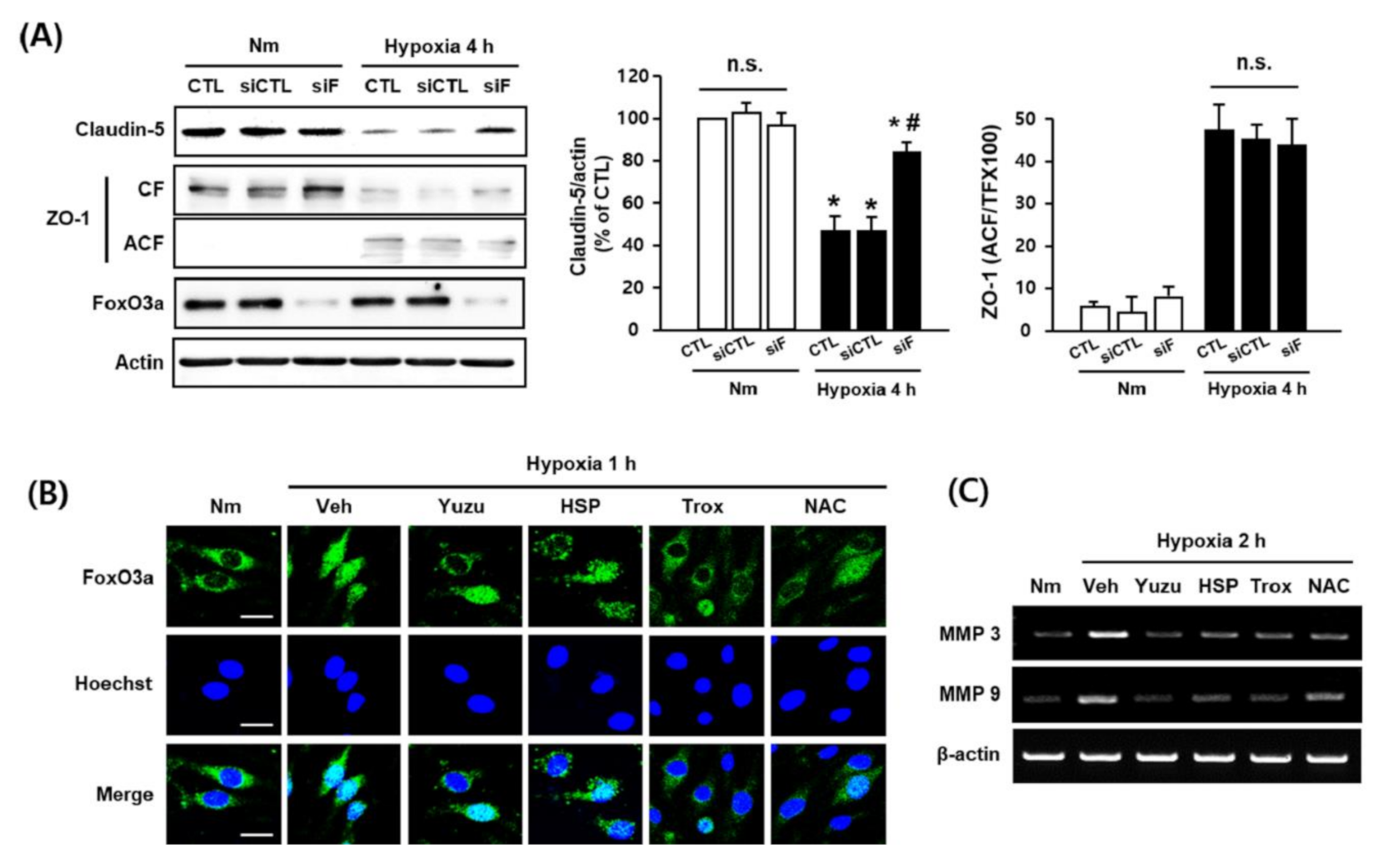
3.5. Protective Effects of Yuzu and HSP on FoxO3a/MMP-Mediated Claudin-5 Degradation are Associated with their Antioxidant Activity in bEnd.3 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke J. Cereb. Circ. 2011, 42, 3323–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Yue, Y.; Li, J.; Li, Z.; Li, X.; Niu, Y.; Xiang, J.; Ding, H. Procyanidin B2 attenuates neurological deficits and blood-brain barrier disruption in a rat model of cerebral ischemia. Mol. Nutr. Food Res. 2015, 59, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Choi, S.M.; Whitcomb, D.J.; Kim, B.C. Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis. 2017, 8, e3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinciguerra, L.; Lanza, G. Update on the Neurobiology of Vascular Cognitive Impairment: From Lab to Clinic. Int. J. Mol. Sci. 2020, 21, 2977. [Google Scholar] [CrossRef] [Green Version]
- Bordet, R.; Ihl, R.; Korczyn, A.D.; Lanza, G.; Jansa, J.; Hoerr, R.; Guekht, A. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: A consensus report. BMC Med. 2017, 15, 107. [Google Scholar]
- Rosenberg, G.A. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin. Sci. Lond. Engl. 1979 2017, 131, 425–437. [Google Scholar] [CrossRef]
- Govindpani, K.; McNamara, L.G.; Smith, N.R.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J. Clin. Med. 2019, 8, 651. [Google Scholar] [CrossRef] [Green Version]
- Bowman, G.L.; Dayon, L.; Kirkland, R.; Wojcik, J.; Peyratout, G.; Severin, I.C.; Henry, H.; Oikonomidi, A.; Migliavacca, E.; Bacher, M.; et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 1640–1650. [Google Scholar] [CrossRef]
- Joseph, C.R. Novel MRI Techniques Identifying Vascular Leak and Paravascular Flow Reduction in Early Alzheimer Disease. Biomedicines 2020, 8, 228. [Google Scholar] [CrossRef]
- Magaki, S.; Tang, Z.; Tung, S.; Williams, C.K.; Lo, D.; Yong, W.H.; Khanlou, N.; Vinters, H.V. The effects of cerebral amyloid angiopathy on integrity of the blood-brain barrier. Neurobiol. Aging 2018, 70, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; van Doorn, R.; Gringhuis, S.I.; van der Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Piontek, J.; et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. Off. Publ. Feder. Am. Soc. Exp. Biol. 2007, 21, 3666–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.Y.; Wang, Z.J.; Sun, D.M.; Wang, Y. Novel Therapeutic Effects of Leonurine on Ischemic Stroke: New Mechanisms of BBB Integrity. Oxid. Med. Cell. Longev. 2017, 2017, 7150376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wetering, S.; van Buul, J.D.; Quik, S.; Mul, F.P.; Anthony, E.C.; ten Klooster, J.P.; Collard, J.G.; Hordijk, P.L. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J. Cell Sci. 2002, 115, 1837–1846. [Google Scholar]
- Kim, T.H.; Kim, H.M.; Park, S.W.; Jung, Y.S. Inhibitory effects of yuzu and its components on human platelet aggregation. Biomol. Ther. 2015, 23, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Raza, S.S.; Khan, M.M.; Ahmad, A.; Ashafaq, M.; Khuwaja, G.; Tabassum, R.; Javed, H.; Siddiqui, M.S.; Safhi, M.M.; Islam, F. Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Res. 2011, 1420, 93–105. [Google Scholar] [CrossRef]
- Ashafaq, M.; Varshney, L.; Khan, M.H.; Salman, M.; Naseem, M.; Wajid, S.; Parvez, S. Neuromodulatory effects of hesperidin in mitigating oxidative stress in streptozotocin induced diabetes. BioMed Res. Int. 2014, 2014, 249031. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.Y.; Ahn, J.H.; Park, S.W.; Jung, Y.S. Preventive effect of yuzu and hesperidin on left ventricular remodeling and dysfunction in rat permanent left anterior descending coronary artery occlusion model. PLoS ONE 2015, 10, e110596. [Google Scholar] [CrossRef]
- Park, J.A.; Oh, J.E.; Cho, M.S. Development of yuja (Citrus junos) beverage based on antioxidant properties and sensory attributes using response surface methodology. J. Food Sci. Technol. 2019, 56, 1854–1863. [Google Scholar] [CrossRef]
- Assefa, A.D.; Ko, E.Y.; Moon, S.H.; Keum, Y.S. Antioxidant and antiplatelet activities of flavonoid-rich fractions of three citrus fruits from Korea. 3 Biotech 2016, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Donato, F.; de Gomes, M.G.; Goes, A.T.; Filho, C.B.; Del Fabbro, L.; Antunes, M.S.; Souza, L.C.; Boeira, S.P.; Jesse, C.R. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res. Bull. 2014, 104, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.W.; Jang, M.; Park, S.W.; Kim, E.J.; Jung, Y.S. Onion (Allium cepa) extract attenuates brain edema. Nutrition 2013, 29, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Lee, M.Y.; Shin, I.S.; Seo, C.S.; Ha, H.; Shin, H.K. Anti-inflammatory effects of Amomum compactum on RAW 264.7 cells via induction of heme oxygenase-1. Arch. Pharm. Res. 2012, 35, 739–746. [Google Scholar] [CrossRef]
- Jung, S.Y.; Choi, S.H.; Yoo, S.Y.; Baek, S.H.; Kwon, S.M. Modulation of Human Cardiac Progenitors via Hypoxia-ERK Circuit Improves their Functional Bioactivities. Biomol. Ther. 2013, 21, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelke, G.V.; Jagtap, J.C.; Kim, D.K.; Shah, R.D.; Das, G.; Shivayogi, M.; Pujari, R.; Shastry, P. TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas. Biomedicines 2017, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Hyun, S.W.; Jung, Y.S. Hypoxia induces FoxO3a-mediated dysfunction of blood-brain barrier. Biochem. Biophys. Res. Commun. 2014, 450, 1638–1642. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.Y.; Wang, Z.B.; Zhang, L.C.; Wei, X.; Li, L. Tight junction in blood-brain barrier: An overview of structure, regulation, and regulator substances. CNS Neurosci. Ther. 2012, 18, 609–615. [Google Scholar] [CrossRef]
- Ferrer, I. Cognitive impairment of vascular origin: Neuropathology of cognitive impairment of vascular origin. J. Neurol. Sci. 2010, 299, 139–149. [Google Scholar] [CrossRef]
- Skrobot, O.A.; O’Brien, J.; Black, S.; Chen, C.; DeCarli, C.; Erkinjuntti, T.; Ford, G.A.; Kalaria, R.N.; Pantoni, L.; Pasquier, F.; et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s Dement. 2017, 13, 624–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrobot, O.A.; Attems, J.; Esiri, M.; Hortobágyi, T.; Ironside, J.W.; Kalaria, R.N.; King, A.; Lammie, G.A.; Mann, D.; Neal, J.; et al. Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain J. Neurol. 2016, 139, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Al-Ahmad, A.J.; Gassmann, M.; Ogunshola, O.O. Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J. Cell. Physiol. 2014, 229, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain endothelial cell-cell junctions: how to open the blood brain barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Luissint, A.C.; Federici, C.; Guillonneau, F.; Chretien, F.; Camoin, L.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.O. Guanine nucleotide-binding protein Galphai2: A new partner of claudin-5 that regulates tight junction integrity in human brain endothelial cells. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2012, 32, 860–873. [Google Scholar] [CrossRef]
- Haileselassie, B.; Joshi, A.U.; Minhas, P.S.; Mukherjee, R.; Andreasson, K.I.; Mochly-Rosen, D. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy. J. Neuroinflamm. 2020, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Leng, Y.; Tsai, L.K.; Leeds, P.; Chuang, D.M. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: The roles of HDAC and MMP-9 inhibition. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2011, 31, 52–57. [Google Scholar] [CrossRef]
- Liu, J.; Jin, X.; Liu, K.J.; Liu, W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 3044–3057. [Google Scholar] [CrossRef]
- Hicks, K.; O’Neil, R.G.; Dubinsky, W.S.; Brown, R.C. TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am. J. Physiol. Cell Physiol. 2010, 298, C1583–C1593. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; Wobben, M.; Marti, H.H.; Renz, D.; Schaper, W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc. Res. 2002, 63, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Pun, P.B.; Lu, J.; Moochhala, S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 2009, 43, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Bulboaca, A.E.; Boarescu, P.M. The Effect of Nano-Epigallocatechin-Gallate on Oxidative Stress and Matrix Metalloproteinases in Experimental Diabetes Mellitus. Antioxidants (Basel) 2020, 9, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Namkoong, K.; Kim, D.H.; Kim, K.J.; Cheong, Y.H.; Kim, S.S.; Lee, W.B.; Kim, K.Y. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc. Res. 2004, 68, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.N.; Schwesinger, C.; Ye, J.; Denker, B.M.; Nigam, S.K. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J. Biol. Chem. 2001, 276, 22048–22055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polter, A.; Yang, S.; Zmijewska, A.A.; van Groen, T.; Paik, J.H.; Depinho, R.A.; Peng, S.L.; Jope, R.S.; Li, X. Forkhead box, class O transcription factors in brain: Regulation and behavioral manifestation. Biol. Psychiatry 2009, 65, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.; Chen, J.; Yuan, Z. Post-translational regulation of FOXO. Acta Biochim. Biophys. Sin. 2012, 44, 897–901. [Google Scholar] [CrossRef] [Green Version]
- Salih, D.A.; Brunet, A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008, 20, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, K.; Shioda, N. Pathophysiological relevance of forkhead transcription factors in brain ischemia. Adv. Exp. Med. Biol. 2009, 665, 130–142. [Google Scholar]
- Zhang, S.; Zhao, Y.; Xu, M.; Yu, L.; Zhao, Y.; Chen, J.; Yuan, Y.; Zheng, Q.; Niu, X. FoxO3a modulates hypoxia stress induced oxidative stress and apoptosis in cardiac microvascular endothelial cells. PLoS ONE 2013, 8, e80342. [Google Scholar]
- Lee, H.Y.; You, H.J.; Won, J.Y.; Youn, S.W.; Cho, H.J.; Park, K.W.; Park, W.Y.; Seo, J.S.; Park, Y.B.; Walsh, K.; et al. Forkhead factor, FOXO3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 302–308. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Tumer, T.B.; Moreira, A.C.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennisi, M.; Lanza, G.; Cantone, M.; D’Amico, E.; Fisicaro, F.; Puglisi, V.; Vinciguerra, L.; Bella, R.; Vicari, E.; Malaguarnera, G. Acetyl-L-Carnitine in Dementia and Other Cognitive Disorders: A Critical Update. Nutrients 2020, 12, 1389. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.K.; Hyun, S.-W.; Jung, Y.-S. Yuzu and Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity. Antioxidants 2020, 9, 843. https://doi.org/10.3390/antiox9090843
Lee BK, Hyun S-W, Jung Y-S. Yuzu and Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity. Antioxidants. 2020; 9(9):843. https://doi.org/10.3390/antiox9090843
Chicago/Turabian StyleLee, Bo Kyung, Soo-Wang Hyun, and Yi-Sook Jung. 2020. "Yuzu and Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity" Antioxidants 9, no. 9: 843. https://doi.org/10.3390/antiox9090843
APA StyleLee, B. K., Hyun, S.-W., & Jung, Y.-S. (2020). Yuzu and Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity. Antioxidants, 9(9), 843. https://doi.org/10.3390/antiox9090843




