Airway Inflammation and Host Responses in the Era of CFTR Modulators
Abstract
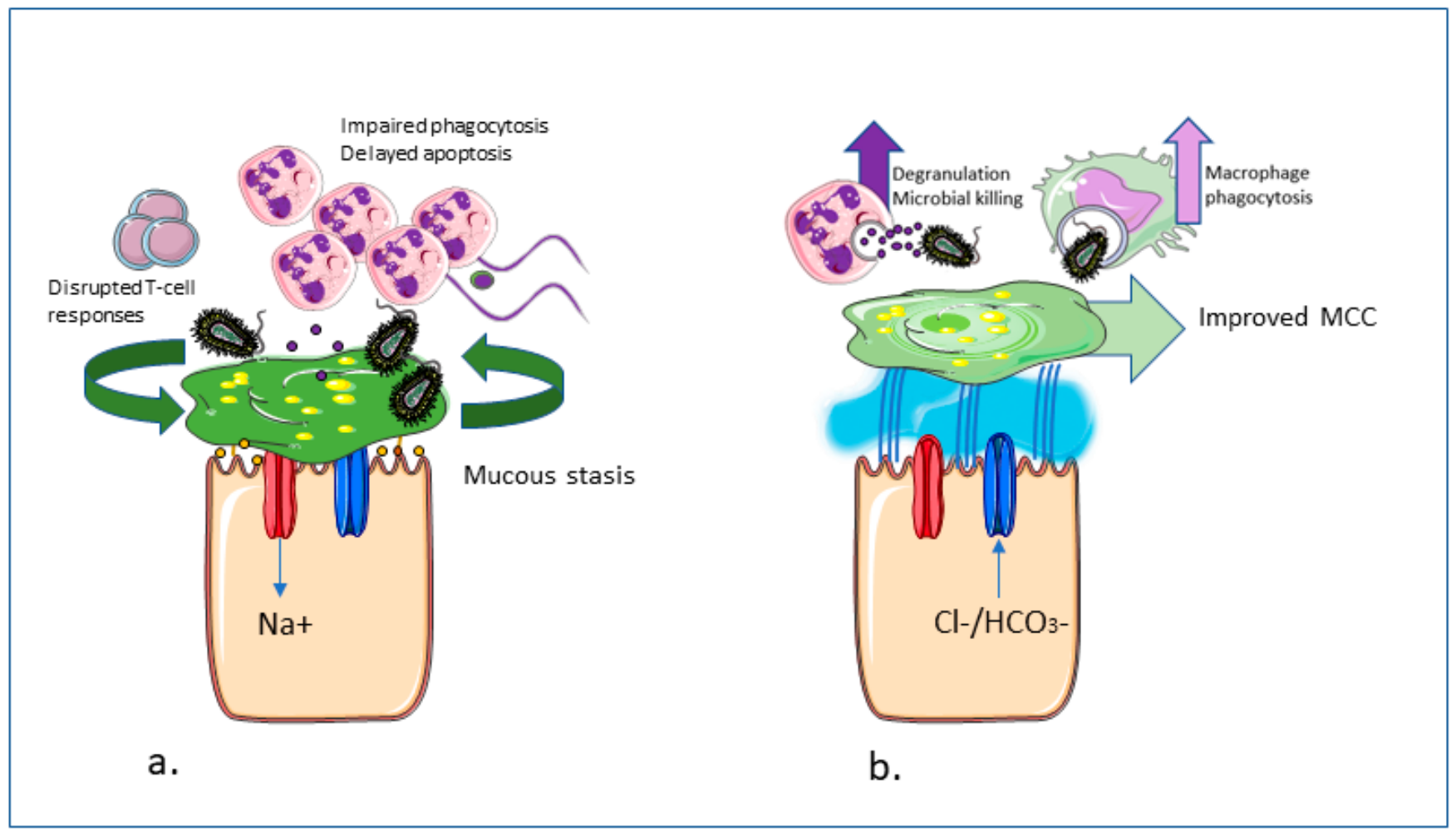
1. Introduction
2. Inflammation in CF
2.1. Pro-Inflammatory Airway Environment
2.2. Abnormal Immune Cell Responses
2.3. Impaired Signalling Mechansims
2.4. Impaired Counter-Inflammatory Mechanisms
3. Effects of CFTR Modulators on Inflammation
3.1. Effects on Immune Cells
3.1.1. Neutrophils
3.1.2. Macrophages
3.1.3. Lymphocytes and the Adaptive Immune Response
3.1.4. Airway Epithelial Cells
3.1.5. Platelets
3.2. Other Effects
4. CFTR Modulators, the Microbiome, and Airway Infection
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASL | Airway surface liquid |
| BAL | Bronchoalveolar lavage |
| CF | Cystic fibrosis |
| CFTR | CF transmembrane conductance regulator |
| DAMP | Damage-associated molecular patterns |
| DCs | Dendritic cells |
| ENaC | Epithelial Na+ channel |
| ER | Endoplasmic reticulum |
| FEV1 | Forced expiratory volume in 1 s |
| HBE | Human bronchial epithelial cell |
| IVA | Ivacaftor |
| LCI | Lung clearance index |
| LPS | Lipopolysaccharide |
| LUM | Lumacaftor |
| MCC | Mucociliary clearance |
| MDM | Monocyte-derived macrophage |
| NE | Neutrophil elastase |
| NO | Nitric oxide |
| Pa | Pseudomonas aeruginosa |
| PEx | Pulmonary exacerbations |
| PWCF | People with CF |
| ROS | Reactive oxygen species |
| TEZ | Tezacaftor |
| TRPC | Transient receptor potential channel (TRPC) |
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Nichols, D.P.; Chmiel, J.F. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 2015, 50, S39–S56. [Google Scholar] [CrossRef] [PubMed]
- Regamey, N.; Jeffery, P.K.; Alton, E.W.F.W.; Bush, A.; Davies, J.C. Airway remodelling and its relationship to inflammation in cystic fibrosis. Thorax 2011, 66, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Sontag, M.K.; Wagener, J.S.; Kapsner, R.K.; Osberg, I.; Accurso, F.J. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J. Pediatr. 2002, 141, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.T.; Flume, P.A. Cystic fibrosis: Translating molecular mechanisms into effective therapies. Ann. Am. Thorac. Soc. 2018, 15, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.C.; Moskowitz, S.M.; Brown, C.; Horsley, A.; Mall, M.A.; McKone, E.F.; Plant, B.J.; Prais, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; et al. VX-659–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two phe508del alleles. N. Engl. J. Med. 2018, 379, 1599–1611. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two phe508del alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; Van Der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Dřevínek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR Potentiator in Patients with Cystic Fibrosis and the G551D Mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Munck, A.; Walker, S.; Faro, A.; Hiatt, P.; Gilmartin, G.; Higgins, M. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J. Cyst. Fibros. 2014, 13, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, P65–P124. [Google Scholar] [CrossRef]
- Sawicki, G.S.; McKone, E.F.; Pasta, D.J.; Millar, S.J.; Wagener, J.S.; Johnson, C.A.; Konstan, M.W. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am. J. Respir. Crit. Care Med. 2015, 192, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.C.; Parsons, F.; Gangell, C.; Brennan, S.; Stick, S.M.; Sly, P.D. Evolution of pulmonary inflammation and nutritional status in infants and young children with cystic fibrosis. Thorax 2011, 66, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.S.; Grimwood, K.; Carlin, J.B.; Carzino, R.; Gutièrrez, J.P.; Hull, J.; Olinsky, A.; Phelan, E.M.; Robertson, C.F.; Phelan, P.D. Lower airway inflammation in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1997, 156, 1197–1204. [Google Scholar] [CrossRef]
- Sly, P.D.; Brennan, S.; Gangell, C.; de Klerk, N.; Murray, C.; Mott, L.; Stick, S.M.; Robinson, P.J.; Robertson, C.F.; Ranganathan, S.C.; et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am. J. Respir. Crit. Care Med. 2009, 180, 146–152. [Google Scholar] [CrossRef]
- Esther, C.R.; Muhlebach, M.S.; Ehre, C.; Hill, D.B.; Wolfgang, M.C.; Kesimer, M.; Ramsey, K.A.; Markovetz, M.R.; Garbarine, I.C.; Gregory Forest, M.; et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 2019, 11, eaav3488. [Google Scholar] [CrossRef]
- Rosenow, T.; Clara Mok, L.; Turkovic, L.; Berry, L.J.; Sly, P.D.; Ranganathan, S.; Tiddens, H.A.W.M.; Stick, S.M. The cumulative effect of inflammation and infection on structural lung disease in early cystic fibrosis. Eur. Respir. J. 2019, 54, 1801771. [Google Scholar] [CrossRef]
- Bedrossian, C.W.M.; Donald Greenberg, S.; Singer, D.B.; Hansen, J.J.; Rosenberg, H.S. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum. Pathol. 1976, 7, 195–204. [Google Scholar] [CrossRef]
- Stick, S.M.; Brennan, S.; Murray, C.; Douglas, T.; von Ungern-Sternberg, B.S.; Garratt, L.W.; Gangell, C.L.; De Klerk, N.; Linnane, B.; Ranganathan, S.; et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J. Pediatr. 2009, 155, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Sly, P.D.; Gangell, C.L.; Chen, L.; Ware, R.S.; Ranganathan, S.; Mott, L.S.; Murray, C.P.; Stick, S.M. AREST CF investigators risk factors for bronchiectasis in children with cystic fibrosis. N. Engl. J. Med. 2013, 368, 1963–1970. [Google Scholar] [CrossRef]
- Pillarisetti, N.; Williamson, E.; Linnane, B.; Skoric, B.; Robertson, C.F.; Robinson, P.; Massie, J.; Hall, G.L.; Sly, P.; Stick, S.; et al. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.F.; Konstan, M.W. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin. Chest Med. 2007, 28, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Perrem, L.; Ratjen, F. Anti-inflammatories and mucociliary clearance therapies in the age of CFTR modulators. Pediatr. Pulmonol. 2019, 54, S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.Z.; Wagener, J.S.; Bost, T.; Martinez, J.; Accurso, F.J.; Riches, D.W.H. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995, 151, 1075–1082. [Google Scholar] [CrossRef]
- Balough, K.; McCubbin, M.; Weinberger, M.; Smits, W.; Ahrens, R.; Fick, R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr. Pulmonol. 1995, 20, 63–70. [Google Scholar] [CrossRef]
- Brennan, S.; Hall, G.L.; Horak, F.; Moeller, A.; Pitrez, P.M.C.; Franzmann, A.; Turner, S.; De Klerk, N.; Franklin, P.; Winfield, K.R.; et al. Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax 2005, 60, 159–163. [Google Scholar] [CrossRef]
- Rosen, B.H.; Evans, T.I.A.; Moll, S.R.; Gray, J.S.; Liang, B.; Sun, X.; Zhang, Y.; Jensen-Cody, C.W.; Swatek, A.M.; Zhou, W.; et al. Infection is not required for mucoinflammatory lung disease in CFTR-Knockout ferrets. Am. J. Respir. Crit. Care Med. 2018, 197, 1308–1318. [Google Scholar] [CrossRef]
- Yoshimura, K.; Nakamura, H.; Trapnell, B.C.; Chu, C.S.; Dakemans, W.; Pavirani, A.; Lecocq, J.P.; Crystal, R.G. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991, 19, 5417–5423. [Google Scholar] [CrossRef]
- Painter, R.G.; Valentine, V.G.; Lanson, N.A.; Leidal, K.; Zhang, Q.; Lombard, G.; Thompson, C.; Viswanathan, A.; Nauseef, W.M.; Wang, G.; et al. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 2006, 45, 10260–10269. [Google Scholar] [CrossRef] [PubMed]
- Coakley, R.D.; Grubb, B.R.; Paradiso, A.M.; Gatzy, J.T.; Johnson, L.G.; Kreda, S.M.; O’Neal, W.K.; Boucher, R.C. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. USA 2003, 100, 16083–16088. [Google Scholar] [CrossRef]
- Mattoscio, D.; Evangelista, V.; De Cristofaro, R.; Recchiuti, A.; Pandolfi, A.; Di Silvestre, S.; Manarini, S.; Martelli, N.; Rocca, B.; Petrucci, G.; et al. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: Impact on mediators and mechanisms of the inflammatory response. FASEB J. 2010, 24, 3970–3980. [Google Scholar] [CrossRef]
- Di, A.; Brown, M.E.; Deriy, L.V.; Li, C.; Szeto, F.L.; Chen, Y.; Huang, P.; Tong, J.; Naren, A.P.; Bindokas, V.; et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 2006, 8, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Bubien, J.K. CFTR may play a role in regulated secretion by lymphocytes: A new hypothesis for the pathophysiology of cystic fibrosis. Pflug. Arch. Eur. J. Physiol. 2001, 443, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tertilt, C.; Krause, A.; Quadri, L.E.N.; Crystal, R.G.; Worgall, S. Influence of the cystic fibrosis transmembrane conductance regulator on expression of lipid metabolism-related genes in dendritic cells. Respir. Res. 2009, 10, 26. [Google Scholar] [CrossRef]
- Van Heeckeren, A.; Walenga, R.; Konstan, M.W.; Bonfield, T.; Davis, P.B.; Ferkol, T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J. Clin. Invest. 1997, 100, 2810–2815. [Google Scholar] [CrossRef]
- Siegmann, N.; Worbs, D.; Effinger, F.; Bormann, T.; Gebhardt, M.; Ulrich, M.; Wermeling, F.; Müller-Hermelink, E.; Biedermann, T.; Tighe, M.; et al. Invariant natural killer T (iNKT) cells prevent autoimmunity, but induce pulmonary inflammation in cystic fibrosis. Cell. Physiol. Biochem. 2014, 34, 56–70. [Google Scholar] [CrossRef]
- Schlesinger, R.B.; Fine, J.M.; Chen, L.C. Interspecies differences in the phagocytic activity of pulmonary macrophages subjected to acidic challenge. Toxicol. Sci. 1992, 19, 584–589. [Google Scholar] [CrossRef]
- Clary-Meinesz, C.; Mouroux, J.; Cosson, J.; Huitorel, P.; Blaive, B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur. Respir. J. 1998, 11, 330–333. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, Z.S.; Sweezey, N.; Grasemann, H.; Palaniyar, N. Progression of cystic fibrosis lung disease from childhood to adulthood: Neutrophils, neutrophil extracellular trap (NET) formation, and NET degradation. Genes 2019, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sun, L.; Kato, T.; Okuda, K.; Martino, M.B.; Abzhanova, A.; Lin, J.M.; Gilmore, R.C.; Batson, B.D.; O’Neal, Y.K.; et al. IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J. Clin. Invest. 2019, 129, 4433–4450. [Google Scholar] [CrossRef] [PubMed]
- Birket, S.E.; Rowe, S.M. Revealing the molecular signaling pathways of mucus stasis in cystic fibrosis. J. Clin. Invest. 2019, 129, 4089–4090. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, M.M.; Rosenfeld, M.; Retsch-Bogart, G.; Gibson, R.; Ramsey, B. Approach to eradication of initial Pseudomonas aeruginosa infection in children with cystic fibrosis. Pediatr. Pulmonol. 2007, 42, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Döring, G. Cystic fibrosis. Lancet 2003, 361, 681–689. [Google Scholar] [CrossRef]
- Rogers, G.B.; Taylor, S.L.; Hoffman, L.R.; Burr, L.D. The impact of CFTR modulator therapies on CF airway microbiology. J. Cyst. Fibros. 2019, 19, 359–364. [Google Scholar] [CrossRef]
- Cox, M.J.; Allgaier, M.; Taylor, B.; Baek, M.S.; Huang, Y.J.; Daly, R.A.; Karaoz, U.; Andersen, G.L.; Brown, R.; Fujimura, K.E.; et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Coburn, B.; Wang, P.W.; Diaz Caballero, J.; Clark, S.T.; Brahma, V.; Donaldson, S.; Zhang, Y.; Surendra, A.; Gong, Y.; Elizabeth Tullis, D.; et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci. Rep. 2015, 5, 10241. [Google Scholar] [CrossRef]
- Dakin, C.J.; Numa, A.H.; Wang, H.; Morton, J.R.; Vertzyas, C.C.; Henry, R.L. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002, 165, 904–910. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Zaslona, Z.; Becker-Flegler, K.; Palsson-McDermott, E.M.; Boland, F.; Gunaratnam, C.; Gulbins, E.; O’Neill, L.A.; Reeves, E.P.; McElvaney, N.G. Specific inhibition of the NLRP3 inflammasome as an antiinflammatory strategy in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 1381–1391. [Google Scholar] [CrossRef]
- DiMango, E.; Zar, H.J.; Bryan, R.; Prince, A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Invest. 1995, 96, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Higashimoto, Y.; Inoue, H.; Shimizu, T.; Kuwano, K. A novel secreted protease from Pseudomonas aeruginosa activates NF-KB through protease-activated receptors. Cell. Microbiol. 2008, 10, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, M.C.; Weldon, S.; McAuley, D.F.; Mall, M.A.; Taggart, C.C. Targeting proteases in cystic fibrosis lung disease paradigms, progress, and potential. Am. J. Respir. Crit. Care Med. 2020, 201, 141–147. [Google Scholar] [CrossRef]
- Smallman, L.A.; Hill, S.L.; Stockley, R.A. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: A serine proteinase effect. Thorax 1984, 39, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Young, L.R.; Wang, Y.; Horger, T.; Rose, M.C.; Fischer, B.M. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 1999, 276, 835–843. [Google Scholar] [CrossRef]
- Fischer, B.M.; Voynow, J.A. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am. J. Respir. Cell Mol. Biol. 2002, 26, 447–452. [Google Scholar] [CrossRef]
- Park, J.-A.; He, F.; Martin, L.D.; Li, Y.; Chorley, B.N.; Adler, K.B. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase cδ-mediated mechanism. Am. J. Pathol. 2005, 167, 651–661. [Google Scholar] [CrossRef]
- Caldwell, R.A.; Boucher, R.C.; Stutts, M.J. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L813–L819. [Google Scholar] [CrossRef]
- Le Gars, M.; Descamps, D.; Roussel, D.; Saussereau, E.; Guillot, L.; Ruffin, M.; Tabary, O.; Hong, S.S.; Boulanger, P.; Paulais, M.; et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am. J. Respir. Crit. Care Med. 2013, 187, 170–179. [Google Scholar] [CrossRef]
- Vandivier, R.W.; Fadok, V.A.; Hoffmann, P.R.; Bratton, D.L.; Penvari, C.; Brown, K.K.; Brain, J.D.; Accurso, F.J.; Henson, P.M. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 2002, 109, 661–670. [Google Scholar] [CrossRef]
- Mayer-Hamblett, N.; Aitken, M.L.; Accurso, F.J.; Kronmal, R.A.; Konstan, M.W.; Burns, J.L.; Sagel, S.D.; Ramsey, B.W. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 175, 822–828. [Google Scholar] [CrossRef]
- Meyer, K.C.; Zimmerman, J. Neutrophil mediators, Pseudomonas, and pulmonary dysfunction in cystic fibrosis. J. Lab. Clin. Med. 1993, 121, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Kummarapurugu, A.B.; Afosah, D.K.; Sankaranarayanan, N.V.; Gangji, R.N.; Zheng, S.; Kennedy, T.; Rubin, B.K.; Voynow, J.A.; Desai, U.R. Molecular principles for heparin oligosaccharide-based inhibition of neutrophil elastase in cystic fibrosis. J. Biol. Chem. 2018, 293, 12480–12490. [Google Scholar] [CrossRef] [PubMed]
- Pohl, K.; Hayes, E.; Keenan, J.; Henry, M.; Meleady, P.; Molloy, K.; Jundi, B.; Bergin, D.A.; McCarthy, C.; McElvaney, O.J.; et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood 2014, 124, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.P.; Valentine, V.G.; Wang, G. CFTR targeting during activation of human neutrophils. J. Leukoc. Biol. 2016, 100, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.P.; Zhou, Y.; Song, K.; Hodges, C.A.; Drumm, M.L.; Wang, G. Neutrophil-mediated phagocytic host defense defect in myeloid Cftr-inactivated mice. PLoS ONE 2014, 9, e106813. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.A.; Li, X.; Seitz, A.; Steinmann, J.; Koch, A.; Schuchman, E.; Kamler, M.; Edwards, M.J.; Caldwell, C.C.; Gulbins, E. Neutrophils kill reactive oxygen species-resistant pseudomonas aeruginosa by sphingosine. Cell Physiol. Biochem. 2017, 43, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Bernut, A.; Loynes, C.A.; Floto, R.A.; Renshaw, S.A. Deletion of cftr leads to an excessive neutrophilic response and defective tissue repair in a zebrafish model of sterile inflammation. BioRxiv 2019, 842195. [Google Scholar] [CrossRef]
- Su, X.; Looney, M.R.; Su, H.; Lee, J.W.; Song, Y.; Matthay, M.A. Role of CFTR expressed by neutrophils in modulating acute lung inflammation and injury in mice. Inflamm. Res. 2011, 60, 619–632. [Google Scholar] [CrossRef]
- Taylor, P.R.; Bonfield, T.L.; Chmiel, J.F.; Pearlman, E. Neutrophils from F508del cystic fibrosis patients produce IL-17A and express IL-23 dependent IL-17RC. Clin. Immunol. 2016, 170, 53–60. [Google Scholar] [CrossRef]
- Taggart, C.; Coakley, R.J.; Greally, P.; Canny, G.; O’Neill, S.J.; McElvaney, N.G. Increased elastase release by CF neutrophils is mediated by tumor necrosis factor-alpha and interleukin-8. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L33–L41. [Google Scholar] [CrossRef]
- Del Porto, P.; Cifani, N.; Guarnieri, S.; Di Domenico, E.G.; Mariggiò, M.A.; Spadaro, F.; Guglietta, S.; Anile, M.; Venuta, F.; Quattrucci, S.; et al. Dysfunctional cftr alters the bactericidal activity of human macrophages against pseudomonas aeruginosa. PLoS ONE 2011, 6, e19970. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Sly, P.D.; Holt, P.G.; Bosco, A.; Ware, R.S.; Logan, J.; Bell, S.C.; Wainwright, C.E.; Fantino, E. CFTR-dependent defect in alternatively-activated macrophages in cystic fibrosis. J. Cyst. Fibros. 2017, 16, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Griese, M.; Kappler, M.; Zissel, G.; Reinhardt, D.; Rebhan, C.; Schendel, D.J.; Krauss-Etschmann, S. Pulmonary TH2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J. Allergy Clin. Immunol. 2006, 117, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Braag, S.A.; Keeler, A.; Hodges, C.; Drumm, M.; Flotte, T.R. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am. J. Respir. Cell Mol. Biol. 2011, 44, 922–929. [Google Scholar] [CrossRef]
- Tiringer, K.; Treis, A.; Fucik, P.; Gona, M.; Gruber, S.; Renner, S.; Dehlink, E.; Nachbaur, E.; Horak, F.; Jaksch, P.; et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2013, 187, 621–629. [Google Scholar] [CrossRef]
- Polverino, F.; Lu, B.; Quintero, J.R.; Vargas, S.O.; Patel, A.S.; Owen, C.A.; Gerard, N.P.; Gerard, C.; Cernadas, M. CFTR regulates B cell activation and lymphoid follicle development. Respir. Res. 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Hector, A.; Schäfer, H.; Pöschel, S.; Fischer, A.; Fritzsching, B.; Ralhan, A.; Carevic, M.; Öz, H.; Zundel, S.; Hogardt, M.; et al. Regulatory T-cell impairment in cystic fibrosis patients with chronic Pseudomonas infection. Am. J. Respir. Crit. Care Med. 2015, 191, 914–923. [Google Scholar] [CrossRef]
- Kushwah, R.; Gagnon, S.; Sweezey, N.B. Intrinsic predisposition of naïve cystic fibrosis T cells to differentiate towards a Th17 phenotype. Respir. Res. 2013, 14, 1–8. [Google Scholar] [CrossRef]
- Zhang, P.-X.; Murray, T.S.; Villella, V.R.; Ferrari, E.; Esposito, S.; D’Souza, A.; Raia, V.; Maiuri, L.; Krause, D.S.; Egan, M.E.; et al. Reduced caveolin-1 promotes hyperinflammation due to abnormal heme oxygenase-1 localization in lipopolysaccharide-challenged macrophages with dysfunctional cystic fibrosis transmembrane conductance regulator. J. Immunol. 2013, 190, 5196–5206. [Google Scholar] [CrossRef]
- Ribeiro, C.M.P.; Paradiso, A.M.; Schwab, U.; Perez-Vilar, J.; Jones, L.; O’Neal, W.; Boucher, R.C. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 2005, 280, 17798–17806. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, J.; Roussel, L.; Nattagh, L.; Rousseau, S. Loss of cystic fibrosis transmembrane conductance regulator function enhances activation of p38 and erk mapks, increasing interleukin-6 synthesis in airway epithelial cells exposed to Pseudomonas aeruginosa. J. Biol. Chem. 2010, 285, 22299–22307. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Issler, A.C.; Cotton, C.U.; Kelley, T.J.; Verkman, A.S.; Davis, P.B. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Tirouvanziam, R.; de Bentzmann, S.; Hubeau, C.; Hinnrasky, J.; Jacquot, J.; Péault, B.; Puchelle, E. Inflammation and infection in naive human cystic fibrosis airway grafts. Am. J. Respir. Cell Mol. Biol. 2000, 23, 121–127. [Google Scholar] [CrossRef]
- Chen, J.; Kinter, M.; Shank, S.; Cotton, C.; Kelley, T.J.; Ziady, A.G. Dysfunction of Nrf-2 in CF epithelia leads to excess intracellular H2O2 and inflammatory cytokine production. PLoS ONE 2008, 3, e3367. [Google Scholar] [CrossRef]
- Bonfield, T.L.; Panuska, J.R.; Konstan, M.W.; Hilliard, K.A.; Hilliard, J.B.; Ghnaim, H.; Berger, M. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995, 152, 2111–2118. [Google Scholar] [CrossRef]
- Zaman, M.M.; Gelrud, A.; Junaidi, O.; Regan, M.M.; Warny, M.; Shea, J.C.; Kelly, C.; O’Sullivan, B.P.; Freedman, S.D. Interleukin 8 secretion from monocytes of subjects heterozygous for the ΔF508 cystic fibrosis transmembrane conductance regulator gene mutation is altered. Clin. Diagn. Lab. Immunol. 2004, 11, 819–824. [Google Scholar] [CrossRef]
- Tang, A.; Sharma, A.; Jen, R.; Hirschfeld, A.F.; Chilvers, M.A.; Lavoie, P.M.; Turvey, S.E. Inflammasome-mediated IL-1β production in humans with cystic fibrosis. PLoS ONE 2012, 7, e37689. [Google Scholar] [CrossRef]
- Bruscia, E.M.; Zhang, P.X.; Ferreira, E.; Caputo, C.; Emerson, J.W.; Tuck, D.; Krause, D.S.; Egan, M.E. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator-/-mice. Am. J. Respir. Cell Mol. Biol. 2009, 40, 295–304. [Google Scholar] [CrossRef]
- Sturges, N.C.; Wikstrom, M.E.; Winfield, K.R.; Gard, S.E.; Brennan, S.; Sly, P.D.; Upham, J.W.; on behalf of AREST CF. Monocytes from children with clinically stable cystic fibrosis show enhanced expression of toll-like receptor 4. Pediatr. Pulmonol. 2010, 45, 883–889. [Google Scholar] [CrossRef]
- Lara-Reyna, S.; Scambler, T.; Holbrook, J.; Wong, C.; Jarosz-Griffiths, H.H.; Martinon, F.; Savic, S.; Peckham, D.; McDermott, M.F. metabolic reprograming of cystic fibrosis macrophages via the ire1α arm of the unfolded protein response results in exacerbated inflammation. Front. Immunol. 2019, 10, 1789. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of inflammasome signaling. Nat. Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; de Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Konstan, M.W.; Berger, M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J. Allergy Clin. Immunol. 1999, 104, 72–78. [Google Scholar] [CrossRef]
- Grasemann, H.; Ratjen, F. Nitric oxide and l-arginine deficiency in cystic fibrosis. Curr. Pharm. Des. 2012, 18, 726–736. [Google Scholar] [CrossRef]
- Balfour-Lynn, I.M.; Laverty, A.; Dinwiddie, R. Reduced upper airway nitric oxide in cystic fibrosis. Arch. Dis. Child. 1996, 75, 319–322. [Google Scholar] [CrossRef]
- Karp, C.L.; Flick, L.M.; Park, K.W.; Softic, S.; Greer, T.M.; Keledjian, R.; Yang, R.; Uddin, J.; Guggino, W.B.; Atabani, S.F.; et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 2004, 5, 388–392. [Google Scholar] [CrossRef]
- Bratcher, P.E.; Rowe, S.M.; Reeves, G.; Roberts, T.; Szul, T.; Harris, W.T.; Tirouvanziam, R.; Gaggar, A. Alterations in blood leukocytes of G551D-bearing cystic fibrosis patients undergoing treatment with ivacaftor. J. Cyst. Fibros. 2016, 15, 67–73. [Google Scholar] [CrossRef]
- Weathington, N.M.; Jackson, P.L.; Gaggar, A.; Xu, X.; Snelgrove, R. Matrix metalloproteinase-9 cleaves surfactant Protein-D, impairing collectin function as a bacterial agglutinin and opsonin in vitro, and such cleavage is seen during acute influenza infection in mice. ATS J. 2010, A5646. [Google Scholar] [CrossRef]
- Birrer, P.; McElvaney, N.G.; Rüdeberg, A.; Wirz Sommer, C.; Liechti-Gallati, S.; Kraemer, R.; Hubbard, R.; Crystal, R.G.; Sommer, C.W.; Liechti-Gallati, S.; et al. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1994, 150, 207–213. [Google Scholar] [CrossRef]
- Weldon, S.; McNally, P.; McElvaney, N.G.; Elborn, J.S.; McAuley, D.F.; Wartelle, J.; Belaaouaj, A.; Levine, R.L.; Taggart, C.C. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J. Immunol. 2009, 183, 8148–8156. [Google Scholar] [CrossRef] [PubMed]
- Guyot, N.; Butler, M.W.; McNally, P.; Weldon, S.; Greene, C.M.; Levine, R.L.; O’Neill, S.J.; Taggart, C.C.; McElvaney, N.G. Elafin, an elastase-specific inhibitor, is cleaved by its cognate enzyme neutrophil elastase in sputum from individuals with cystic fibrosis. J. Biol. Chem. 2008, 283, 32377–32385. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.R.R.; Kajbafzadeh, M.; Desai, S.; Yang, C.L.; Skolnik, K.; Quon, B.S. A systematic review of the clinical efficacy and safety of cftr modulators in cystic fibrosis. Sci. Rep. 2019, 9, 7234. [Google Scholar] [CrossRef] [PubMed]
- Hisert, K.B.; Heltshe, S.L.; Pope, C.; Jorth, P.; Wu, X.; Edwards, R.M.; Radey, M.; Accurso, F.J.; Wolter, D.J.; Cooke, G.; et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am. J. Respir. Crit. Care Med. 2017, 195, 1617–1628. [Google Scholar] [CrossRef]
- Rowe, S.M.; Heltshe, S.L.; Gonska, T.; Donaldson, S.H.; Borowitz, D.; Gelfond, D.; Sagel, S.D.; Khan, U.; Mayer-Hamblett, N.; van Dalfsen, J.M.; et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 175–184. [Google Scholar] [CrossRef]
- Jarosz-Griffiths, H.H.; Scambler, T.; Wong, C.H.; Lara-Reyna, S.; Holbrook, J.; Martinon, F.; Savic, S.; Whitaker, P.; Etherington, C.; Spoletini, G.; et al. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. Elife 2020, 9, e54556. [Google Scholar] [CrossRef]
- Donaldson, S.H.; Laube, B.L.; Corcoran, T.E.; Bhambhvani, P.; Zeman, K.; Ceppe, A.; Zeitlin, P.L.; Mogayzel, P.J.; Boyle, M.; Locke, L.W.; et al. Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR. JCI Insight 2018, 3, e122695. [Google Scholar] [CrossRef]
- Altes, T.A.; Johnson, M.; Fidler, M.; Botfield, M.; Tustison, N.J.; Leiva-Salinas, C.; de Lange, E.E.; Froh, D.; Mugler, J.P. Use of hyperpolarized helium-3 MRI to assess response to ivacaftor treatment in patients with cystic fibrosis. J. Cyst. Fibros. 2017, 16, 267–274. [Google Scholar] [CrossRef]
- Davies, J.; Sheridan, H.; Lee, P.; Song, T.; Stone, A.; Ratjen, F. S121 lung clearance index to evaluate the effect of ivacaftor on lung function in subjects with cf who have the g551d-cftr mutation and mild lung disease. Thorax 2012, 67, A58–A59. [Google Scholar] [CrossRef][Green Version]
- Royal College of Surgeons in Ireland. A Real-World Study in Cystic Fibrosis. Available online: https://recovercf.ie/ (accessed on 29 July 2020).
- White, M.M.; Geraghty, P.; Hayes, E.; Cox, S.; Leitch, W.; Alfawaz, B.; Lavelle, G.M.; McElvaney, O.J.; Flannery, R.; Keenan, J.; et al. Neutrophil membrane cholesterol content is a key factor in cystic fibrosis lung disease. EBioMedicine 2017, 23, 173–184. [Google Scholar] [CrossRef]
- Gray, R.D.; Hardisty, G.; Regan, K.H.; Smith, M.; Robb, C.T.; Duffin, R.; Mackellar, A.; Felton, J.M.; Paemka, L.; McCullagh, B.N.; et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax 2018, 73, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shrestha, C.L.; Kopp, B.T. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, R.; Koeppen, K.; Nymon, A.; Hampton, T.H.; Berwin, B.; Ashare, A.; Stanton, B.A. Lumacaftor (VX-809) restores the ability of CF macrophages to phagocytose and kill Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L432–L438. [Google Scholar] [CrossRef] [PubMed]
- Hisert, K.B.; Schoenfelt, K.Q.; Cooke, G.; Grogan, B.; Launspach, J.L.; Gallagher, C.G.; Donnelly, S.C.; Welsh, M.J.; Singh, P.K.; McKone, E.F.; et al. Ivacaftor-induced proteomic changes suggest monocyte defects may contribute to the pathogenesis of cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 54, 594–599. [Google Scholar] [CrossRef]
- Hisert, K.B.; Birkland, T.P.; Schoenfelt, K.Q.; Long, M.E.; Grogan, B.; Carter, S.; Liles, W.C.; McKone, E.F.; Becker, L.; Manicone, A.M. Ivacaftor decreases monocyte sensitivity to interferon-γ in people with cystic fibrosis. ERJ Open Res. 2020, 6, 00318–02019. [Google Scholar] [CrossRef]
- Favia, M.; Gallo, C.; Guerra, L.; De Venuto, D.; Diana, A.; Polizzi, A.M.; Montemurro, P.; Mariggiò, M.A.; Leonetti, G.; Manca, A.; et al. Treatment of cystic fibrosis patients homozygous for F508del with lumacaftor-ivacaftor (Orkambi®) restores defective CFTR channel function in circulating mononuclear cells. Int. J. Mol. Sci. 2020, 21, 2398. [Google Scholar] [CrossRef]
- Kopp, B.T.; Zhang, S.; Shrestha, C. Influence of cftr modulators on immune responses in cystic fibrosis. In Proceedings of the American Thoracic Society 2019 International Conference, Dallas, TX, USA, 17–19 May 2019; p. A4008. [Google Scholar] [CrossRef]
- Kopp, B.T.; Fitch, J.; Jaramillo, L.; Shrestha, C.L.; Robledo-Avila, F.; Zhang, S.; Palacios, S.; Woodley, F.; Hayes, D.; Partida-Sanchez, S.; et al. Whole-blood transcriptomic responses to lumacaftor/ivacaftor therapy in cystic fibrosis. J. Cyst. Fibros. 2019, 19, 245–254. [Google Scholar] [CrossRef]
- Borcherding, D.C.; Siefert, M.E.; Lin, S.; Brewington, J.; Sadek, H.; Clancy, J.P.; Plafker, S.M.; Ziady, A.G. Clinically approved CFTR modulators rescue Nrf2 dysfunction in cystic fibrosis airway epithelia. J. Clin. Invest. 2019, 129, 3448–3463. [Google Scholar] [CrossRef]
- Adam, D.; Bilodeau, C.; Sognigbé, L.; Maillé, É.; Ruffin, M.; Brochiero, E. CFTR rescue with VX-809 and VX-770 favors the repair of primary airway epithelial cell cultures from patients with class II mutations in the presence of Pseudomonas aeruginosa exoproducts. J. Cyst. Fibros. 2018, 17, 705–714. [Google Scholar] [CrossRef]
- Ruffin, M.; Roussel, L.; Maillé, É.; Rousseau, S.; Brochiero, E. Vx-809/Vx-770 treatment reduces inflammatory response to Pseudomonas aeruginosa in primary differentiated cystic fibrosis bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L635–L641. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.A.; Coutermarsh, B.; Barnaby, R.; Hogan, D. Pseudomonas aeruginosa reduces VX-809 stimulated F508del-CFTR chloride secretion by airway epithelial cells. PLoS ONE 2015, 10, e0127742. [Google Scholar] [CrossRef]
- Veit, G.; Bossard, F.; Goepp, J.; Verkman, A.S.; Galietta, L.J.V.; Hanrahan, J.W.; Lukacs, G.L. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol. Biol. Cell 2012, 23, 4188–4202. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, P.; Lorentzen, D.; Excoffon, K.J.D.A.; Zabner, J.; McCray, P.B.; Nauseef, W.M.; Dupuy, C.; Bánfi, B. A novel host defense system of airways is defective in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 175, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, V.; Ulrich, M.; Endlich, N.; Riethmüller, J.; Wilker, B.; De Oliveira-Munding, C.C.; van Heeckeren, A.M.; Barr, M.L.; Von Kürthy, G.; Schmid, K.W.; et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 2008, 14, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Freedman, S.D.; Blanco, P.G.; Zaman, M.M.; Shea, J.C.; Ollero, M.; Hopper, I.K.; Weed, D.A.; Gelrud, A.; Regan, M.M.; Laposata, M.; et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 2004, 350, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.H.; Lee, M.M.; Yacono, P.W.; Cannon, C.L.; Alev Gerçeker, A.; Golan, D.E.; Pier, G.B. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-κB translocation. Proc. Natl. Acad. Sci. USA. 2002, 99, 6907–6912. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.T.N.; Bardou, O.; Privé, A.; Maillé, E.; Adam, D.; Lingée, S.; Ferraro, P.; Desrosiers, M.Y.; Coraux, C.; Brochiero, E. Improvement of defective cystic fibrosis airway epithelial wound repair after CFTR rescue. Eur. Respir. J. 2012, 40, 1390–1400. [Google Scholar] [CrossRef]
- Jabr, S.; Gartner, S.; Milne, G.L.; Roca-Ferrer, J.; Casas, J.; Moreno, A.; Gelpí, E.; Picado, C. Quantification of major urinary metabolites of PGE2 and PGD2 in cystic fibrosis: Correlation with disease severity. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 121–126. [Google Scholar] [CrossRef]
- O’Connor, M.G.; Seegmiller, A. The effects of ivacaftor on CF fatty acid metabolism: An analysis from the GOAL study. J. Cyst. Fibros. 2017, 16, 132–138. [Google Scholar] [CrossRef]
- Ortiz-Muñoz, G.; Yu, M.A.; Lefrançais, E.; Mallavia, B.; Valet, C.; Tian, J.J.; Ranucci, S.; Wang, K.M.; Liu, Z.; Kwaan, N.; et al. Cystic fibrosis transmembrane conductance regulator dysfunction in platelets drives lung hyperinflammation. J. Clin. Invest. 2020, 130, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Burrell, A.; Ansusinha, E.; Peng, D.; Chaney, H.; Sami, I.; Perez, G.F.; Koumbourlis, A.C.; McCarter, R.; Freishtat, R.J.; et al. Airway microbial diversity is decreased in young children with cystic fibrosis compared to healthy controls but improved with CFTR modulation. Heliyon 2020, 6, e04104. [Google Scholar] [CrossRef] [PubMed]
- Bernarde, C.; Keravec, M.; Mounier, J.; Gouriou, S.; Rault, G.; Férec, C.; Barbier, G.; Héry-Arnaud, G. Impact of the CFTR-Potentiator ivacaftor on airway microbiota in cystic fibrosis patients carrying a G551D mutation. PLoS ONE 2015, 10, e0124124. [Google Scholar] [CrossRef]
- Reznikov, L.R.; Abou Alaiwa, M.H.; Dohrn, C.L.; Gansemer, N.D.; Diekema, D.J.; Stoltz, D.A.; Welsh, M.J. Antibacterial properties of the CFTR potentiator ivacaftor. J. Cyst. Fibros. 2014, 13, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.E.; Dubois, A.V.; Ingram, R.J.; Weldon, S.; Taggart, C.C.; Elborn, J.S.; Tunney, M.M. Activity of innate antimicrobial peptides and ivacaftor against clinical cystic fibrosis respiratory pathogens. Int. J. Antimicrob. Agents 2017, 50, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.K.; Azad, M.A.K.; Han, M.L.; Zhou, Q.; Wang, J.; Huang, J.X.; Cooper, M.A.; Doi, Y.; Baker, M.A.; Bergen, P.J.; et al. An “unlikely” Pair: The antimicrobial synergy of polymyxin b in combination with the cystic fibrosis transmembrane conductance regulator drugs KALYDECO and ORKAMBI. ACS Infect. Dis. 2016, 2, 478–488. [Google Scholar] [CrossRef]
- Millar, B.C.; McCaughan, J.; Rendall, J.C.; Downey, D.G.; Moore, J.E. Pseudomonas aeruginosa in cystic fibrosis patients with c.1652G›A (G551D)-CFTR treated with ivacaftor—Changes in microbiological parameters. J. Clin. Pharm. Ther. 2018, 43, 92–100. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Syed, S.A.; Rossi, L.; Garg, M.; Needham, B.; Avolio, J.; Young, K.; Surette, M.G.; Gonska, T. Impact of CFTR modulation with Ivacaftor on Gut Microbiota and Intestinal Inflammation. Sci. Rep. 2018, 8, 17834. [Google Scholar] [CrossRef]
- Sergeev, V.; Chou, F.Y.; Lam, G.Y.; Hamilton, C.M.; Wilcox, P.G.; Quon, B.S. The extrapulmonary effects of cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis. Ann. Am. Thorac. Soc. 2020, 17, 147–154. [Google Scholar] [CrossRef]
- Gifford, A.H.; Heltshe, S.L.; Goss, C.H. CFTR modulator use is associated with higher hemoglobin levels in individuals with cystic fibrosis. Ann. Am. Thorac. Soc. 2019, 16, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Chioccioli, M.; Feriani, L.; Kotar, J.; Bratcher, P.E.; Cicuta, P. Phenotyping ciliary dynamics and coordination in response to CFTR-modulators in Cystic Fibrosis respiratory epithelial cells. Nat. Commun. 2019, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Hajj, R.; Lesimple, P.; Nawrocki-Raby, B.; Birembaut, P.; Puchelle, E.; Coraux, C. Human airway surface epithelial regeneration is delayed and abnormal in cystic fibrosis. J. Pathol. 2007, 211, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yi, Y.; Yan, Z.; Rosen, B.H.; Liang, B.; Winter, M.C.; Evans, T.I.A.; Rotti, P.G.; Yang, Y.; Gray, J.S.; et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Trimble, A.T.; Donaldson, S.H. Ivacaftor withdrawal syndrome in cystic fibrosis patients with the G551D mutation. J. Cyst. Fibros. 2018, 17, e13–e16. [Google Scholar] [CrossRef]
- Gostelie, R.; Stegeman, I.; Berkers, G.; Bittermann, J.; Ligtenberg-van der Drift, I.; van Kipshagen, P.-J.; de Winter-de Groot, K.; Speleman, L. The impact of ivacaftor on sinonasal pathology in S1251N-mediated cystic fibrosis patients. PLoS ONE 2020, 15, e0235638. [Google Scholar] [CrossRef]
- Torphy, T.J.; Allen, J.; Cantin, A.M.; Konstan, M.W.; Accurso, F.J.; Joseloff, E.; Ratjen, F.A.; Chmiel, J.F. Considerations for the conduct of clinical trials with antiinflammatory agents in cystic fibrosis: A cystic fibrosis foundation workshop report. Ann. Am. Thorac. Soc. 2015, 12, 1398–1406. [Google Scholar] [CrossRef]
- Elborn, J.S.; Ahuja, S.; Springman, E.; Mershon, J.; Grosswald, R.; Rowe, S.M. EMPIRE-CF: A phase II randomized placebo-controlled trial of once-daily, oral acebilustat in adult patients with cystic fibrosis—Study design and patient demographics. Contemp. Clin. Trials 2018, 72, 86–94. [Google Scholar] [CrossRef]
- Springman, E.; Grosswald, R.; Philpot, E.; MacGregor, G.; Horsley, A.; Bilton, D.; Stewart, J.; Elborn, J.S. 126 A phase 1 clinical study of CTX-4430 in cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, S90. [Google Scholar] [CrossRef]
- Chmiel, J.F.; Elborn, J.S.; Constantine, S.; White, B. A Double-Blind, Placebo-Controlled Phase 2 Study in Adults with Cystic Fibrosis of Anabasum, A Selective Cannabinoid Receptor Type 2 Agonist. Pediatr. Pulmonol. 2017, 52, 317. [Google Scholar] [CrossRef]
- Heltshe, S.L.; Mayer-Hamblett, N.; Burns, J.L.; Khan, U.; Baines, A.; Ramsey, B.W.; Rowe, S.M. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin. Infect Dis. 2015, 60, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Currie, A.J.; Main, E.T.; Wilson, H.M.; Armstrong-James, D.; Warris, A. CFTR Modulators Dampen Aspergillus-Induced Reactive Oxygen Species Production by Cystic Fibrosis Phagocytes. Front Cell Infect Microbiol. 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keown, K.; Brown, R.; Doherty, D.F.; Houston, C.; McKelvey, M.C.; Creane, S.; Linden, D.; McAuley, D.F.; Kidney, J.C.; Weldon, S.; et al. Airway Inflammation and Host Responses in the Era of CFTR Modulators. Int. J. Mol. Sci. 2020, 21, 6379. https://doi.org/10.3390/ijms21176379
Keown K, Brown R, Doherty DF, Houston C, McKelvey MC, Creane S, Linden D, McAuley DF, Kidney JC, Weldon S, et al. Airway Inflammation and Host Responses in the Era of CFTR Modulators. International Journal of Molecular Sciences. 2020; 21(17):6379. https://doi.org/10.3390/ijms21176379
Chicago/Turabian StyleKeown, Karen, Ryan Brown, Declan F. Doherty, Claire Houston, Michael C. McKelvey, Shannice Creane, Dermot Linden, Daniel F. McAuley, Joseph C. Kidney, Sinéad Weldon, and et al. 2020. "Airway Inflammation and Host Responses in the Era of CFTR Modulators" International Journal of Molecular Sciences 21, no. 17: 6379. https://doi.org/10.3390/ijms21176379
APA StyleKeown, K., Brown, R., Doherty, D. F., Houston, C., McKelvey, M. C., Creane, S., Linden, D., McAuley, D. F., Kidney, J. C., Weldon, S., Downey, D. G., & Taggart, C. C. (2020). Airway Inflammation and Host Responses in the Era of CFTR Modulators. International Journal of Molecular Sciences, 21(17), 6379. https://doi.org/10.3390/ijms21176379






