1. Introduction
Leishmaniases are neglected tropical diseases caused by the infection with
Leishmania parasites, and are transmitted by the bite of a sand fly belonging to the genera
Lutzomyia and
Phlebotomus. Leishmaniases are endemic in large areas of the tropics, subtropics, and the Mediterranean basin, and are among the major neglected tropical diseases causing morbidity worldwide. Recently, it has broken out of its traditional boundaries and has been reported in new geographic locations with atypical disease manifestations involving novel parasite variants. Cutaneous leishmaniasis (CL) is endemic in more than 70 countries, with an estimated annual incidence of 1.5–2 million new cases, and clinical manifestations ranging from small skin nodules to massive destruction of the mucous tissues. CL is mainly caused by
Leishmania major in the Old World and by
L. amazonensis and
L. braziliensis in the New World, specifically in Brazil [
1]. In spite of the high prevalence, and advances in the chemotherapy for leishmaniasis, the current available drugs, including pentavalent antimonials, amphotericin B, miltefosine, paromomycin, and pentamidine are compromised by the emergence of resistance, variable sensitivity between species, adverse side effects, requirements for long courses of administration, and high cost [
2]. These drawbacks and the absence of vaccines underline the urgent need for searching alternative treatments with acceptable efficacy and safety profile.
Natural products are an important source of leishmanicidal drugs owing to their accessibility, structural diversity, low cost, and possible rapid biodegradation [
3,
4,
5]. In South America, where resorting to medicinal plants represents a primary health care measure of the native population, several species of
Piper genus are widely used as a remedy to relieve the symptoms of leishmaniasis disease. Thus, the leaves of
Piper aduncum,
P. loretoanum, and
P. hispidum are used as poultices for healing wounds and to treat the symptoms of CL [
6,
7]. In addition,
Piper species are used as culinary spices, and as a food preservative to control food spoilage and pathogenic microorganisms. In particular,
P. nigrum (black pepper) is worldwide popular as a flavoring for food [
8]. Phytochemical investigations of
Piper species have reported numerous metabolites with ecological and medicinal properties, including amides, pyrones, lignanes, terpenes, and flavonoids [
8]. Alkamides, also named piperamides, are characteristic bioactive constituents in
Piper species [
9]. In particular, (
E)-piplartine, also called piperlongumine, is the major natural alkaloid from
P. longum and
P. tuberculatum, and in vitro and in vivo studies have demonstrated its promising pharmacological properties such as antioxidant, anxiolytic, anti-atherosclerosis, antidiabetic, and antiparasitic against neglected tropical diseases [
10]. Moreover, (
E)-piplartine is reported to kill a large variety of cancer cells while remaining nontoxic to normal cells, highlighting its therapeutic potential [
11,
12].
In previous investigations, we reported the isolation of an unprecedented chlorine-containing piperamide along with several known compounds and their antileihmanicidal activity from
Piper pseudoarboreum [
13]. In continuous research toward the discovery of natural occurring leihmanicidal agents, we report herein on the isolation and structure elucidation of six known alkamides from the leaves of
P. pseudoarboreum Yunker through a bioassay-guided fractionation carried out against four promastigote strains of
Leishmania. Compounds
2 and
3 were further evaluated on intracellular amastigotes of
L amazonensis and
L. infantum. (
E)-piplartine (
3) was selected to be assayed in an in vivo model for cutaneous leishmaniasis.
2. Materials and Methods
2.1. General Experimental Procedures
The structure of the isolated compounds were elucidated using spectrometric and spectroscopic methods, and comparison with data previously reported. The Nuclear Magnetic Resonance (NMR) experiments were recorded on Bruker Avance 400 and 500 spectrometers (Bruker Co. Billerica, MA, USA); chemical shifts were referred to the residual solvent signal (CDCl3: δH 7.26, δC 77.36) (acetone d6: δH 2.09, δC 30.60 and 205.87), using trimethylsilane (TMS) as internal standard. Electron Impact Mass Spectrometry (EIMS) and High Resolution Electron Impact Mass Spectrometry (HREIMS) were recorded on a Micromass Autospec spectrometer (Micromass, Manchester, UK). Silica gel 60 (15–40 mm) and silica gel 60 F254 for column chromatography and Thin Layer Chromatography (TLC), respectively, were purchased from Panreac (Barcelona, Spain). Sephadex LH-20 was obtained from Pharmacia Biotech (Pharmacia, Uppsala, Sweden). Centrifugal planar chromatography was carried out in a Chromatotron instrument (model 7924T, Harrison Research Inc., Palo Alto, CA, USA) on manually coated silica gel 60 GF254 (Merck, Darmstadt, Germany) using 4-mm plates. The spots were visualized by UV light and heating silica gel plates sprayed with H2O-H2SO4-AcOH (1:4:20).
2.2. Chemicals and Reagents
All solvents used were of analytical grade and purchased from Panreac (Barcelona, Spain). (E)-Piplartine, Scheneider’s insect medium, RPMI-1640, fetal bovine serum (FBS), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), resazurin sodium salt, and sodium dodecyl sulphate (Sigma-Aldrich, St Louis, MO, USA), L-glutamine (Avantor Performance Material Inc., PA, USA), trypsin (Merck, Darmstadt, Germany), penicillin Penilevel® 100.000 U.I. (ERN laboratories, Barcelona, Spain), streptomycin sulphate (Reig Jofré laboratories, Barcelona, Spain), and Glucantime® (Merial Laboratories, Barcelona, Spain).
2.3. Plant Material
Leaves of Piper pseudoarboreum Yunck. were collected in November 2009 at Iquitos, Maynas Province, Department of Loreto, Perú. The plant material was identified by the botanist Juan Celedonio Ruiz Macedo, and a voucher specimen (AMZ 11114) was deposited at the Amazonense Herbarium of the Universidad Nacional de la Amazonia Peruana, Iquitos, Perú.
2.4. Extraction, Bioassay-Guided Fractionation and Isolation
The dried leaves of P. pseudoarboreum (200.3 g) were powdered and extracted in a Soxhlet apparatus with 5 L of 96% ethanol. The solvent was evaporated to give 42.9 g (21.4%) of extract. The ethanolic extract (EtOH) was partitioned into dichlorometane (DCM), ethyl acetate (EtOAc), and water (H2O). After removing the organic solvents under reduced pressure, the DCM (9.2 g, 4.6%) and EtOAc (1.2 g, 0.6%) fractions were obtained, whereas the aqueous-soluble extract was lyophilized providing the H2O fraction (8.9 g, 4.5%). The most active organic fraction (DCM, 9.2 g) was chromatographed over silica gel column eluting with mixtures of hexanes-EtOAc (10:0 to 0:10, 1 L each one) to obtain seven sub-fractions (F1–F7). The most active fraction, F6 (1.5 g), was subjected to column chromatography over Sephadex LH-20 by isocratic elution (MeOH-CHCl3, 1:1) to afford fifteen sub-fractions, which were combined based on their TLC profiles (F6A to F6F). Preliminary nuclear magnetic resonance (NMR) studies revealed that sub-fraction F6B was rich in aromatic alkamides, and were further investigated. Thus, F6B (448.1 mg) was chromatographed by centrifugal planar chromatography on 4-mm silica gel plates, using mixtures of hexanes-EtOAc (60:40 to 50:40) as eluent to give eleven sub-fractions (F6B1 to F6B11). Sub-fraction F6B2 (21.5 mg) was further purified on silica gel by preparative TLC (3 × development, hexanes-2-propanol, 8:2) to give compounds 1 (1.7 mg) and 5 (1.4 mg). Purification of sub-fraction F6B4 (18.3 mg) by preparative TLC (2 × development, CH2Cl2-Et2O, 95:5) yielded compounds 3 (11.4 mg) and 4 (2.2 mg), whereas sub-fraction F6B7 (23.8 mg) gave compounds 2 (19.8 mg) and 6 (0.9 mg) after purification by preparative TLC (2 × development, hexanes-2-propanol, 8:2). The compounds were identified by NMR spectroscopy and comparison with data reported in the literature.
2.5. Biological Studies
2.5.1. Parasites
Autochthonous isolates of Leishmania infantum (MCAN/ES/92/BCN83) were obtained from an asymptomatic dog from the Priorat region (Catalunya, Spain), and kindly provided by Prof. Montserrat Portús (University of Barcelona). L. braziliensis (2903), L. amazonensis (MHOM/Br/79/Maria) and L. guyanensis (141/93) were kindly given by Prof. Alfredo Toraño (Instituto de Salud Carlos III, Madrid).
2.5.2. Cells
J774 murine macrophages were grown and maintained in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, penicillin G (100 U/mL), and streptomycin (100 μg/mL) at 37 °C and 5% CO2 air atmosphere.
2.5.3. Animals
Male BALB/c mice of 20–25 g body weigh were purchased from Harlan Interfauna Ibérica (Barcelona, Spain). All rodents were housed in plastic cages in a 12 h dark–light cycle under controlled temperature (25 °C) and humidity (70%) conditions. During the study, animals had unrestricted access to food and water.
2.5.4. In Vitro Promastigotes Susceptibility Assay
In vitro antileishmanial assay was performed using a method described elsewhere [
14]. Briefly, promastigotes were grown in vitro in a Schneider’s insect medium supplemented with 20% heat-inactivated FBS, penicillin (100 U/mL) and streptomycin (100 μg/mL) at 26 °C in 25 mL in tissue culture flasks, and were cultured in 96-well plastic plates (2.5 × 10
5 parasites/well). Compounds dissolved in dimethylsulfoxide 1% (DMSO) at the suitable concentration to be tested in serial dilutions (a first screening using 100 μg/mL, and then 100, 50, 25, 12.5, 6.25, 3.12, 1.56 and 0.78 μg/mL) to get a final volume of 200 μL were added to each well. After an incubation of 48 h at 26 °C, 20 μL of 2.5 mM resazurin solution was added. Plates were then analyzed by fluorescence emission (535
ex–590
em nm) using a fluorometer Infinite 200 (Tecan i-Control, Tecan Group Ltd, Männedorf, Switzerland). All tests were carried out in triplicate, and miltefosine was used as the reference drug. The antileishmanial activity of each compound was estimated by calculating the GI% (percentage of growth inhibition) and then the IC
50 value (concentration of the compound that produced a 50% reduction in parasites).
2.5.5. Cytotoxicity Assay
The cytotoxicity assay of the tested compounds was performed according to a previously described method [
14]. Briefly, J774 macrophages (5 × 10
4 cells/well) were placed in 96-well flat-bottom plates with 100 μL of RPMI-1640 medium, and allowed to attach at 37 °C and 5% CO
2 for 2 h. Afterwards, 100 μL of RPMI-1640 medium containing the test compound in varying concentrations (100, 50, 25, 12.5, 6.25, 3.12, 1.56, and 0.78 µg/mL) were added to the cells and incubated for another 48 h. Growth controls and signal-to-noise were included. Following the aforementioned incubation time, 20 μL of 2.5 mM resazurin solution in PBS was added, and the plates were placed again in the incubator for another 3 h to evaluate cell viability. The ability of cells to reduce resazurin was determined by fluorometry as in the promastigote assay. Each concentration was assayed in triplicate. Cytotoxicity was expressed as the 50% reduction of cell viability of treated culture cells with respect to untreated culture (CC
50).
2.5.6. In Vitro Amastigote Assay
The effectiveness against intracellular amastigotes was evaluated using a fluorometric method described elsewhere [
15]. Briefly, macrophages (5 × 10
4 cells) and stationary
Leishmania promastigotes in a ratio of 1:10 (macrophage/parasite) were seeded in each well of a microtiter plate, suspended in 200 μL of culture medium and incubated at 33 °C and 5% CO
2 for 24 h. After this incubation time, the temperature was increased up to 37 °C for another 24 h. Cells were washed with medium several times in order to remove free non-infective promastigotes, and the supernatant was replaced by 200 μL/well of culture medium containing two-fold serial dilutions of the test compounds (ranging from 5 to 0.038 μg/mL) and the reference drug (ranging from 50 to 0.38 μg/mL). The culture medium was removed carefully to be replaced by 200 μL/well of the lysis solution (RPMI-1640 with 0.048% HEPES and 0.006% sodium dodecyl sulfate (SDS)) and incubated at room temperature for 20 min. Thereafter, the plates were centrifuged at 3500×
g for 5 min and the lysis solution was replaced by 200 μL/well of Schneider’s insect medium. The culture plates were incubated at 26 °C for another 3 days for the transformation of viable promastigotes into amastigotes. Afterwards, 20 μL/well of 2.5 mM resazurin was added and incubated for 3 h. Plates were analyzed by fluorescence emission, and IC
50 was determined as described above. All tests were carried out in triplicate. Miltefosine was used as reference drug and was evaluated at the same conditions.
2.5.7. In Vivo Experiments
BALB/c mice were infected subcutaneously at the left hand-foot with 1 × 10
7 promastigotes of
L. amazonensis on day 0. Right hind paw was used as a negative control (no infection). Thirty five days after infection, chronic cutaneous leishmaniasis was developed, and animals were randomly divided into three groups (
n = 8/group): animals treated with (
E)-piplartine received in the foot lesions (intralesion) doses of 25 mg/kg/day for 4 days in a 15 µL volume of phosphate saline dilution/propylene glycol (9:1), a group treated with glucantime receiving 25 mg/kg/day for 4 days by intraperitoneal route, and the control group. The measurement of cutaneous lesion was monitored at 0, 35, 50, and 100 days post-infection, using a Vernier calliper to measure footpad size. The number of viable
L. amazonensis parasites in the spleen of the different groups of mice was estimated using the limiting dilution assay method at the end of the experiment (day 100 post-infection) [
16]. Mice were sacrificed, and the spleen were aseptically removed, weighed, and homogenized in Schneider’s medium supplemented with 10% FBS. Briefly, serial dilutions were prepared and distributed to 96-well microtiter plates under sterile conditions, and incubated at 26 °C. On day 7 post-incubation, wells were analyzed using an inverted microscope. The number of parasites per milligram of tissue was determined based on the tissue weight and the parasite load from the culture dilutions [
17].
2.5.8. Ethical Consideration
All animals were handled according to the European Union legislation Directive 2010/63/EU and Spanish law Real Decreto 53/2013 on the protection of animals used for scientific purposes. The experimental protocols involving the use of animals were approved by the local ethical committee of the University Complutense of Madrid (CEXAN170415)
http://147.96.70.122/Web/Actas/CEXAN170415.pdf.
2.5.9. Statistical Analysis
For in vitro assays, the antileishmanial activity (IC50) and cytotoxic activity (CC50) of compounds were analyzed by Probit test, using SPSS v20.0 software. All results were expressed as means ± standard error of the mean (S.E.M). For in vivo assays, results were analyzed by Shapiro-Wilk’s normality test, and then by one-way ANOVA with Tukey’s HSD post-hoc test. Significant differences were considered at p-value < 0.05, using SPSS v20.0 and Microsoft Excel 2010 software.
3. Results and Discussion
The ethanolic extract of the leaves of P. pseudoarboreum was evaluated against promastigote forms of L. amazonensis, L. braziliensis, L. guyanensis, and L. infantum. The active EtOH crude extract was further fractionated by liquid–liquid partition to obtain DCM, EtOAc, and H2O fractions, which were assayed for their in vitro activity against the four Leishmania strains.
The DCM fraction showed an improved profile compared to the crude extract, displaying IC
50 values ranging from 14.7 to 19.1 µg/mL for the four
Leishmania strains assayed, whereas the EtOAc and H
2O fractions showed to be inactive (IC
50 > 50 µg/mL). Thus, DCM fraction was further fractionated to yield seven sub-fractions. Sub-fractions F1–F4 showed to be inactive (IC
50 > 100 µM), whereas sub-fraction F5 showed some degree of activity on the four
Leishmania strains (IC
50 15.7–20.8 µg/mL) and F7 exhibited only slight potency on
L. amazonensis and
L. brazilensis. Moreover, the most active sub-fraction F6 exhibited higher potency than miltefosine, used as the reference drug (IC
50 ranging from 2.2 to 3.4 µM vs. 17.7 to 30.7 µM), although showed a slightly low selectivity index taking J774 macrophages as reference mammalian cells (CC
50 values ranging from 1.9 to 3.0 vs. 4.4 to 7.7) (
Table 1).
Therefore, sub-fraction F6 was submitted to multiple chromatographic steps on silica gel and Sephadex LH-20 affording the known alkamides
1–
6 (
Figure 1). Their chemical structures were elucidated on the basis of their spectroscopic data (
Supplementary Materials Figures S1–S6) and comparison with data reported in the literature. Thus, the isolated metabolites were identified as sintenpyridone (
1) [
18], (
E)-demethoxypiplartine (
2) [
19], (
E)-piplartine (also known as piperlongumine,
3) [
19], (
Z)-piplartine (
4) [
20], 3,4-epoxy-8,9-dihydropiplartine (
5) [
21], and 10,11-dihydropiperine (
6) [
20].
Alkamides
1–
6 were tested by in vitro assays against the four strains of
Leishmania promastigotes. The results indicated that alkamides
2 and
3 were 4.7 to 18-fold more potent (IC
50 ranging from 1.6 to 3.8 µM) than miltefosine (IC
50 ranging from 17.7 to 30.7 µM), and exhibited a selectivity index ranging from 3.0 to 6.6 for all
Leishmania strains tested (
Table 1). Recently, Araújo-Vilges and co-workers reported that (
E)-piplartine was able to reduce the growth of
L. amazonensis promastigotes (MHOM/BR/pH8) in a dose-dependent pattern, exhibiting an IC
50 value of 179.0 µg/mL [
22]. On the other hand, Capello et al. [
23] reported that no antileishmanial activity on macrophages infected with
L. (L.) amazonensis was found for (
E)-piplartine at 50 µg/mL. We assume that such differences in potency depend to a great extent on the infecting
Leishmania strain used in the assay and cell culture procedures.
Regarding the influence of the substitution pattern in the alkamide scaffold on the leishmanicidal activity, it seems that α,β-unsaturated carbonyl groups in both, the acyl chain and the lactam ring are critical functionalities for the activity (2, 3, and 4 vs. 1, 5, and 6). Moreover, isomerization of the unsaturated acyl chain leads to slight changes in the activity (3 vs. 4). No straightforward conclusion can be drawn from the type of functional group on the aromatic ring.
Based on the in vitro results on promastigote forms, alkamides
2 and
3 were selected to be evaluated on intracellular amastigotes of
L. amazonensis and
L. infantum. The results revealed that compounds
2 and
3 exhibited some degree of activity, showing two-fold higher potency on
L. amazonesis (IC
50 9.1 and 8.2 µM, respectively) than on
L. infantum (IC
50 17.1 and 16.1 µM, respectively). Moreover, both compounds exhibited higher activity than miltefosine on both assayed
Leishmania strains. Furthermore, these compounds were 5- to 6-fold more potent than miltefosine on
L. amazonensis (
Table 2).
Taking into consideration its potency and efficacy on
Leishmania promastigote and amastigote forms, and although a poor selectivity index, (
E)-piplartine was selected for in vivo assays to investigate its potential as a lead compound targeting CL since previous toxicological studies indicate a good safety profile in murine models [
10]. Previous works report the in vitro evaluation of (
E)-piplartine against
Leishmania spp. promastigotes [
22,
24] and
L. amazonensis intracellular amastigote forms [
25] as well as an in vivo study against
L. donovani in a hamster model of visceral leishmaniasis [
24]. However, to our knowledge, in vivo studies on CL have not been reported.
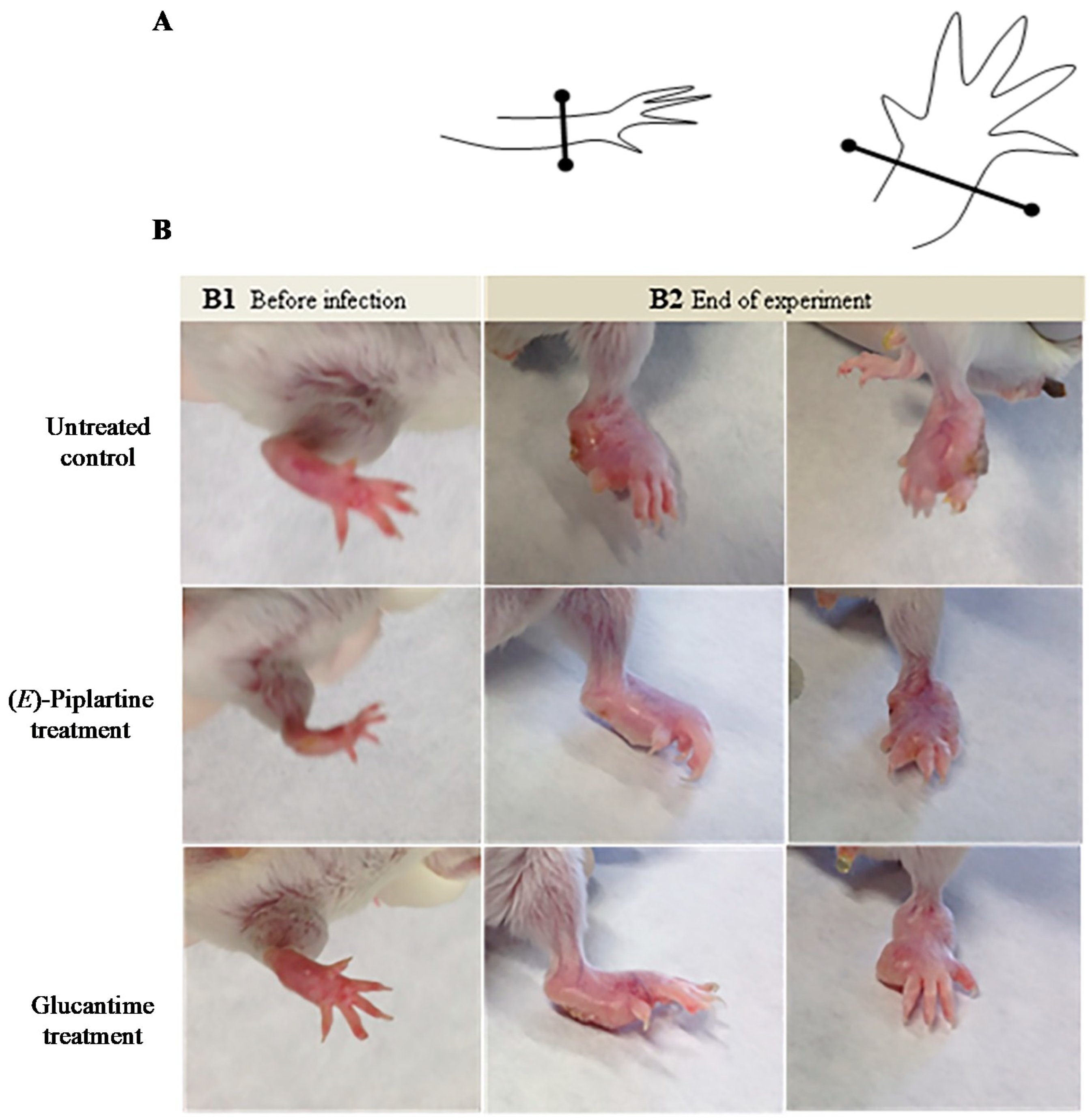
The in vivo assay in BALB/c mice infected with
L. amazonensis for CL was performed by treatment of three randomly separated mice groups (8 mice per group). Thirty five days after infection, the treated mice group with (
E)-piplartine received in the foot lesions (intralesion) a dose of 25 mg/kg/day for 4 days, whereas the treated group with glucantime received by intraperitoneal route 25 mg/kg/day for 4 days. The lesion size footpad was measured four times before infection and treatment, after treatment and at the end of the experiment (days 0, 35, 50, and 100) (
Figure 2 and
Figure 3). The individual lesion size was calculated from two measurements (differences between the left and the right footpad).
Moreover, with the aim to establish the visceralization of the chronic infection disease, all mice were sacrificed at the end of the experiment to determine parasite burden in spleen by culture on microtiter plates. This in vivo assay indicated that from the day of infection to the day before treatment with (
E)-piplartine, the progress of the lesion size was similar in the three mice groups (day 35), whereas after the end of treatment (day 50), the mean progress of lesions within groups treated with (
E)-piplartine and glucantime were reduced by around 35% with respect to the untreated group. At the end of the experiment, both treated groups showed more than 40% reduction in the lesion size and 55% in spleen parasite burden compared to untreated mice group. The intralesion (
E)-piplartine treatment efficacy was comparable to the intraperitoneal glucantime treatment, with
p-values of 0.800 and 0.832 for the lesion size and spleen parasite burden, respectively, and significantly higher than the untreated control group, with
p-values of 0.045 and 0.027, respectively (
Table 3).
Thus, the results indicated that (
E)-piplartine was effective in the in vivo assay (
Figure 2 and
Figure 3, and
Table 3) as evidenced by a significant reduction in the lesion size footpad after infection, and in the spleen parasite burden at the end of the experiment (day 100 post-infection). These findings, together with previous safety [
22,
25] and pharmacokinetic studies [
10,
26], provide additional experimental evidence of the potential of (
E)-piplartine as a promising leishmanicidal lead compound.
In this study, the intralesional route for (
E)-piplartine was chosen in order to develop a prospective formulation for topical administration. This administration route is an attractive alternative for CL, offering significant advantages over systemic therapy with: fewer adverse side effects, easy administration, and low costs [
27]. This later point is relevant as in regions with limited resources there are no dispensaries or qualified personnel for intramuscular or intravenous drug administration [
28]. In addition, topical formulations can penetrate over the skin to diminish disease progression at the beginning of the infection [
29].
Although the mechanism of action of (
E)-piplartine has not been established on
Leishmania parasites, previous studies performed on cancer cell lines [
12,
30,
31] have demonstrated that this alkamide is able to inhibit the proliferative process by activation of mitochondrial apoptosis pathways and induction of reactive oxygen species. Considering these studies, the effect of (
E)-piplartine on
Leishmania parasites could also be related to the activation of apoptotic events. Moreover, further studies should be undertaken in order to determine the leishmanicidal mechanism of action of this promising lead compound.