Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia
Abstract
1. Introduction
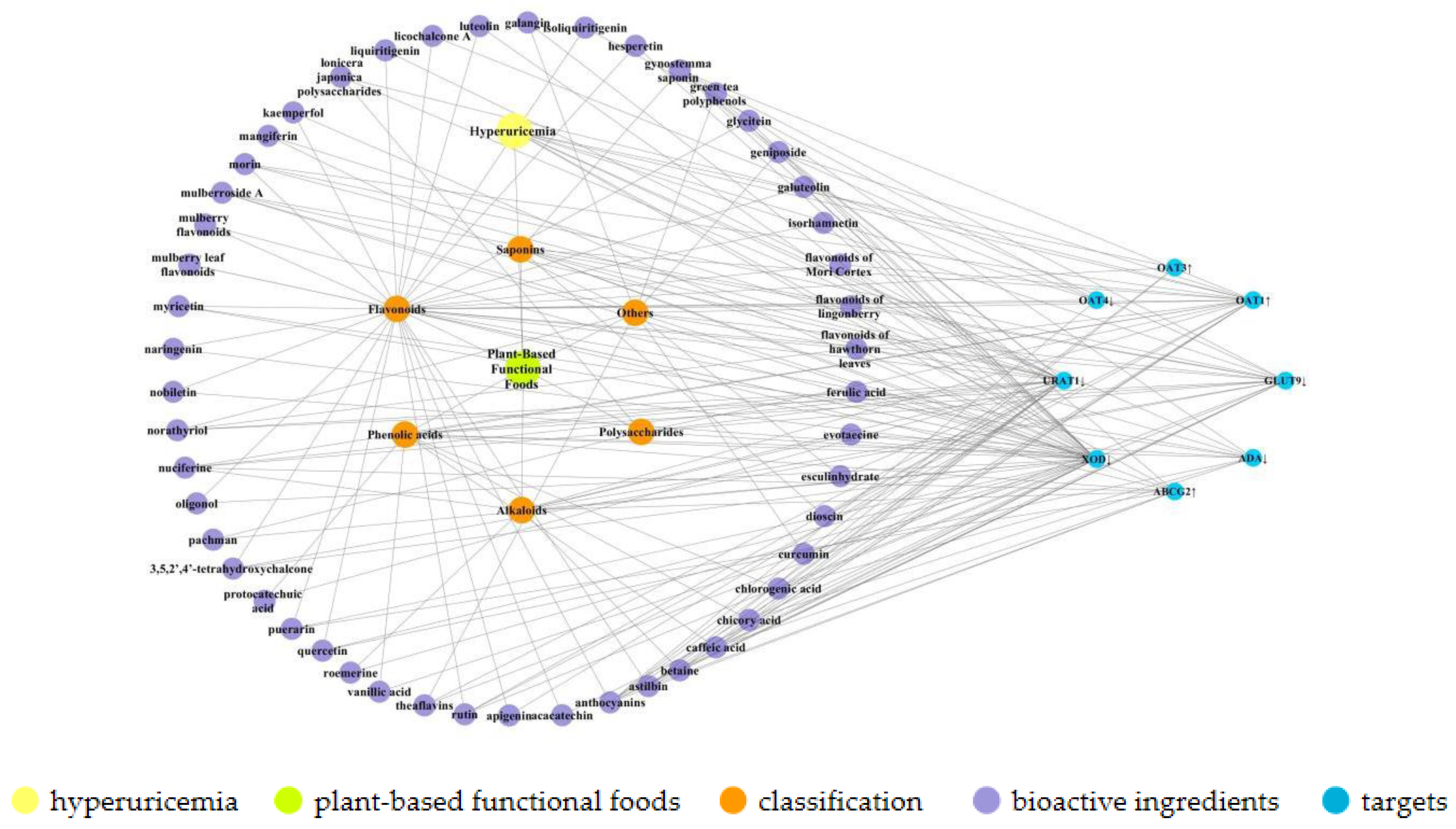
2. Bioactive Components of Plant-Based Functional Foods
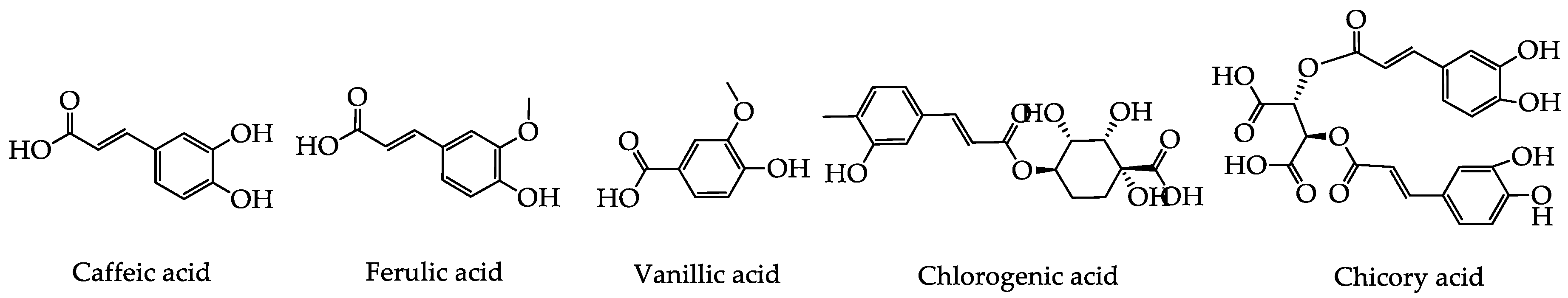
2.1. Phenolic Acids
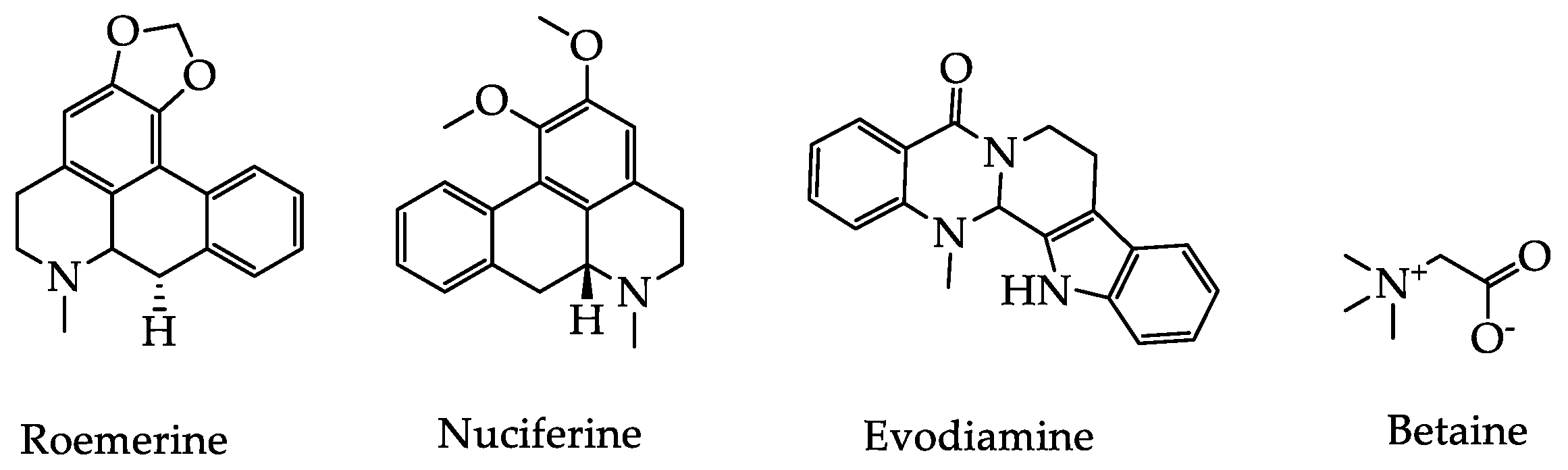
2.2. Alkaloids
2.3. Saponins
2.4. Polysaccharides
2.5. Others
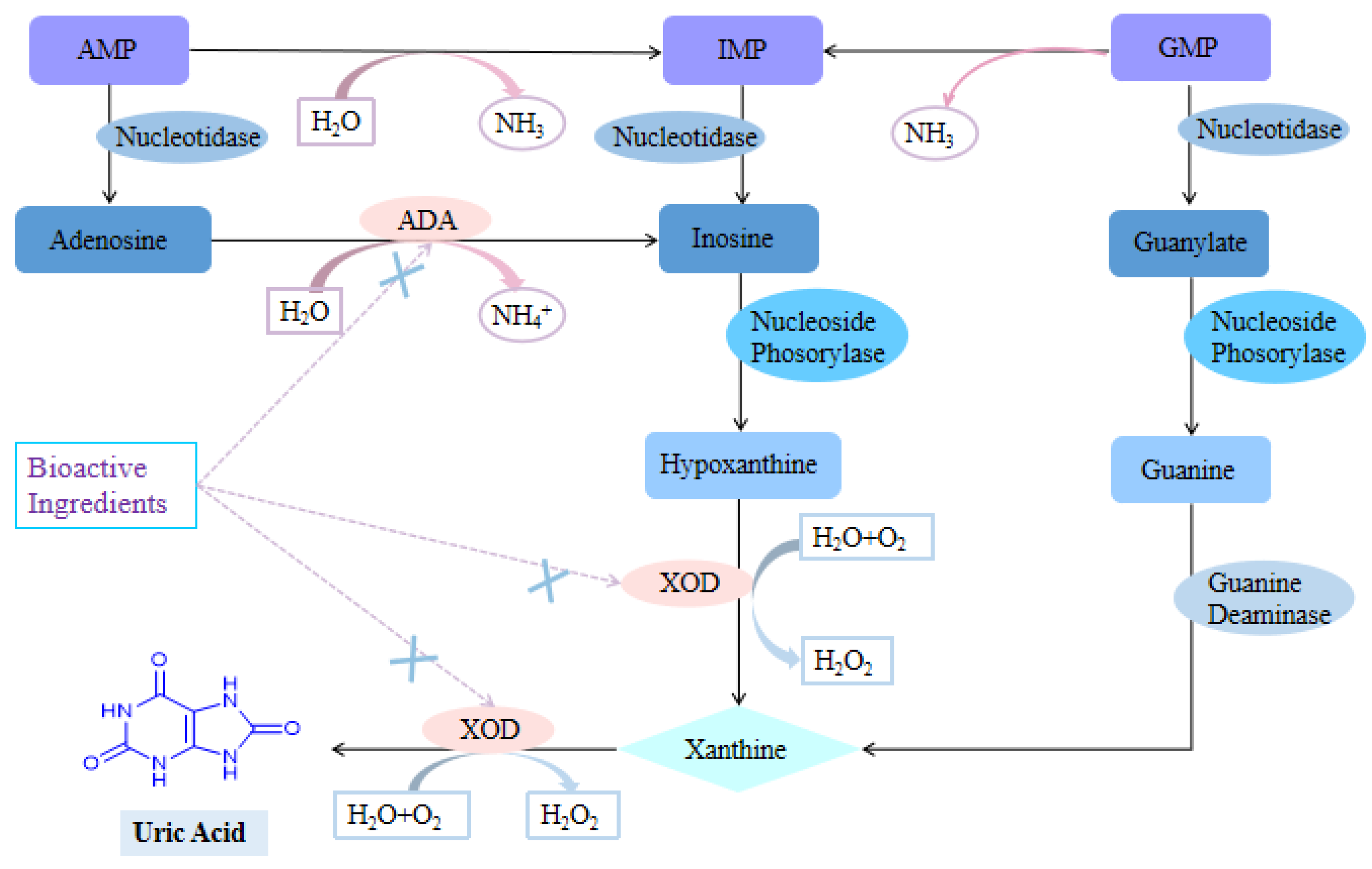
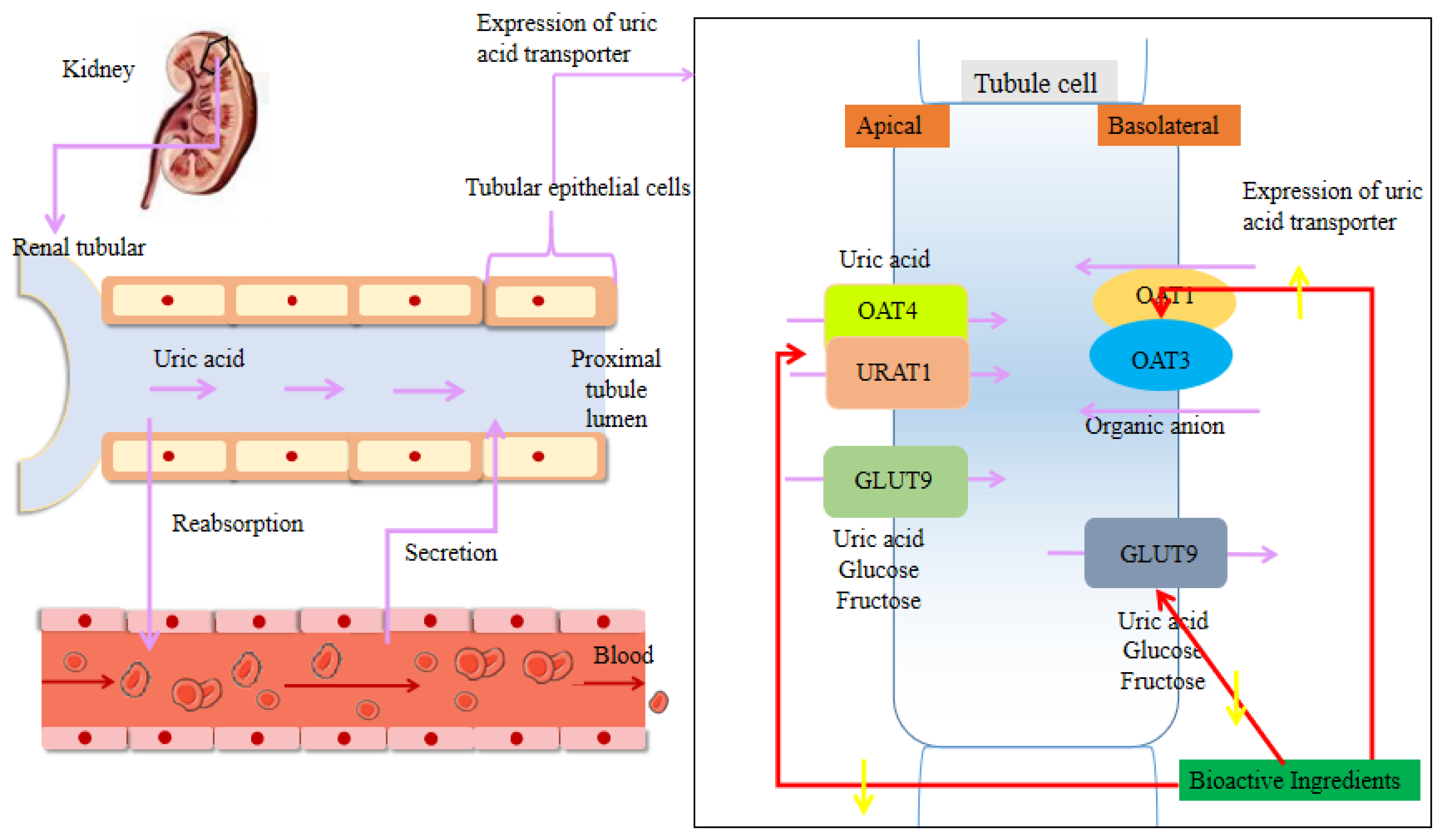
3. Uric Acid Reduction Effects of Plant-Based Functional Foods
3.1. Inhibition of Uric Acid Production
3.2. Regulation of the Renal Uric Acid Transporter
3.3. Enhancement in Intestinal UA Secretion
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.N.; Zhao, M.; Xin, Y.; Liu, J.J.; Wang, M.; Zhao, C.J. 1H-NMR and MS based metabolomics study of the therapeutic effect of Cortex Fraxini on hyperuricemic rats. J. Ethnopharmacol. 2016, 185, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Su, H.; Zhang, J.; Kong, J. The effects of ginsenosides and anserine on the up-regulation of renal aquaporins 1–4 in hyperuricemic mice. Am. J. Chin. Med. 2019, 47, 1–15. [Google Scholar] [CrossRef]
- Corey-Bloom, J.; Haque, A.; Aboufadel, S.; Snell, C.; Fischer, R.S.; Granger, S.W.; Granger, D.A.; Thomas, E.A. Uric acid as a potential peripheral biomarker for disease features in huntington’s patients. Front. Neurosci. 2020, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.A.; Forsythe, A.; Smeeding, J.E.; Lawrence Edwards, N. Chronic gout: Epidemiology, disease progression, treatment and disease burden. Curr. Med. Res. Opin. 2010, 26, 2813–2821. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wu, Q.J.; Wang, H.Y.; Zhang, S.; Jiang, Y.T.; Gong, T.T.; Xu, X.; Chang, Q.; Niu, K.J.; Zhao, Y. Global, regional and national burden of gout, 1990–2017: A systematic analysis of the Global Burden of Disease Study. Rheumatology 2019, 59, 1529–1538. [Google Scholar] [CrossRef]
- Michael, C.X.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary prevalence of gout and hyperuricemia in the united states and decadal trends: The national health and nutrition examination survey 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Mu, Z.P.; Wang, W.; Wang, J.; Lv, W.S.; Chen, Y.; Wang, F.; Yu, X.L.; Wang, Y.G.; Cheng, B.F.; Wang, Z.C. Predictors of poor response to urate-lowering therapy in patients with gout and hyperuricemia: A post-hoc analysis of a multicenter randomized trial. Clin. Rheumatol. 2019, 38, 3511–3519. [Google Scholar] [CrossRef]
- Chen, S.; Guo, X.F.; Dong, S.Y.; Yu, S.S.; Chen, Y.T.; Zhang, N.J.; Sun, Y.X. Association between the hypertriglyceridemic waist phenotype and hyperuricemia: A cross-sectional study. Clin. Rheumatol. 2017, 36, 1111–1119. [Google Scholar] [CrossRef]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef]
- Li, C.G.; Hsieh, M.C.; Chang, S.J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef]
- Petreski, T.; Ekart, R.; Hojs, R.; Bevc, S. Asymptomatic hyperuricemia and cardiovascular mortality in patients with chronic kidney disease who progress to hemodialysis. Int. Urol. Nephrol. 2019, 51, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Du, G.L.; Song, N.; Ma, Y.T.; Li, X.M.; Gao, X.M.; Yang, Y.N. Hyperuricemia and its association with adiposity and dyslipidemia in Northwest China: Results from cardiovascular risk survey in Xinjiang (CRS 2008–2012). Lipids Health Dis. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Oh, J.; Shin, D.H. Urate-lowering agents for asymptomatic hyperuricemia in stage 3–4 chronic kidney disease: Controversial role of kidney function. PLoS ONE 2019, 14, e0218510. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.F.; Chen, X.; Lei, S.S.; Li, B.; Zhang, N.Y.; Ge, H.Z.; Yang, K.; Lv, G.Y.; Chen, S.H. Effects and mechanisms of Dendrobium officinalis six nostrum for treatment of hyperuricemia with hyperlipidemia. Evid. Based Complement. Altern. Med. 2020, 2020, 2914019. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lv, J.M.; Yao, Q. Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors: An overview. Med. Sci. Monit. 2016, 22, 2501–2512. [Google Scholar] [CrossRef]
- Han, S.; Wei, R.H.; Han, D.; Zhu, J.X.; Luo, W.Z.; Ao, W.; Zhong, G.Y. Hypouricemic effects of extracts from Urtica hyperborea Jacq. ex Wedd. in hyperuricemia mice through XOD, URAT1, and OAT1. BioMed Res. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Liu, X.R.; Huang, S.S.; Xu, W.D.; Zhou, A.J.; Li, H.; Zhang, R.; Liu, Y.; Yang, Y.; Jia, H. Association of dietary patterns and hyperuricemia: A cross-sectional study of the Yi ethnic group in China. Food Nutr. Res. 2018, 62, 1380. [Google Scholar] [CrossRef]
- Li, R.R.; Yu, K.; Li, C.W. Dietary factors and risk of gout and hyperuricemia: A meta-analysis and systematic review. Asia. Pac. J. Clin. Nutr. 2018, 27, 1344–1356. [Google Scholar] [CrossRef]
- Büsing, F.; Hägele, F.A.; Nas, A.; Döbert, L.V.; Fricker, A.; Dörner, E.; Podlesny, D.; Aschoff, J.; Pöhnl, T.; Schweiggert, R.; et al. High intake of orange juice and cola differently affects metabolic risk in healthy subjects. Clin. Nutr. 2018, 38, 812–819. [Google Scholar] [CrossRef]
- Perez-Ruiz, F.; Dalbeth, N.; Bardin, T. A review of uric acid, crystal deposition disease, and gout. Adv. Ther. 2015, 32, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ristic, B.; Sikder, M.O.F.; Bhutia, Y.D.; Ganapathy, V. Pharmacologic inducers of the uric acid exporter ABCG2 as potential drugs for treatment of gouty arthritis. Asian J. Pharm. Sci. 2020, 15, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Q.; Shi, Y.F.; Zhuang, S.G.; Liu, N. Recent advances on uric acid transporters. Oncotarget 2017, 8, 100852–100862. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.K.; Liu, S.; Gunic, E.; Miner, J.N. Discovery and characterization of verinurad, a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout. Sci. Rep. 2017, 7, 665. [Google Scholar] [CrossRef] [PubMed]
- DeBosch, B.J.; Kluth, O.; Fujiwara, H.; Schürmann, A.; Moley, K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat. Commun. 2014, 5, 4642. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.X.; Wang, Y.; Yang, M.F.; Bian, W.X.; Zeng, L.; Yin, S.G.; Xiong, Z.Q.; Hu, Y.; Wang, S.Y.; Meng, B.L.; et al. New rice-derived short peptide potently alleviated hyperuricemia induced by potassium oxonate in rats. J. Agric. Food Chem. 2018, 67, 220–228. [Google Scholar] [CrossRef]
- Gliozzi, M.; Malara, N.; Muscoli, S.; Mollace, V. The treatment of hyperuricemia. Int. J. Cardiol. 2016, 213, 23–27. [Google Scholar] [CrossRef]
- Pinela, J.; Carocho, M.; Dias, M.I.; Caleja, C.; Barros, L.; Ferreira, I.C.F.R. Wild plant-based functional foods, drugs, and nutraceuticals. Wild Plants Mushrooms Nuts. 2016, 315–351. [Google Scholar] [CrossRef]
- Kumar, A.; Mosa, K.A.; Ji, L.Y.; Kage, U.; Dhokane, D.; Karre, S.; Madalageri, D.; Pathania, N. Metabolomics assisted biotechnological interventions for developing plant-based functional foods and nutraceuticals. Crit. Rev. Food. Sci. Nutr. 2018, 58, 1791–1807. [Google Scholar] [CrossRef]
- Ji, M.Y.; Bo, A.; Yang, M.; Xu, J.F.; Jiang, L.L.; Zhou, B.C.; Li, M.H. The pharmacological effects and health benefits of Platycodon grandiflorus-A medicine food homology species. Foods 2020, 9, 142. [Google Scholar] [CrossRef]
- Gong, X.; Ji, M.Y.; Xu, J.P.; Zhang, C.H.; Li, M.H. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. Nutr. 2019, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and atherosclerosis: Human trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Zhao, L.; Wang, C.T.; Nadeem, M.; Raza, A.; Ali, N.; Shah, A.A. Management of hyperuricemia through dietary polyphenols as a natural medicament: A comprehensive review. Crit. Rev. Food Sci. 2019, 59, 1433–1455. [Google Scholar] [CrossRef]
- Arimboor, R.; Arumughan, C. Effect of polymerization on antioxidant and xanthine oxidase inhibitory potential of sea buckthorn (H. rhamnoides) proanthocyanidins. J. Food Sci. 2012, 77, C1036–C1041. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.Y.; Gong, X.; Li, X.; Wang, C.C.; Li, M.H. Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae Species—A Review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Wu, J.L.; Li, J.X.; Ma, Z.C. Effect of lemon water soluble extract on hyperuricemia in mouse model. Food Funct. 2019, 10, 6000–6008. [Google Scholar] [CrossRef]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef]
- Lin, S.Y.; Zhang, G.W.; Liao, Y.J.; Pan, J.H. Inhibition of chrysin on xanthine oxidase activity and its inhibition mechanism. Int. J. Biol. Macromol. 2015, 81, 274–282. [Google Scholar] [CrossRef]
- Patra, J.C.; Chua, B.H. Artificial neural network-based drug design for diabetes mellitus using flavonoids. J. Comput. Chem. 2011, 32, 555–567. [Google Scholar] [CrossRef]
- Lin, S.Y.; Zhang, G.W.; Liao, Y.J.; Pan, J.H. Dietary flavonoids as xanthine oxidase inhibitors: Structure-Affinity and Structure-Activity relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, C.S.; Chen, C.T.; Liang, Y.C.; Lin, J.K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002, 294, 167–172. [Google Scholar] [CrossRef]
- Cheng, L.C.; Murugaiyah, V.; Chan, K.L. Flavonoids and phenylethanoid glycosides from Lippia nodiflora as promising antihyperuricemic agents and elucidation of their mechanism of action. J. Ethnopharmacol. 2015, 176, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure-antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Wang, C.P.; Wang, X.; Zhang, X.; Shi, Y.W.; Liu, L.; Kong, L.D. Morin improves urate excretion and kidney function through regulation of renal organic ion transporters in hyperuricemic mice. J. Pharm. Pharm. Sci. 2010, 13, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.H.; Ma, Y.C.; Li, X.P.; Zhang, B.; Zhang, M.D.; Wan, S.M.; Yang, X.; Yang, T.F.; Jiang, J.W.; Bao, R. Research progress of puerarin and its derivatives on anti-inflammatory and anti-gout activities. China J. Chin. Mater. Med. 2017, 42, 3703–3708. [Google Scholar] [CrossRef]
- Xie, K.L.; Li, Z.H.; Dong, X.Z.; Gong, M.X. Research progress of quercetin on inhibiting the activity of xanthine oxidase. Lishizhen Med. Mater. Med. Res. 2019, 30, 2223–2225. [Google Scholar] [CrossRef]
- Shi, Y.L.; Williamson, G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: A randomised, double-blinded, placebo-controlled, cross-over trial. Brit. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Zhang, G.W.; Gong, D.M. Mechanistic insights into the inhibition of quercetin on xanthine oxidase. Int. J. Biol. Macromol. 2018, 112, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Su, G.H.; Luo, C.L.; Pang, Y.L.; Wang, L.; Li, X.; Zhang, J.L. Effects of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) on the serum uric acid level and xanthine oxidase activity in hyperuricemic mice. Food Funct. 2015, 6, 3045–3055. [Google Scholar] [CrossRef]
- Qian, X.Y.; Wang, X.; Luo, J.; Liu, Y.; Pang, J.; Zhang, H.Y.; Xu, Z.L.; Xie, J.W.; Jiang, X.W.; Ling, W. Hypouricemic and nephroprotective roles of anthocyanins in hyperuricemic mice. Food Funct. 2019, 10, 867–878. [Google Scholar] [CrossRef]
- Meehmood, A.; Zhao, L.; Chengtao, W.; Hossen, I.; Raka, R.N.; Zhang, H. Stevia residue extract increases intestinal uric acid excretion via interacting with intestinal urate transporters in hyperuricemic mice. Food Funct. 2019, 10, 7900–7912. [Google Scholar] [CrossRef]
- Miao, M.X.; Wang, X.; Lu, Y.; Wang, X. Mechanism Study on effects of apigenin on reducing uric acid and renal protection in oteracil potassium-induced hyperuricemia mice. China Pharm. 2016, 27, 4794–4796. [Google Scholar] [CrossRef]
- Hao, Y.; Jiao, A.N.; Yu, M.; Gao, J.Z.; He, X.; Zhang, M.H.; Jiao, L.Q.; Zhang, J. Activity screening of thirty flavonoids on the inhibition of xanthine oxidase. Chin. Tradit. Pat. Med. 2019, 41, 55–59. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, D.; Wu, H.L. Pu-erh ripened tea resists to hyperuricemia through xanthine oxidase and renal urate transporters in hyperuricemic mice. J. Funct. Foods. 2017, 29, 201–207. [Google Scholar] [CrossRef]
- Wang, Z.; Ci, X.Y.; Cui, T.; Wei, Z.H.; Zhang, H.B.; Liu, R.; Li, Y.Z.; Yi, X.L.; Zhang, T.J.; Gu, Y.; et al. Effects of Chinese herb ingredients with different properties on OAT4, URAT1 and serum uric acid level in acute hyperuricemia mice. Chin. Trad. Herb. Drugs 2019, 50, 1157–1163. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhan, J.; Wang, X.B.; Zou, L. Research progress in treatment of hyperuricemia with active ingredients of traditional Chinese medicine. Chin. J. Pharmacol. Toxicol. 2015, 29, 471–476. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, N.; Chen, J.H. Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed. Pharm. 2016, 83, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhang, R.T.; Shang, X.Y.; Wang, N.; Li, S.; Zhang, Z.S. Effect of puerarin on serum uric acid in hyperuricemic rat. Food Sci. Technol. 2014, 39, 216–220. [Google Scholar] [CrossRef]
- Pu, J.Y.; Niu, Y.F.; Gao, L.H.; Lin, H.; Tu, C.X.; Li, L. Effects of 3,5,2′,4′-tetrahydroxychalcone on urate excretion in hyperuricemic mice. Chin. Pharmacol. Bull. 2015, 31, 1091–1095. [Google Scholar] [CrossRef]
- Dang, Y.X.; Liang, D.L.; Zhou, X.X.; Qin, Y.; Gao, Y.; Li, W.M. Protective effect of Mori Cortex on kidney in rats with hyperlipidemia and hyperuricemia based on molecular docking technique. Chin. Trad. Herb. Drugs 2019, 50, 1175–1181. [Google Scholar] [CrossRef]
- Zhang, H.C.; Zhang, Y.; Lv, G.F.; Wang, E.P.; Chen, X. The puerarin impact on the expression of ABCG2 in human renal proximal tubule epithelial cells. SH J. TCM Mar. 2016, 50, 74–77. [Google Scholar] [CrossRef]
- Wang, K.; Wang, R.P.; Li, J.; Zhao, D.; Wang, J.Q.; Ran, X.; Qu, W.J. The preventive and therapeutic effects of mulberry leaf flavonoids on adenine induced hyperuricemia and kidney injury in rats. Nat. Prod. Res. Dev. 2012, 24, 172–175, 202. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Yang, H. Effects of total flavone of hawthorn leaf on serum uric acid and vascular endothelial cell function in hyperuricemia rats. Chin. J. Exp. Trad. Med. Formulae 2012, 18, 259–261. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, L.Y.; He, G.H.; Zhang, X.; Song, X.H.; Cui, G.L.; Liao, S.Q. Quality evaluation of Flos Sophorae Immaturus from different habitats by HPLC coupled with chemometrics and anti-oxidant ability. Chin. Trad. Herb. Drugs 2018, 49, 4644–4652. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hu, Q.H.; Zhang, X.; Zhu, Q.; Kong, L.D. Beneficial effect of rutin on oxonate-induced hyperuricemia and renal dysfunction in mice. Pharmacology 2013, 92, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.I.; Kondo, S.; Sato, Y.; Yoshizawa, F.; Yagasaki, K. Anti-hyperuricemic effect of isorhamnetin in cultured hepatocytes and model mice: Structure-activity relationships of methylquercetins as inhibitors of uric acid production. Cytotechnology 2019, 71, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, W.; Guo, B.H.; Gao, H.; Liu, Y.; Liu, X.H.; Yao, H.L.; Cheng, K. Chemical evidence for potent xanthine oxidase inhibitory activity of ethyl acetate extract of Citrus aurantium L. dried immature fruits. Molecules 2016, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, M.; Madeswaran, A.; Asokkumar, K. Virtual screening analysis and in-vitro xanthine oxidase inhibitory activity of some commercially available flavonoids. Iran. J. Pharm. Res. 2013, 12, 317–323. [Google Scholar] [CrossRef]
- Yu, S.H.; Song, H.P.; Gao, W.; Zhang, H. Study on the inhibitory activity of flavonoids in Carthami Flos on xanthine oxidase. Chin. J. Ethnomed. Ethnopharm. 2017, 26, 23–26. [Google Scholar]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- Irondi, E.A.; Agboola, S.O.; Oboh, G.; Boligon, A.A.; Athayde, M.L.; Shode, F.O. Guava leaves polyphenolics-rich extract inhibits vital enzymes implicated in gout and hypertension in vitro. J. Intercult. Ethnopharm. 2016, 5, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.S.; Zhang, B.; Lin, Z.J.; Bai, Y.F. Pharmacodynamics authentication research on uric acid-lowering active ingredients of Cichorium intybus L. China J. Trad. Chin. Med. Pharm. 2018, 33, 4933–4936. [Google Scholar]
- Zhu, C.S.; Lin, Z.J.; Zhang, B.; Wang, H.B.; Wang, X.J.; Niu, H.J.; Wang, Y.; Niu, A.Z. Spectrum-effect relationships on uric acid lowering effect of Cichorium intybus. Chin. Trad. Herb. Drugs 2015, 46, 3386–3389. [Google Scholar] [CrossRef]
- Zhao, M.M.; Zhu, D.S.; Sun-Waterhouse, D.X.; Su, G.W.; Lin, L.Z.; Wang, X.; Dong, Y. In vitro and in vivo studies on adlay-derived seed extracts: Phenolic profiles, antioxidant activities, serum uric acid suppression, and xanthine oxidase inhibitory effects. J. Agric. Food Chem. 2014, 62, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, F.; Zou, B.; Shen, Y.F.; Li, Y.Z.; Zhang, A.X.; Fu, G.M. Molecular mechanism underlying the ability of caffeic acid to decrease uric acid levels in hyperuricemia rats. J. Funct. Foods. 2019, 57, 150–156. [Google Scholar] [CrossRef]
- Zhu, C.S.; Lin, Z.J.; Zhang, B.; Bai, Y.F. Uric acid-lowering active ingredients and mechanism of Cichorium intybus. Chin. Trad. Herb. Drugs 2017, 48, 957–961. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, H.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Zou, L.; Feng, F.Q. Research progress of uric acid-lowering bioactive compounds in food and their mechanisms. Sci. Technol. Food Ind. 2019, 40, 352–357, 364. [Google Scholar] [CrossRef]
- Sang, M.M.; Du, G.Y.; Hao, J.; Wang, L.L.; Liu, E.W.; Zhang, Y.; Wang, T.; Gao, X.M.; Han, L. Modeling and optimizing inhibitory activities of Nelumbinis folium extract on xanthine oxidase using response surface methodology. J. Pharm. Biomed. 2017, 139, 37–43. [Google Scholar] [CrossRef]
- Wang, M.X.; Liu, Y.L.; Yang, Y.; Zhang, D.M.; Kong, L.D. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol. 2015, 747, 59–70. [Google Scholar] [CrossRef]
- Tao, Z.Y.; Cheng, Y.; Tang, Y.; Tan, Y.M.; Li, J. Effect of evodiamine on the animal model of Hyperuricemia. Pharm. Clin. Chin. Mater. Med. 2014, 5, 69–71. [Google Scholar]
- Song, Y.; Li, J.; Cheng, Y.; Lin, Z.; He, B.Y.; Wang, C.Y. Lowering effect of evodiamine dispersible tablets on uric acid in chickens. Chin. J. New Drugs 2015, 24, 1057–1060. [Google Scholar]
- Hu, M.; Liu, J.W.; Song, Y.; Zeng, N. Effect and mechanism study of evodiamine on hyperuricemia model quail. Pharmacol. Clin. Chin. Mater. Med. 2014, 30, 38–40. [Google Scholar] [CrossRef]
- Tan, L.; Ji, T.; Cao, J.Y.; Hu, F.Z. Determination of betaine contents in Fructus Lycii from different origins by dual wavelength TLC scanning. Nat. Prod. Res. Dev. 2014, 26, 388–391, 397. [Google Scholar] [CrossRef]
- Liu, Y.L.; Pan, Y.; Wang, X.; Fan, C.Y.; Zhu, Q.; Li, J.M.; Wang, S.J.; Kong, L.D. Betaine Reduces Serum Uric Acid Levels and Improves Kidney Function in Hyperuricemic Mice. Planta Med. 2013, 80, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, Z.B.; Chen, M.M.; Song, J.; Cui, H.X. Research progress of therapeutic drug of hyperuricemia and its action target. China Mod. Med. 2018, 25, 16–19. [Google Scholar]
- Gong, L.X.; Chi, J.W.; Wang, J.; Ren, Y.Q.; Sun, B.G. Research progress on main functional component and action mechanism of Dioscorea opposita. Sci. Technol. Food Ind. 2019, 40, 312–319. [Google Scholar] [CrossRef]
- Su, J.X.; Wei, Y.H.; Liu, M.L.; Liu, T.X.; Li, J.H.; Ji, Y.C.; Liang, J.P. Anti-hyperuricemic and nephroprotective effects of Rhizoma Dioscoreae septemlobae extracts and its main component dioscin via regulation of mOAT1, mURAT1 and mOCT2 in hypertensive mice. Arch. Pharm. Res. 2014, 37, 1336–1344. [Google Scholar] [CrossRef]
- Shi, S.; Wang, N.; Shang, X.Y.; Zhang, R.T.; Li, S.; Zhang, Z.S. Effect of gypenoside on serum uric acid of hyperuricemic rats. Nat. Prod. Res. Dev. 2014, 26, 1285–1289, 1315. [Google Scholar] [CrossRef]
- Pang, M.X.; Fang, Y.Y.; Chen, S.H.; Zhu, X.X.; Shan, C.W.; Su, J.; Yu, J.J.; Li, B.; Yang, Y.; Chen, B.; et al. Gypenosides inhibits xanthine oxidoreductase and ameliorates urate excretion in hyperuricemic rats induced by high cholesterol and high fat food (Lipid Emulsion). Med. Sci. Monit. 2017, 23, 1129–1140. [Google Scholar] [CrossRef]
- Meng, F.C.; Li, Q.; Qi, Y.M.; He, C.W.; Wang, C.M.; Zhang, Q.W. Characterization and immunoregulatory activity of two polysaccharides from the root of Ilex asprella. Carbohydr. Polym. 2018, 197, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, S.S.; Wang, X.X.; Zhu, Y.F.; Zhang, J.J.; Liu, H.; Jia, L. Characterization, anti-oxidation and anti-inflammation of polysaccharides by Hypsizygus marmoreus against LPS-induced toxicity on lung. Int. J. Biol. Macromol. 2018, 111, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.X.; Wang, Q.L.; Deng, W.W.; Sun, C.Y.; Wei, Q.Y.; Adu-Frimpong, M.; Shi, J.X.; Yu, J.N.; Xu, X.M. Anti-hyperuricemic and anti-gouty arthritis activities of polysaccharide purified from Lonicera japonica in model rats. Int. J. Biol. Macromol. 2019, 123, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Yan, J.X.; Wang, P.; Zhou, Y.; Wu, X.A. Effects of pachman on the expression of renal tubular transporters rURAT1, rOAT1 and rOCT2 of the rats with hyperuricemia. West. J. Tradit. Chin. Med. 2019, 32, 10–14. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Ishimoto, T.; Cicerchi, C.; Tamura, Y.; Roncal-Jimenez, C.A.; Chen, W.; Johnson, R.J. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 2526–2538. [Google Scholar] [CrossRef]
- Lecoultre, V.; Egli, L.; Theytaz, F.; Despland, C.; Schneiter, P.; Tappy, L. Fructose-induced hyperuricemia is associated with a decreased renal uric acid excretion in humans. Diabetes Care 2013, 36, e149–e150. [Google Scholar] [CrossRef]
- Cirillo, P.; Gersch, M.S.; Mu, W.; Scherer, P.M.; Kim, K.M.; Gesualdo, L.; Henderson, G.N.; Johnson, R.J.; Sautin, Y.Y. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J. Am. Soc. Nephrol. 2009, 20, 545–553. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, C.; Li, F. Research advances in mechanism of active components of traditional Chinese medicine for reducing uric acid. Chin. Pharmacol. Bull. 2018, 34, 19–22. [Google Scholar] [CrossRef]
- Zeng, J.X.; Xu, B.B.; Wang, J.; Bi, Y.; Wang, X.Y.; Zhong, G.Y.; Ren, G.; Zhu, J.X.; Li, M.; Zhu, Y.Y. Hypouricemic effects of acteoside and isoacteoside from Plantaginis Semen on mice with acute hyperuricemia and their possible mechanisms. Chin. Tradit. Pat. Med. 2016, 38, 1449–1454. [Google Scholar] [CrossRef]
- Moriwaki, Y.J.; Okuda, C.; Yamamoto, A.; Ka, T.; Tsutsumi, Z.; Takahashi, S.; Yamamoto, T.; Kitadate, K.; Wakame, K. Effects of oligonol, an oligomerized polyphenol formulated from lychee fruit, on serum concentration and urinary excretion of uric acid. J. Func. Foods 2011, 3, 13–16. [Google Scholar] [CrossRef]
- Nugraheni, P.W.; Rahmawati, F.; Mahdi, C.; Prasetyawan, S. Green tea extract (Camellia sinensis L.) effects on uric acid levels on hyperuricemia rats (Rattus norvegicus). J. Pure App. Chem. Res. 2017, 6, 246–254. [Google Scholar] [CrossRef][Green Version]
- Huang, C.G.; Shang, Y.J.; Zhang, J.; Zhang, J.R.; Li, W.J.; Jiao, B.H. Hypouricemic effects of phenylpropanoid glycosides acteoside of scrophularia ningpoensis on serum uric acid levels in potassium oxonate-pretreated mice. Am. J. Chin. Med. 2008, 36, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Wang, Y.; Wang, X.; Zhang, X.; Ye, J.F.; Hu, L.S.; Kong, L.D. Mulberroside A possesses potent uricosuric and nephroprotective effects in hyperuricemic mice. Planta Med. 2011, 77, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.W.; Niu, Y.F.; Gao, L.H.; Li, L.; Lin, H. Analysis of hypouricemic mechanism of mangiferin based on intestinal urate transporter ABCG2. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 145–149. [Google Scholar] [CrossRef]
- Yang, H.; Gao, L.H.; Niu, Y.F.; Zhou, Y.F.; Lin, H.; Jiang, J.; Kong, X.F.; Liu, X.; Li, L. Mangiferin inhibits renal urate reabsorption by modulating urate transporters in experimental hyperuricemia. Biol. Pharm. Bull. 2015, 38, 1591–1598. [Google Scholar] [CrossRef]
- Lin, H.; Tu, C.X.; Niu, Y.F.; Li, F.S.; Yuan, L.X.; Li, N.; Xu, A.P.; Gao, L.H.; Li, L. Dual actions of norathyriol as a new candidate hypouricaemic agent: Uricosuric effects and xanthine oxidase inhibition. Eur. J. Pharmacol. 2019, 853, 371–380. [Google Scholar] [CrossRef]
- Chen, Y.E.; Li, C.T.; Duan, S.N.; Yuan, X.; Liang, J.; Hou, S.Z. Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharmacother. 2019, 118, 109195. [Google Scholar] [CrossRef]
- Ao, G.Z.; Zhou, M.Z.; Li, Y.Y.; Li, S.N.; Wang, H.N.; Wan, Q.W.; Li, H.Q.; Hu, Q.H. Discovery of novel curcumin derivatives targeting xanthine oxidase and urate transporter 1 as anti-hyperuricemic agents. Bioorg. Med. Chem. 2017, 25, 166–174. [Google Scholar] [CrossRef]
- Li, X.Z.; Zheng, L.L.; Ai, B.L.; Zheng, X.Y.; Yang, Y.; Yang, J.S.; Sheng, Z.W. The inhibitory kinetics and mechanism of xanthine oxidase by screened polyphenols. Food Res. Dev. 2020, 41, 12–19, 97. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Q.; Zhao, K.; Zhao, M. Identification of the free phenolic profile of Adlay bran by UPLC-QTOF-MS/MS and inhibitory mechanisms of phenolic acids against xanthine oxidase. Food Chem. 2018, 253, 108–118. [Google Scholar] [CrossRef]
- Lin, L.C.; Pai, Y.F.; Tsai, T.H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, G.; Hu, Y.; Ma, Y. Effect of luteolin on xanthine oxidase: Inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem. 2013, 141, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.Q.; Wang, Q.L.; Xu, X.M.; Yu, J.N. Separation, purification of galangin and its effect on reducing uric acid. J. Jiangsu Univ. 2017, 27, 338–343. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, G.W.; Pan, J.H.; Gong, D.M. Galangin competitively inhibits xanthine oxidase by a ping-pong mechanism. Food Res. Int. 2016, 89, 152–160. [Google Scholar] [CrossRef]
- Komazawa, H.; Yamaguchi, H.; Hidaka, K.; Ogura, J.; Kobayashi, M.; Iseki, K. Renal Uptake of substrates for organic anion transporters Oat1 and Oat3 and organic cation transporters Oct1 and Oct2 is altered in rats with adenine-induced chronic renal failure. J. Pharm. Sci. 2013, 102, 1086–1094. [Google Scholar] [CrossRef]
- Nakanishi, T.; Fukushi, A.; Sato, M.; Yoshifuji, M.; Gose, T.; Shirasaka, Y.; Ohe, K.; Kobayashi, M.; Kawai, K.; Tamai, I. Functional characterization of apical transporters expressed in rat proximal tubular cells (PTCs) in primary culture. Mol. Pharm. 2011, 8, 2142–2150. [Google Scholar] [CrossRef]
- Tai, L.L.; Liu, Z.H.; Sun, M.H.; Xie, Q.J.; Cai, X.Q.; Wang, Y.; Dong, X.; Xu, Y. Anti-hyperuricemic effects of three theaflavins isolated from black tea in hyperuricemic mice. J. Funct. Foods. 2020, 66, 103803. [Google Scholar] [CrossRef]
- Yin, Y.C.; Ma, C.C.; Wu, J.; Yu, S.L.; Guo, X.Z.; Hou, L.A.; You, T.T.; Wang, D.C.; Li, H.L.; Xu, T.; et al. Association of SLC22A12 and SLC2A9 genetic polymorphisms with hypouricemia in Ningxia populatio. Basic Clin. Med. 2018, 38, 638–642. [Google Scholar] [CrossRef]
- Liu, D.P. Hypouricemia. Chin. J. Cardiovasc. Med. 2016, 21, 104–107. [Google Scholar] [CrossRef]
- Dong, S.T.; Chen, Y.; Gao, Q.Y. Research progress on bioactive compounds and function of sea buckthorn berry. Chin. Brew. 2020, 39, 26–32. [Google Scholar] [CrossRef]
- Pei, J.B.; Li, X.Y.; Wang, J.H. Study on variation of sugar, acid, vitamin C and pigments contents during fruit development of blueberries. J. Northeast Agric. Univ. 2011, 42, 76–79. [Google Scholar] [CrossRef]
- Mandal, A.K.; Mount, D.B. The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 2015, 77, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Stiburkova, B.; Pavelcova, K.; Pavlikova, M.; Ješina, P.; Pavelka, K. The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients. Arthritis Res. Ther. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Matsuo, H.; Nakaoka, H.; Nakamura, T.; Nakashima, H.; Takada, Y.; Oikawa, Y.; Takada, T.; Sakiyama1, M.; Shimizu1, S.; et al. Common dysfunctional variants of ABCG2 have stronger impact on hyperuricemia progression than typical environmental risk factors. Sci. Rep. 2014, 4, 5227. [Google Scholar] [CrossRef]
- Matsuo, H.; Yamamoto, K.; Nakaoka, H.; Nakayama, A.; Sakiyama, M.; Chiba, T. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann. Rheum. Dis. 2015, 75, 652–659. [Google Scholar] [CrossRef]
- Morimoto, C.; Tamura, Y.; Asakawa, S.; Kuribayashi-Okuma, E.; Nemoto, Y.; Li, J.P.; Murase, T.; Nakamura, T.; Hosoyamada, M.; Uchida, S.; et al. ABCG2 expression and uric acid metabolism of the intestine in hyperuricemia model rat. Nucleosides Nucleotides Nucleic Acids 2020, 39, 744–759. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Zhang, B.; Nie, A.Z.; Bian, M. Cichorium intybus L. promotes intestinal uric acid excretion by modulating ABCG2 in experimental hyperuricemia. Nut. Metab. 2017, 14, 1–11. [Google Scholar] [CrossRef]

| No. | Drug | Animal | Dosage (mg/kg) | Mode of Administration |
|---|---|---|---|---|
| A | Potassium oxonate | Mice | - | Intragastric administration |
| B | Potassium oxonate | Rats | 200 | Intragastric administration |
| C | Potassium oxonate | Mice | 250 | Oral gavage |
| D | Potassium oxonate | Mice | 250 | Intragastric administration |
| E | Potassium oxonate | Mice | 270 | Intragastric administration |
| F | Potassium oxonate | Mice | 300 | Intragastric administration |
| G | Potassium oxonate | Mice | 500 | Intragastric administration |
| H | Adenine | Mice | 75 | Intragastric administration |
| I | Adenine + potassium oxonate | Mice | 100 + 250 | Intragastric administration |
| J | Adenine + ethylamine butanol | Rats | - | Intragastric administration |
| K | Inosine + potassium oxonate | Rats | 400 + 280 | Intragastric administration |
| L | Yeast + potassium oxonate | Rats | 1500 + 200 | Intragastric administration |
| M | Purine | Mice | 300 | Intragastric administration |
| N | Uric acid | Rats | 150 | Intragastric administration |
| O | Uric acid | Rats | 180 | Intragastric administration |
| P | High purine diet | Rats | - | Oral gavage |
| Q | Yeast | Quails | 6 mL | Oral gavage |
| R | High purine diet | Quails | - | Oral gavage |
| Source | Bioactive Compound | Model | Dose | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Apium graveolens L./Celery | Apigenin | C | 40 and 80 mg/kg | SUA, urinary UA and the protein expression of URAT1 levels were significantly decreased, while 24 h urinary creatinine were significantly increased | This is associated with promoting renal excretion of UA by down-regulating the expression of URAT1 | [52] |
| Apium graveolens L./Celery | Kaempferol | E | 150 and 300 mg/kg | Significantly decreased SUA | Inhibit UA production by inhibiting XOD | [53] |
| Camellia sinensis var. Assamica/Pu-erh tea | Myricetin | D | 4 mg/kg | Significantly lowered SUA level, it also markedly inhibited liver XOD and ADA activities | It is mainly involved in inhibiting UA production by inhibiting XOD and ADA activities | [54] |
| Glycyrrhiza uralensis Fisch/Liquorice Root | Liquiritigenin | G | 10 mg/kg | SUA level significantly reduced, fractional excretion of UA was increased | This is related to promoting renal excretion of UA by down-regulating the transport expression of URAT1 | [55] |
| Glycyrrhiza uralensis Fisch/Liquorice Root | Isoliquiritigenin | G | 10 mg/kg | SUA level significantly reduced, fractional excretion of UA was increased | This is related to inhibiting UA reabsorption by down-regulating the transport of OAT4 | [55] |
| Glycyrrhiza uralensis Fisch/Liquorice Root | Licochalcone A | G | 10 mg/kg | SUA level significantly reduced, fractional excretion of UA was increased | This is related to inhibiting UA reabsorption by down-regulating the transport of OAT4 | [55] |
| Vaccinium vitisidaea L./Lingonberry | Flavonoids from fruit residues of lingonberry | B | 100 and 200 mg/kg | SUA was significantly reduced at 100 mg/kg, while 200 mg/kg inhibited the activity of XOD in liver | It is mainly involved in inhibiting XOD activity | [56] |
| Smilax china L./Rhizome Glabrous Greenbrier | Astilbin | B | 10 and 20 mg/kg | SUA, Scr, and BUN were significantly reduced, and urinary UA and renal UA excretion effectively increased | It is related to promoting renal excretion of UA by suppressing role in GLUT9 and URAT1 expression and up-regulating the expression of ABCG2, OAT1, OAT3 | [57] |
| Pueraria lobata (Willd.) Ohwi/Pueraria | Puerarin | L | 200 mg/kg | SUA, and BUN were significantly reduced | It is mainly involved in inhibiting XOD activity to inhibit UA production | [58] |
| Glycyrrhiza uralensis Fisch./Liquorice Root | 3,5,2′,4′-tetrahydroxychalcone | N | 4 mg/kg | SUA and the content of Hepatic XOD were significantly reduced | It is mainly involved in inhibiting XOD activity to inhibit UA production and down-regulating the protein expression of GLUT9 to inhibit UA re-absorption | [59] |
| Morus alba L./Mori Cortex | Flavonoids of Mori Cortex | H | 1 mg/kg | URAT1 was significantly decreased, the content of OAT1 mRNA was significantly increased | It may be related to the down-regulation of URAT1 and the up-regulation OAT1 to promote renal excretion of UA | [60] |
| Morus alba L./Mulberry Leaf | Morusin | J | 40 and 80 mg/kg | Increased urinary UA/creatinine ratio and resulting in reduction of SUA level | Down-regulated of renal mGLUT9 and mURAT1, and increased urate secretion via up-regulating of renal mOAT1 to promote renal excretion of UA | [44] |
| Morus alba L./Mulberry Leaf | Mulberry leaf flavonoids | H | 50, 100, and 200 mg/kg | SUA and urea nitrogen were effectively lowered, XOD was inhibited | It is related to inhibiting the activity of XOD to inhibit UA production | [61] |
| Morus alba L./Mulberry | Mulberry flavonoids | H | 200 mg/kg | SUA were effectively lower | It is related to inhibiting the activity of XOD to inhibit UA production | [56] |
| Crataegus pinnatifida Bge./Hawthorn | Flavonoids of hawthorn leaves | J | 3, 6, and 9 mg/kg | SUA was effectively lowered, XOD was inhibited | It is related to inhibiting the activity of XOD to inhibit UA production | [62] |
| Sophora japonica L./Sophora Japonica | Rutin | D | 50 and 100 mg/kg | Significantly decreased SUA, BUN, and Scr, and increased urine creatinine excretion | It is related to promoting renal excretion of UA by down-regulating mRNA and protein levels of URAT1 and GLUT9, and up-regulating mRNA and protein levels of OAT1 | [63,64] |
| Hippophae rhamnoides L./Seabuckthorn | Isorhamnetin | M | 300 mg/kg | Significantly reduced plasma and hepatic UA level, also decreased hepatic XOD activity | It is related to inhibiting the activity of XOD to inhibit UA production | [65] |
| Source | Bioactive Compound | Model | Dose | IC50 | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|
| Pueraria lobata (Willd.) Ohwi/Pueraria | Puerarin | Human renal proximal tubular epithelial cells (HK2 cells) | 100 mg/L | 16.48 µM | Effectively promoted ABCG2 protein expression in HK2 cells | It is related to up-regulating of ABCG2 to promote renal excretion of UA | [66] |
| Citrus aurantium L./Fructus Aurantii | Hesperetin | XOD inhibitor screening model in vitro | 20 µM | 16.48 µM | Significantly inhibited XOD activity | This is related to inhibit XOD to inhibit UA production | [67] |
| Citrus aurantium L./Fructus Aurantii | Nobiletin | XOD inhibitor screening model in vitro | 20 µM | 16.48 µM | Significantly inhibited XOD activity | This is related to inhibit XOD to inhibit UA production | [67] |
| Citrus reticulata Blanco/Citrus | Acacatechin | XOD model in vitro | 100 µg/mL | 27 ± 1.16 µg/mL | Significantly inhibited XOD activity | It showed competitive type of XOD inhibition to inhibit UA production | [68] |
| Citrus reticulata Blanco/Citrus | Glycitein | XOD model in vitro | 100 µg/mL | 12 ± 0.86 µg/mL | Significantly inhibited XOD activity | It showed competitive type of XOD inhibition to inhibit UA production | [68] |
| Citrus reticulata Blanco/Citrus | Myricetin | XOD model in vitro | 100 µg/mL | 26 ± 0.72 µg/mL | Significantly inhibited XOD activity | It showed competitive type of XOD inhibition to inhibit UA production | [68] |
| Carthamus tinctorius L./Carthami Flos | Galuteolin | XOD inhibitor screening model in vitro | 100 µg/mL | 12 ± 0.86 µg/mL | Significantly inhibited XOD activity | This is related to inhibiting XOD to inhibit UA production | [68] |
| Citrus reticulata Blanco/Citrus | Naringenin | XOD model in vitro | 100 µg/mL | 22 ± 0.64 µg/mL | Significantly inhibited XOD activity | It showed competitive type of XOD inhibition to inhibit UA production | [68] |
| Carthamus tinctorius L./Carthami Flos | Kaemperfol | XOD inhibitor screening model in vitro | 100 µg/mL | 12 ± 0.86 µg/mL | Significantly inhibited XOD activity | This is related to inhibiting XOD to inhibit UA production | [69] |
| Source | Bioactive Compound | Model | Dose | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Cichorium intybus L./Chicory | Chlorogenic acid | R | 50 and 150 mg/kg | SUA level significantly was reduced, XOD and ADA levels showed different degrees of inhibition | This is related to promoting UA excretion by down-regulating the expression of mURAT1 and inhibiting XOD and ADA | [76] |
| Glycyrrhiza uralensis Fisch/Liquorice Root | Protocatechuic acid | F | 10 mg/kg | SUA level significantly reduced, fractional excretion of uric acid was increased | This is related to down-regulation the transport activity of URAT1 by inhibiting UA re-absorption | [55] |
| Coix lachryma-jobi L./Adlay | Vanillic acid | B | 166 mg/kg | SUA level significantly reduced, XOD was inhibited | This is related to inhibiting the activity of XOD | [74] |
| Coix lachryma-jobi L./Adlay | Ferulic acid | B | 166 mg/kg | SUA level significantly reduced, XOD was inhibited | This is related to inhibiting the activity of XOD | [74] |
| Source | Bioactive Compound | Model | Dose | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Evodia rutaecarpa (Juss.) Benth./Euodiae Fructus | Evodiamine | Q | 8 mg/kg | SUA and XOD could be significantly reduced | This is related to inhibiting the activity of XOD to inhibit of UA production | [83] |
| Lycium barbarum L./Lycii Fructus | Betaine | D | 10, 20, and 40 mg/kg | SUA, BUN, and Scr levels significantly reduced, fractional excretion of uric acid was increased | This is related to down-regulating mRNA and protein levels of URAT1 and GLUT9, and up-regulating mRNA and protein levels of OAT1 to promote uric acid excretion | [84,85] |
| Source | Bioactive Compound | Model | Dose | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Camellia sinensis L./Green tea | Green tea polyphenols | P | 600 mg/kg | Decreased SUA and increased excretion of exceeding UA significantly | It can inhibit XOD activities | [101] |
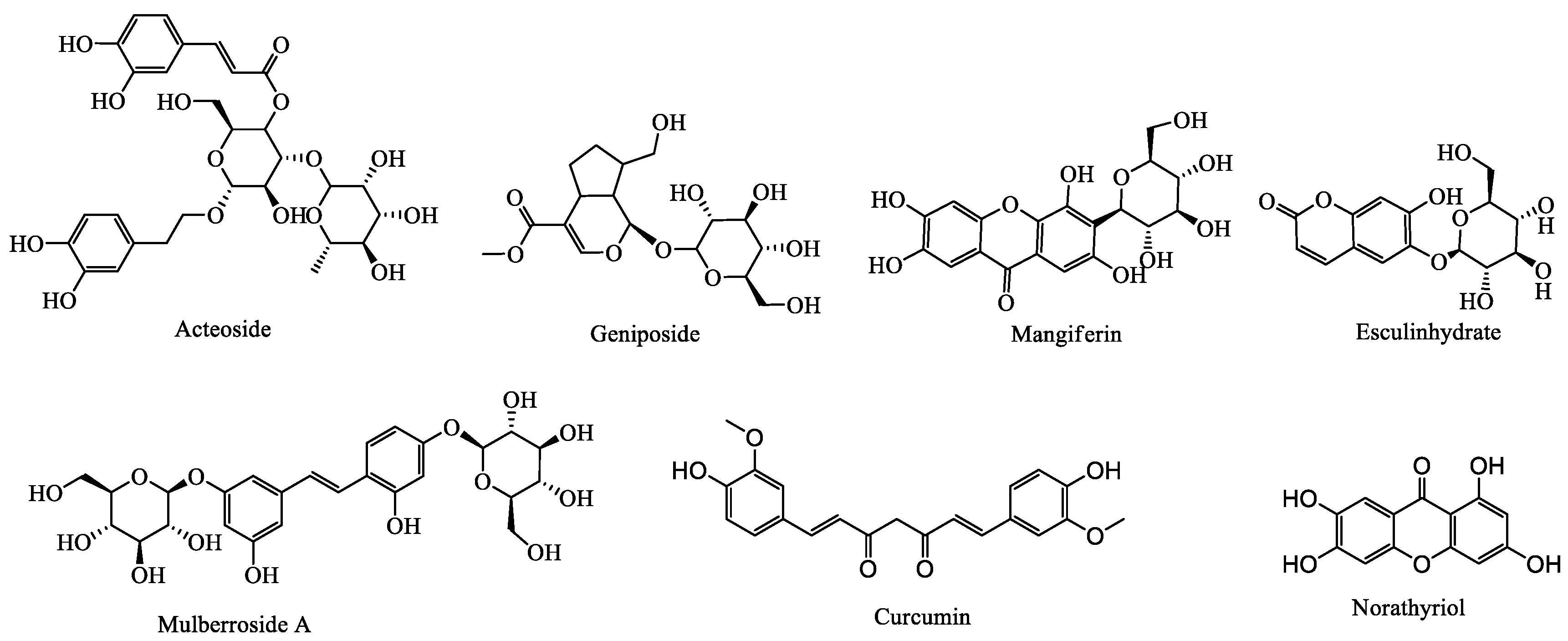
| Plantago asiatica L./Plantaginis Semen | Acteoside | D | 200 mg/kg | UA and creatinine levels were obviously reduced and the activity of hepatic XOD was inhibited. Furthermore, the mRNA expression of URAT1 and GLUT9 were obviously down-regulated | The mechanism of lowering SUA level can inhibit XOD activity and down-regulate the mRNA expression of URAT1 and GLUT9 | [99,102] |
| Morus alba L./Mori Cortex | Mulberroside A | C | 10, 20 and 40 mg/kg | Decreased SUA level and increased urinary UA excretion and fractional excretion of UA. Furthermore, down-regulated mRNA and protein levels of mGLUT9 and mURAT1, and upregulated mRNA and protein levels of mOAT1, mOCT1, mOCT2, mOCTN1, and mOCTN2 | Hypouricemic effect is achieved by down-regulating mRNA and protein levels of mGLUT9 and mURAT1, and upregulating mRNA and protein levels of mOAT1 to promote UA excretion | [103] |
| Cichorium intybus L./Chicory | Esculinhydrate | M | 50 and 150 mg/kg | SUA level significantly increased, XOD and ADA levels showed different degrees of inhibition | This is related to down-regulation the expression of mURAT1 to promote UA excretion | [76] |
| Gardenia jasminoides Ellis/Cape Jasmine | Geniposide | B | 50 and 100 mg/kg | The protein and mRNA expression of URAT1 and GLUT9 and serum UA significantly decreased, while 24 h urinary, the protein and mRNA expression of OAT1 were significantly increased | Down-regulated URAT1 and GLUT9, and up-regulated OAT1 to promote UA excretion | [98] |
| Mangifera indica L./Mango | Mangiferin | B | 6 mg/kg | SUA and the protein expression of URAT1, and GLUT9 were significantly decreased, while 24 h urinary creatinine, the expression of mABCG2 were significantly increased | This is related to down-regulation the protein expression of URAT1, GLUT9 and up-regulation the expression of ABCG2 to promote UA excretion | [104,105] |
| Mangifera indica L./Mango | Norathyriol | O | 4 mg/kg | Decreased SUA and markedly increased the fractional excretion of UA | The mechanism of lowering SUA can inhibit XOD activity and up-regulated OAT1. | [106] |
| Curcuma longa L./Turmeric | Curcumin | G | 20 and 40 mg/kg | Decreased SUA markedly increased | The mechanism of lowering SUA can inhibit XOD activity | [107,108] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.-L.; Gong, X.; Ji, M.-Y.; Wang, C.-C.; Wang, J.-H.; Li, M.-H. Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia. Foods 2020, 9, 973. https://doi.org/10.3390/foods9080973
Jiang L-L, Gong X, Ji M-Y, Wang C-C, Wang J-H, Li M-H. Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia. Foods. 2020; 9(8):973. https://doi.org/10.3390/foods9080973
Chicago/Turabian StyleJiang, Lin-Lin, Xue Gong, Ming-Yue Ji, Cong-Cong Wang, Jian-Hua Wang, and Min-Hui Li. 2020. "Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia" Foods 9, no. 8: 973. https://doi.org/10.3390/foods9080973
APA StyleJiang, L.-L., Gong, X., Ji, M.-Y., Wang, C.-C., Wang, J.-H., & Li, M.-H. (2020). Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia. Foods, 9(8), 973. https://doi.org/10.3390/foods9080973





