Immune Dysfunction and Multiple Treatment Modalities for the SARS-CoV-2 Pandemic: Races of Uncontrolled Running Sweat?
Abstract
1. Introduction
2. Immune Response in SARS-CoV-2 Pathogenesis
2.1. Innate and Adaptive Immune Cells in COVID-19 Patients
2.2. High-Throughput Sequencing Approach to Understand Immune Cell Dysfunctions in SARS-CoV-2-Infected Patients
2.3. Hyper-Cytokine Activation
2.4. SARS-COV-2 Severity in Cancer Patients
2.5. Immune Responses of Asymptomatic Patients with SARS-CoV-2 Infection
3. Treatment Options for SARS-CoV-2-Infected Patient
3.1. Convalescent Plasma (CP) Therapy and ACE2 Blocker
3.2. Targeting Deregulated Signaling in SARS-COV-2-Infected Patients and Treatment Strategies
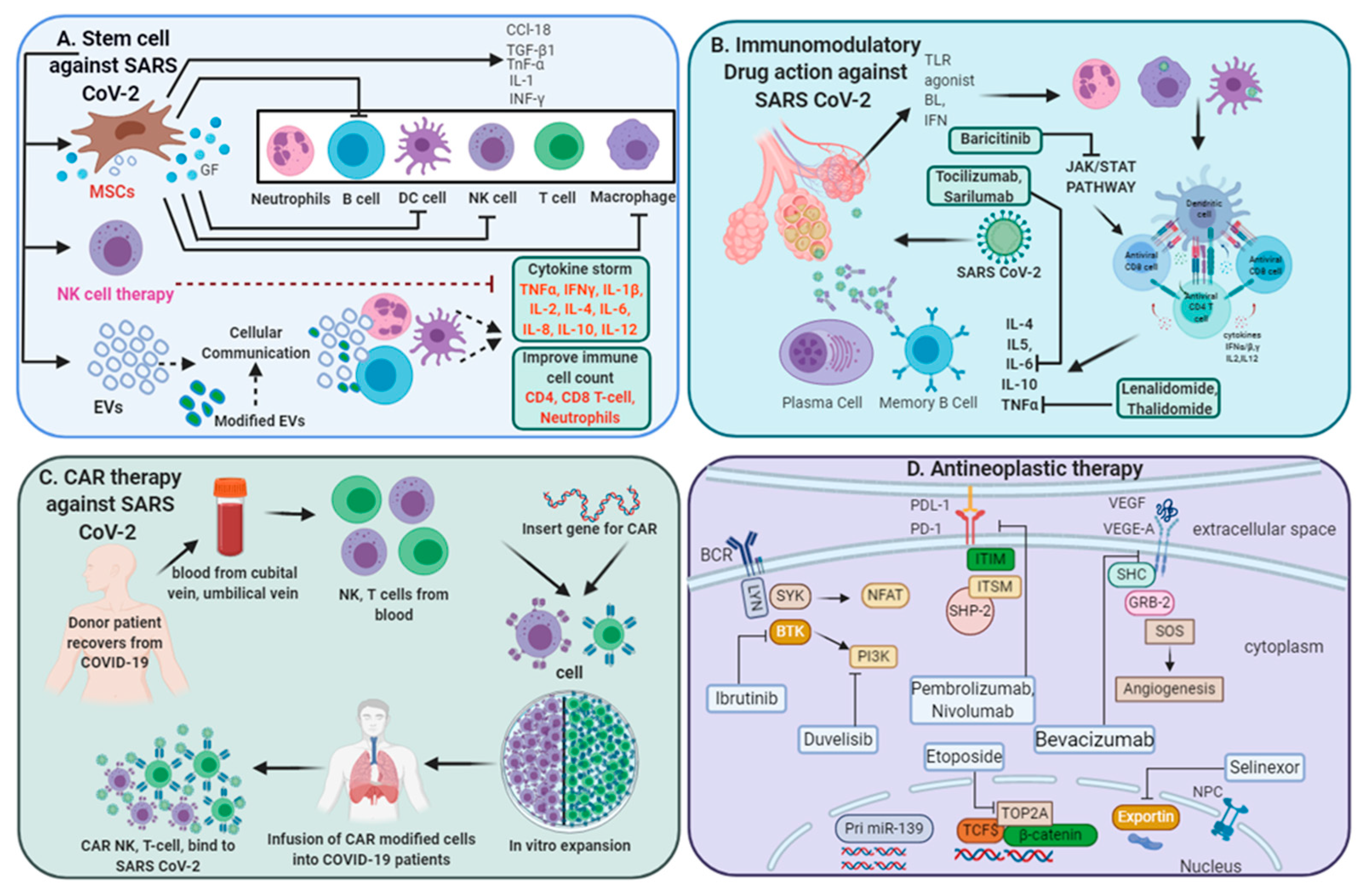
3.3. Regenerative Medicine in COVID-19 Treatment
3.3.1. Stem Cell-Based Therapeutic Interventions
3.3.2. Extracellular Vesicles (EVs) for COVID-19 Treatment
3.3.3. NK Cell-Based Therapy
3.3.4. Chimeric Antigen Receptor (CAR) T-Cell Therapy
3.4. Antineoplastic Therapy for SARS-CoV-2-Infected Patients
3.5. Vaccine Development for COVID-19
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Paules, C.I.; Marston, H.D.; Fauci, A.S. Coronavirus Infections-More Than Just the Common Cold. JAMA 2020, 323, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Chavez, S.; Long, B.; Koyfman, A.; Liang, S.Y. Coronavirus Disease (COVID-19): A primer for emergency physicians. Am. J. Emerg. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Chan, J.F.; Azhar, E.I.; Hui, D.S.; Yuen, K.Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Boll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Van de Haar, J.; Hoes, L.R.; Coles, C.E.; Seamon, K.; Frohling, S.; Jager, D.; Valenza, F.; de Braud, F.; De Petris, L.; Bergh, J.; et al. Caring for patients with cancer in the COVID-19 era. Nat. Med. 2020, 26, 665–671. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Perlman, S.; Dandekar, A.A. Immunopathogenesis of coronavirus infections: Implications for SARS. Nat. Rev. Immunol. 2005, 5, 917–927. [Google Scholar] [CrossRef]
- Zumla, A.; Hui, D.S.; Perlman, S. Middle East respiratory syndrome. Lancet 2015, 386, 995–1007. [Google Scholar] [CrossRef]
- Duijf, P.H.G. Baseline pulmonary levels of CD8+ T cells and NK cells inversely correlate with expression of the SARS-CoV-2 entry receptor ACE2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.M.; Li, S.R.; Twelkmeyer, T.; Wang, W.H.; Zhang, S.Y.; Wang, S.F.; Chen, J.Z.; Jin, X.; Wu, Y.Z.; et al. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat. Commun. 2019, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.P.; Liu, J.P.; Tao, W.Q.; Li, H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020, 84, 106504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Su, W.; Tang, H.; Le, W.; Zhang, X.; Zheng, Y.; Liu, X.; Xie, L.; Li, J.; Ye, J.; et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Xu, D.; Ma, M.; Xu, Y.; Su, Y.; Ong, S.B.; Hu, X.; Chai, M.; Zhao, M.; Li, H.; Chen, Y.; et al. Single-cell Transcriptome Analysis Indicates New Potential Regulation Mechanism of ACE2 and NPs signaling among heart failure patients infected with SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell 2020, 53, 514–529.e513. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells 2020, 9, 920. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.M.; Raoult, D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Su, B.; Pang, L.; Qiao, L.; Feng, Y.; Ouyang, Y.; Guo, X.; Shi, H.; Wei, F.; Su, X.; et al. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Alakwaa, F.M. Repurposing Didanosine as a Potential Treatment for COVID-19 Using Single-Cell RNA Sequencing Data. mSystems 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C.; et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 2020. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Ho, Y.C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Kabashneh, S.; Ali, H.; Alkassis, S. Multi-Organ Failure in a Patient With Diabetes due to COVID-19 With Clear Lungs. Cureus 2020, 12, e8147. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Belizna, C.; Selva-O’Callaghan, A.; Pardos-Gea, J.; Quintana, A.; Mekinian, A.; Anunciacion-Llunell, A.; Miro-Mur, F. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun. Rev. 2020, 19, 102569. [Google Scholar] [CrossRef]
- Costa, F.F.; Rosario, W.R.; Ribeiro Farias, A.C.; de Souza, R.G.; Duarte Gondim, R.S.; Barroso, W.A. Metabolic syndrome and COVID-19: An update on the associated comorbidities and proposed therapies. Diabetes Metab. Syndr. 2020, 14, 809–814. [Google Scholar] [CrossRef]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obs. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Cancer and SARS-CoV-2 Infection: Diagnostic and Therapeutic Challenges. Cancers 2020, 12, 1581. [Google Scholar] [CrossRef]
- Gosain, R.; Abdou, Y.; Singh, A.; Rana, N.; Puzanov, I.; Ernstoff, M.S. COVID-19 and Cancer: A Comprehensive Review. Curr. Oncol. Rep. 2020, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Che, D.; Cao, J.; Shen, J.; Jin, S.; Zhou, Y.; Liu, F.; Gu, K.; Man, Y.; Shang, L.; et al. Interleukin-17 levels correlate with poor prognosis and vascular endothelial growth factor concentration in the serum of patients with non-small cell lung cancer. Biomarkers 2015, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Patera, A.C.; Pesnicak, L.; Bertin, J.; Cohen, J.I. Interleukin 17 modulates the immune response to vaccinia virus infection. Virology 2002, 299, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Hui, D.S.; Azhar, E.I.; Memish, Z.A.; Maeurer, M. Reducing mortality from 2019-nCoV: Host-directed therapies should be an option. Lancet 2020, 395, e35–e36. [Google Scholar] [CrossRef]
- Weitz, J.S.; Beckett, S.J.; Coenen, A.R.; Demory, D.; Dominguez-Mirazo, M.; Dushoff, J.; Leung, C.Y.; Li, G.; Magalie, A.; Park, S.W.; et al. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat. Med. 2020, 26, 849–854. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Helgason, A.; Jonsson, H.; Magnusson, O.T.; Melsted, P.; Norddahl, G.L.; Saemundsdottir, J.; Sigurdsson, A.; Sulem, P.; Agustsdottir, A.B.; et al. Spread of SARS-CoV-2 in the Icelandic Population. N. Engl. J. Med. 2020, 382, 2302–2315. [Google Scholar] [CrossRef]
- Cao, W.C.; Liu, W.; Zhang, P.H.; Zhang, F.; Richardus, J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 2007, 357, 1162–1163. [Google Scholar] [CrossRef]
- Choe, P.G.; Perera, R.; Park, W.B.; Song, K.H.; Bang, J.H.; Kim, E.S.; Kim, H.B.; Ko, L.W.R.; Park, S.W.; Kim, N.J.; et al. MERS-CoV Antibody Responses 1 Year after Symptom Onset, South Korea, 2015. Emerg. Infect. Dis. 2017, 23, 1079–1084. [Google Scholar] [CrossRef]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.S.; Zimmerman, M.; Kauffman, R.; Mantus, G.; Linderman, S.; Vanderheiden, A.; Nyhoff, L.; Davis, C.; Adekunle, S.; Affer, M.; et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020, 20, 398–400. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef]
- Salazar, E.; Perez, K.K.; Ashraf, M.; Chen, J.; Castillo, B.; Christensen, P.A.; Eubank, T.; Bernard, D.W.; Eagar, T.N.; Long, S.W.; et al. Treatment of COVID-19 Patients with Convalescent Plasma in Houston, Texas. medRxiv 2020. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020, 16, 1718–1723. [Google Scholar] [CrossRef]
- Gurwitz, D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020. [Google Scholar] [CrossRef]
- Choudhary, S.; Malik, Y.S.; Tomar, S. Identification of SARS-CoV-2 Cell Entry Inhibitors by Drug Repurposing Using in silico Structure-Based Virtual Screening Approach. Front. Immunol. 2020, 11, 1664. [Google Scholar] [CrossRef]
- Manolis, A.S.; Manolis, T.A.; Manolis, A.A.; Melita, H. The Controversy of Renin-Angiotensin-System Blocker Facilitation Versus Countering COVID-19 Infection. J. Cardiovasc. Pharm. 2020. [Google Scholar] [CrossRef]
- Yamaya, M.; Shimotai, Y.; Hatachi, Y.; Lusamba Kalonji, N.; Tando, Y.; Kitajima, Y.; Matsuo, K.; Kubo, H.; Nagatomi, R.; Hongo, S.; et al. The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells. Pulm. Pharm. Ther. 2015, 33, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, J.; Hume, A.J.; Muhlberger, E. Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog. 2018, 14, e1007390. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; Hlh Across Speciality Collaboration. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Lee, C.; Lim, H.K.; Sakong, J.; Lee, Y.S.; Kim, J.R.; Baek, S.H. Janus kinase-signal transducer and activator of transcription mediates phosphatidic acid-induced interleukin (IL)-1beta and IL-6 production. Mol. Pharm. 2006, 69, 1041–1047. [Google Scholar] [CrossRef]
- Sebba, A. Tocilizumab: The first interleukin-6-receptor inhibitor. Am. J. Health Syst. Pharm. 2008, 65, 1413–1418. [Google Scholar] [CrossRef]
- Innes, A.J.; Cook, L.B.; Marks, S.; Bataillard, E.; Crossette-Thambiah, C.; Sivasubramaniam, G.; Apperley, J.; Milojkovic, D. Ruxolitinib for tocilizumab-refractory severe COVID-19 infection. Br. J. Haematol. 2020. [Google Scholar] [CrossRef]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-kappaB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef]
- George, B.; Chowdhury, S.M.; Hart, A.; Sircar, A.; Singh, S.K.; Nath, U.K.; Mamgain, M.; Singhal, N.K.; Sehgal, L.; Jain, N. Ibrutinib Resistance Mechanisms and Treatment Strategies for B-Cell lymphomas. Cancers 2020, 12, 1328. [Google Scholar] [CrossRef]
- Wang, W.; Ye, L.; Ye, L.; Li, B.; Gao, B.; Zeng, Y.; Kong, L.; Fang, X.; Zheng, H.; Wu, Z.; et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007, 128, 1–8. [Google Scholar] [CrossRef] [PubMed]
- DeDiego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeno, J.M.; Fernandez-Delgado, R.; Fett, C.; Castano-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of NF-kappaB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014, 88, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J.; Nguyen, T.L.; Arguello, M.; Nakhaei, P.; Paz, S. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene 2006, 25, 6844–6867. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef]
- Wu, X.; Dao Thi, V.L.; Huang, Y.; Billerbeck, E.; Saha, D.; Hoffmann, H.H.; Wang, Y.; Silva, L.A.V.; Sarbanes, S.; Sun, T.; et al. Intrinsic Immunity Shapes Viral Resistance of Stem Cells. Cell 2018, 172, 423–438.e425. [Google Scholar] [CrossRef]
- Villa, N.Y.; Bais, S.; Chan, W.M.; Meacham, A.M.; Wise, E.; Rahman, M.M.; Moreb, J.S.; Rosenau, E.H.; Wingard, J.R.; McFadden, G.; et al. Ex vivo virotherapy with myxoma virus does not impair hematopoietic stem and progenitor cells. Cytotherapy 2016, 18, 465–480. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Takeuchi, S.; Iwasawa, T.; Kumagai, M.; Sato, T.; Motegi, S.; Ishii, Y.; Koseki, Y.; Tomiyoshi, K.; Natsui, K.; et al. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm. Regen. 2020, 40, 14. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tsuchiya, A.; Seino, S.; Kawata, Y.; Kojima, Y.; Ikarashi, S.; Starkey Lewis, P.J.; Lu, W.Y.; Kikuta, J.; Kawai, H.; et al. Mesenchymal Stem Cells and Induced Bone Marrow-Derived Macrophages Synergistically Improve Liver Fibrosis in Mice. Stem Cells Transl. Med. 2019, 8, 271–284. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Ren, S.; Wang, W.; Yang, Y.; Li, S.; Meng, M.; Wu, T.; Liu, D.; Tian, S.; et al. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 2020, 11, 207. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, S.; Wu, S.; Chen, L. Exosomes Modulate the Viral Replication and Host Immune Responses in HBV Infection. Biomed. Res. Int. 2019, 2019, 2103943. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Benthin, C.; Zeno, B.; Albertson, T.E.; Boyd, J.; Christie, J.D.; Hall, R.; Poirier, G.; Ronco, J.J.; Tidswell, M.; et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care 2017, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Inal, J.M. Decoy ACE2-expressing extracellular vesicles that competitively bind SARS-CoV-2 as a possible COVID-19 therapy. Clin. Sci. 2020, 134, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.A.; Lotvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Ferrarotti, I.; Saracino, L.; Perteghella, S.; Torre, M.L.; Corsico, A.G. Mesenchymal Stromal Cell Secretome for Severe COVID-19 Infections: Premises for the Therapeutic Use. Cells 2020, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Pellegrini, C.; Blandizzi, C. NKG2A and COVID-19: Another brick in the wall. Cell Mol. Immunol. 2020, 17, 672–674. [Google Scholar] [CrossRef]
- Zhang, M.; Wen, B.; Anton, O.M.; Yao, Z.; Dubois, S.; Ju, W.; Sato, N.; DiLillo, D.J.; Bamford, R.N.; Ravetch, J.V.; et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc. Natl. Acad. Sci. USA 2018, 115, E10915–E10924. [Google Scholar] [CrossRef]
- Ma, L.; Dichwalkar, T.; Chang, J.Y.H.; Cossette, B.; Garafola, D.; Zhang, A.Q.; Fichter, M.; Wang, C.; Liang, S.; Silva, M.; et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 2019, 365, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.T.; Schreiber, S. Adoptive T-cell therapy for HBV-associated HCC and HBV infection. Antivir. Res. 2020, 176, 104748. [Google Scholar] [CrossRef] [PubMed]
- Wisskirchen, K.; Kah, J.; Malo, A.; Asen, T.; Volz, T.; Allweiss, L.; Wettengel, J.M.; Lutgehetmann, M.; Urban, S.; Bauer, T.; et al. T cell receptor grafting allows virological control of Hepatitis B virus infection. J. Clin. Investig. 2019, 129, 2932–2945. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.L.; Chia, A.; Chang, C.X.; Leong, H.N.; Ling, K.L.; Grotenbreg, G.M.; Gehring, A.J.; Tan, Y.J.; Bertoletti, A. Engineering T cells specific for a dominant severe acute respiratory syndrome coronavirus CD8 T cell epitope. J. Virol. 2011, 85, 10464–10471. [Google Scholar] [CrossRef]
- Bian, Y.; Shang, S.; Siddiqui, S.; Zhao, J.; Joosten, S.A.; Ottenhoff, T.H.M.; Cantor, H.; Wang, C.R. MHC Ib molecule Qa-1 presents Mycobacterium tuberculosis peptide antigens to CD8+ T cells and contributes to protection against infection. PLoS Pathog. 2017, 13, e1006384. [Google Scholar] [CrossRef]
- Bobin, A.; Gardeney, H.; Sabirou, F.; Gruchet, C.; Levy, A.; Nsiala, L.; Cailly, L.; Tomowiak, C.; Torregrosa, J.; Guidez, S.; et al. The Role of Immunotherapy in Non-transplant Eligible Multiple Myeloma. Front. Oncol. 2020, 10, 676. [Google Scholar] [CrossRef]
- Chen, C.; Qi, F.; Shi, K.; Li, Y.; Li, J.; Chen, Y.; Pan, J.; Zhou, T.; Lin, X.; Zhang, J.; et al. Thalidomide combined with low-dose short-term glucocorticoid in the treatment of critical Coronavirus Disease 2019. Clin. Transl. Med. 2020. [Google Scholar] [CrossRef]
- Podar, K.; Shah, J.; Chari, A.; Richardson, P.G.; Jagannath, S. Selinexor for the treatment of multiple myeloma. Expert Opin. Pharm. 2020, 21, 399–408. [Google Scholar] [CrossRef]
- Uddin, M.H.; Zonder, J.A.; Azmi, A.S. Exportin 1 inhibition as antiviral therapy. Drug Discov. Today 2020. [Google Scholar] [CrossRef]
- Sun, Y.; Saha, S.; Wang, W.; Saha, L.K.; Huang, S.N.; Pommier, Y. Excision repair of topoisomerase DNA-protein crosslinks (TOP-DPC). DNA Repair. 2020, 89, 102837. [Google Scholar] [CrossRef]
- Montero-Baladía, M.; Buzón, L.; Astigarraga, I.; Delgado, P.; Iglesias, E.; Callejo, F.; López-Veloso, M.; Minguito, J.; Fernández-Regueras, M.; Ubeira, M.; et al. Etoposide treatment adjunctive to immunosuppressants for critically ill COVID-19 patients: Etoposide for severe COVID-19 patients. J. Infect. 2020. [Google Scholar] [CrossRef]
- Hamizi, K.; Aouidane, S.; Belaaloui, G. Etoposide-based therapy for severe forms of COVID-19. Med. Hypotheses 2020, 142, 109826. [Google Scholar] [CrossRef] [PubMed]
- Frohman, E.M.; Villemarette-Pittman, N.R.; Cruz, R.A.; Longmuir, R.; Rowe, V.; Rowe, E.S.; Varkey, T.C.; Steinman, L.; Zamvil, S.S.; Frohman, T.C. Part II. high-dose methotrexate with leucovorin rescue for severe COVID-19: An immune stabilization strategy for SARS-CoV-2 induced ‘PANIC’ attack. J. Neurol. Sci. 2020, 415, 116935. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Han, J.; Wu, X.; Zeng, H.; Liu, J.; Zhang, H. VEGF-D: A novel biomarker for detection of COVID-19 progression. Crit. Care 2020, 24, 373. [Google Scholar] [CrossRef]
- Chi, Y.; Ge, Y.; Wu, B.; Zhang, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y.; et al. Serum Cytokine and Chemokine profile in Relation to the Severity of Coronavirus disease 2019 (COVID-19) in China. J. Infect. Dis. 2020, 222, 746–754. [Google Scholar] [CrossRef]
- Treon, S.P.; Castillo, J.J.; Skarbnik, A.P.; Soumerai, J.D.; Ghobrial, I.M.; Guerrera, M.L.; Meid, K.; Yang, G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 2020, 135, 1912–1915. [Google Scholar] [CrossRef]
- Roschewski, M.; Lionakis, M.S.; Sharman, J.P.; Roswarski, J.; Goy, A.; Monticelli, M.A.; Roshon, M.; Wrzesinski, S.H.; Desai, J.V.; Zarakas, M.A.; et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Rolla, S.; Maglione, A.; De Mercanti, S.F.; Clerico, M. The Meaning of Immune Reconstitution after Alemtuzumab Therapy in Multiple Sclerosis. Cells 2020, 9, 1396. [Google Scholar] [CrossRef]
- Matias-Guiu, J.; Montero-Escribano, P.; Pytel, V.; Porta-Etessam, J.; Matias-Guiu, J.A. Potential COVID-19 infection in patients with severe multiple sclerosis treated with alemtuzumab. Mult. Scler. Relat. Disord. 2020, 44, 102297. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Kindrachuk, J.; Ork, B.; Hart, B.J.; Mazur, S.; Holbrook, M.R.; Frieman, M.B.; Traynor, D.; Johnson, R.F.; Dyall, J.; Kuhn, J.H.; et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015, 59, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S. Inhaled biguanides and mTOR inhibition for influenza and coronavirus (Review). World Acad Sci. J. 2020, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.S.; Liu, P.; Baleeiro, R.; Lemoine, N.R.; Yuan, M.; Wang, Y.H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2018, 32, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Yekeduz, E.; Dursun, B.; Aydin, G.C.; Yazgan, S.C.; Ozturk, H.H.; Azap, A.; Utkan, G.; Urun, Y. Clinical course of COVID-19 infection in elderly patient with melanoma on nivolumab. J. Oncol. Pharm. Prac. 2020, 26, 1289–1294. [Google Scholar] [CrossRef]
- O’Kelly, B.; McGettrick, P.; Angelov, D.; Fay, M.; McGinty, T.; Cotter, A.G.; Sheehan, G.; Lambert, J.S. Outcome of a patient with refractory Hodgkin lymphoma on pembrolizumab, infected with SARS-CoV-2. Br. J. Haematol. 2020, 190, e1–e3. [Google Scholar] [CrossRef]
- Mullard, A. COVID-19 vaccine development pipeline gears up. Lancet 2020, 395, 1751–1752. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gomez Roman, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Choi, Y.; Chang, J. Viral vectors for vaccine applications. Clin. Exp. Vaccine Res. 2013, 2, 97–105. [Google Scholar] [CrossRef]
- Garofalo, M.; Staniszewska, M.; Salmaso, S.; Caliceti, P.; Pancer, K.W.; Wieczorek, M.; Kuryk, L. Prospects of Replication-Deficient Adenovirus Based Vaccine Development against SARS-CoV-2. Vaccines 2020, 8, 293. [Google Scholar] [CrossRef]
- Cintolo, J.A.; Datta, J.; Mathew, S.J.; Czerniecki, B.J. Dendritic cell-based vaccines: Barriers and opportunities. Future Oncol. 2012, 8, 1273–1299. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: Russia approves vaccine without large scale testing or published results. BMJ 2020, 370. [Google Scholar] [CrossRef]
| Trial No | Clinical Stage | COVID-19 Patient (n) | Interventions | Primary Endpoint (day) | Brief Description of Study | Location |
|---|---|---|---|---|---|---|
| NK Cell-Based Therapy | ||||||
| NCT04324996 | Phase (I/II) | 90 | NKG2D CAR-NK cells, NKG2D-ACE2 CAR-NK cells (108 cells/kg, IV) | CR, safety and tolerability (day 28) | NK cells from umbilical cord blood and engineered genetically, secreting IL15 and GM-CSF. | China |
| NCT04375176 | Recruiting | 150 | Functional analysis of monocytes and NK cells | Immune cells activity (6 months) | Phenotypic and functional analysis of monocytes and NK cells | Italy |
| NCT04280224 | Phase (I) | 30 | NK cells (0.1–2 × 108 cells/kg, twice per week) | CR, safety and tolerability (day 28) | NK cells in combination with standard therapy | China |
| NCT04365101 | Phase (I/II) | 86 | CYNK-001 allogeneic NK cells (days 1, 4, and 7) | AE and clinical improvement (day 28) | CD56+/CD3- NK cells derived from human placental CD34+ cells and culture-expanded | USA (multiple locations) |
| NCT04363346 | Phase (I) | 12 | FT516 (9 × 107 cells day-1, 3 × 108 cells day-4, 9 × 108 cells day-7). | MTD of FT516 using 3 dose-escalation strategies, Dose Limiting Toxicity Events (day 36) | NK cell product derived from an iPSC transduced with ADAM17 non-cleavable CD16 | USA |
| NCT04344548 | Phase (I/II) | 10 | Three doses of allogeneic NK cell transfer | AE and safety | Safety and immunogenicity of allogeneic NK cells from PBMCs of healthy donors in patients infected with COVID-19 collected by apheresis | Colombia |
| Mesenchymal Stem Cell-Based Therapy in Phase ¾ of Clinical Trial | ||||||
| NCT04444271 | Phase (II) | 20 | MSCs, 2 × 106 cells/kg, day 1 and 7 | Overall survival | Efficacy of MSCs as an add-on therapy to standard supportive treatment | Pakistan |
| NCT04416139 | Phase (II) | 10 | MSCs 1 million/kg single dose, IV | CR, safety and tolerability | Efficacy of MSCs | Mexico |
| NCT04366063 | Phase (II/III) | 60 | Two doses MSCs 100 × 106, IV+CT. | AE and safety, 28 days | MSCs for ARDS | Iran |
| NCT04336254 | Phase (I/II) | 20 | 3 × 107 human dental pulp stem cells, day 1,4,7 | CR, safety and tolerability | Human dental pulp MSCs | China |
| NCT04366323 | Phase (I/II) | 26 | Two doses, 80 million MSCs | AE and safety, 28 day | Allogeneic adult MSCs of expanded adipose tissue | Spain |
| NCT04366271 | Phase (II) | 106 | 1 infusion of undifferentiated allogeneic MSCs | AE and safety, 28 days | MSCs from umbilical cord tissue | Spain |
| NCT04390139 | Phase (I/II) | 30 | 2 infusions of Wharton-Jelly MSCs, 1 × 106 cells/kg.dose | AE and safety, 28 days | Drug: XCEL-UMC-BETA | Spain |
| NCT04333368 | Phase (I/II) | 40 | 1 million/kg, day 1,3,5 | CR, safety and tolerability | Umbilical Cord-derived MSCs | France |
| Convalescent Plasma in Phase ¾ of Clinical Trial | ||||||
| NCT04348656 | Phase (III) or Phase (II/III) | 1200 | 200–500 mL ABO compatible CP plasma or two doses of 250 mL CP | Recovery or decrease mortality in hospital | CONCOR-1 trial for efficacy of transfusion of COVID-19 CP | The multicenter USA and Canada |
| NCT04362176 | 500 | Passive Immunity Trial of Nashville II | ||||
| NCT04376034 | 240 | CP treatment in pediatric and adults | ||||
| NCT04361253 | 220 | Infusion of high-titer COVID-19 CP | ||||
| NCT04425915 | 400 | India | ||||
| NCT04342182 | 426 | CONCOVID Study | Netherlands | |||
| NCT04374526 | 182 | LIFESAVER | Italy | |||
| NCT04385043 | 400 | COV2-CP | ||||
| NCT04345289 | 1500 | Six parallel treatment arms of CP, sarilumab, hydroxychloroquine, baricitinib, IV and SC | Denmark | |||
| Extracellular Vesicle as COVID-19 Therapy | ||||||
| NCT04276987 | Phase (I) | 30 | 5 times aerosol inhalation of MSCs-derived Exo (2 × 108 particles) | AE, safety and tolerability (day 28) | Exo derived from allogeneic adipose MSCs | China |
| NCT04384445 | Phase (I/II) | 20 | Organicell Flow, 2–5 × 1011 particle/mL, IV. | AE, safety and tolerability (day 60) | Organicell Flow derived from human amniotic fluid contain chemokines, cytokines and EVs | USA |
| NCT04389385 | Phase (I) | 60 | 5 times aerosol inhalation of CSTC-Exo (2 × 108 particles) | AE, safety and tolerability (day 28) | CSTC-Exo, in vitro expanded and cultured with virus peptide and GFs. | Turkey |
| Adoptive Cell Therapy (Chimeric Antigen Receptor CAR Therapy) | ||||||
| NCT04351659 | NA | 8 | Donors who had tested positive for SARS-CoV-2 in the past and have recovered are suitable for blood donation. | The success rate in production of SARS-CoV-2 specific T cells from a convalescent donor | Development of treatment protocol for adoptive T-cell therapy | Singapore |
| Drug Class | Active Agent | Targetable Action | Cancer Treatment | Trial Number | Size | Clinical Phase |
|---|---|---|---|---|---|---|
| Corticosteroid | Dexamethasone | Anti-inflammatory Immunosuppressive | Lymphomas, leukemias | NCT04325061 | 200 | Phase 4 |
| NCT04347980 | 122 | Phase 3 | ||||
| Methylprednisolone | NCT04341038 | 84 | Phase 3 | |||
| NCT04438980 | 72 | Phase 3 | ||||
| NCT04244591 | 80 | Phase 3 | ||||
| NCT04263402 | 100 | Phase 4 | ||||
| Immunomodulators | Lenalidomide | Antiangiogenic, TNF-α signaling modulation | Multiple myeloma, myelodysplastic syndromes | NCT04361643 | 120 | Phase 4 |
| Thalidomide | NCT04273529 | 100 | Phase 2 | |||
| NCT04273581 | 40 | Phase 2 | ||||
| Nuclear export inhibitor | Selinexor | Binds to exportin 1 | Relapsed/refractory multiple myeloma | NCT04355676 | 80 | Phase 2 |
| NCT04349098 | 230 | Phase 2 | ||||
| Chemotherapy | Methotrexate | Antimetabolite for antifolate type | Multiple cancers including breast, lymphoma, epidermoid, small-cell lung cancer, head and neck, squamous cell lung cancer. | NCT04352465 | 42 | Phase ½ |
| Etoposide | Inhibits topoisomerase-II | NCT04356690 | 64 | Phase 2 | ||
| Anti-vascular endothelial growth factor | Bevacizumab | The humanized monoclonal antibody that blocks angiogenesis by inhibiting VEGF-A | Number of types of cancers and a specific eye disease | NCT04305106 | 140 | NA |
| NCT04344782 | 130 | Phase 2 | ||||
| NCT04275414 | 20 | Phase 2/3 | ||||
| Immune check-point inhibitors | Pembrolizumab | Block PD1 receptor | Multiple cancer types | NCT04335305 | 24 | Phase 2 |
| Nivolumab | NCT04413838 | 120 | Phase 2 | |||
| NCT04343144 | 92 | Phase 2 | ||||
| NCT04356508 | 15 | Phase 2 | ||||
| Kinase inhibitors | Ibrutinib | Irreversible covalent BTK inhibitor | B-cell lymphoma, including CLL, MCL, ABC-DLBCLs | NCT04375397 | 46 | Phase 2 |
| NCT04439006 | 72 | Phase 2 | ||||
| Acalabrutinib | NCT04380688 | 60 | Phase 2 | |||
| NCT04346199 | 140 | Phase 2 | ||||
| Duvelisib | inhibitor of PI3K | CLL, SLL | NCT04372602 | 25 | Phase 2 | |
| Imatinib | Inhibits bcr-abl tyrosine kinase | CML, ALL | NCT04394416 | 204 | Phase 3 | |
| NCT04422678 | 30 | Phase 3 | ||||
| NCT04346147 | 165 | Phase 2 |
| Company/Sponsor | Vaccine | Trial Number | Size | Clinical Stage | Brief Description | Intervention | Location |
|---|---|---|---|---|---|---|---|
| DNA-Based Vaccine for COVID-19 | |||||||
| Genexine, Inc. | GX-19 | NCT04445389 | 190 | Phase ½ | For safety, tolerability, and immunogenicity in HV | Expressing SARS-CoV-2 S-protein antigen, IM via EP | Korea |
| Inovio Pharma. | INO-4800 | NCT04336410 | 120 | Phase 1 | For safety, tolerability, and immunological profile in HV | A Prophylactic Vaccine Against SARS-CoV-2, ID then EP | USA |
| Symvivo Corp. | bacTRL-Spike | NCT04334980 | 112 | Phase 1 | For safety, tolerability, and immunity in HV | Bacterial medium with live Bifidobacterium longum, with plasmid expressing SARS-CoV-2 spike protein, 1–10 billion CFU | USA, Canada |
| Zydus Cadila | ZyCoV-D | - | - | Phase ½ | For safety, tolerability | India | |
| mRNA-Based Vaccine for COVID-19 | |||||||
| CureVac AG | CVnCoV | NCT04449276 | 168 | Phase 1 | Reactogenicity, Immunogenicity in HV | Participants will receive an IM injection in deltoid area | Germany |
| NIAID, | mRNA-1273 | NCT04283461 | 155 | Phase 1 | Safety Immunogenicity in HV | LNP dispersion containing mRNA for spike protein of SARS-CoV-2, IM, 10–250 microgram | USA |
| ModernaTX,Inc. | mRNA-1273 | NCT04405076 | 600 | Phase 2 | Dose-confirmation study in HV | mRNA-1273: 50 microgram, IM | USA |
| Biontech SE | BNT162 | NCT04368728 | 7600 | Phase ½ | Safety, tolerability, immunogenicity in HV | Anti-viral RNA vaccine for active immunization against COVID-19, IM various doses | USA |
| Adenovirus-Based Vaccine for COVID-19 | |||||||
| PLA of China | Ad5-nCoV | NCT04341389 | 508 | Phase 2 | Immunogenicity and safety in HV | Encodes for a full-length spike (S) protein of SARS-CoV-2, IM, 0.5–1 × 1011 VP | China |
| CanSino Biologics | Ad5-nCoV | NCT04313127 | 108 | Phase 1 | Dose-escalating, in HV | IM, 0.5–1.5 × 1011 VP | China |
| CanSino Biologics | Ad5-nCoV | NCT04398147 | 696 | Phase ½ | Safety, tolerability, and immunogenicity, in HV | Single dose, 5–10 × 1010 VP, IM | Canada |
| Gamaleya Research Institute | Gam-COVID-Vac Lyo | NCT04437875 NCT04436471 | 38 | Phase 1–2 * | Safety, tolerability, and immunogenicity in HV | rAd26, type 26 adenovirus, containing the SARS-CoV-2 S protein gene, IM | Russia |
| Altimmune, Inc. | NasoVAX | NCT04442230 | 96 | Phase 2 | Safety and effectiveness in early COVID-19-infected people | Replication-deficient adenovirus vectors in suspension | USA |
| Inactivated SARS-CoV-2 | |||||||
| Chinese Academy of Medical Sciences | Unname | NCT04412538 | 942 | Phase ½ | Safety and immunogenicity in HV | SARS-CoV-2 inactivated vaccine, two doses at 0, 28 day, 50–150 U/0.5 mL | China |
| Sinovac R & D Ltd. | Unname | NCT04352608 | 744 | Phase ½ | Safety and immunogenicity in HV | Two doses at day 0, 28, 600–1200 SU/0.5 mL | China |
| Bharat Biotech | COVAXIN | - | - | Phase ½ | Safety and immunogenicity | - | India |
| Dendritic cell-based vaccine for COVID-19 | |||||||
| Shenzhen Geno-Immune Medical Institute | LV-SMENP-DC | NCT04276896 | 100 | Phase ½ | Covid-19 minigenes engineered using lentiviral vector system (NHP/TYF) to modify DCs | 5 × 106 LV-DC vaccine and 1 × 108 CTLs via sub-cutaneous injections and IV infusions respectively | China |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothari, A.; Singh, V.; Nath, U.K.; Kumar, S.; Rai, V.; Kaushal, K.; Omar, B.J.; Pandey, A.; Jain, N. Immune Dysfunction and Multiple Treatment Modalities for the SARS-CoV-2 Pandemic: Races of Uncontrolled Running Sweat? Biology 2020, 9, 243. https://doi.org/10.3390/biology9090243
Kothari A, Singh V, Nath UK, Kumar S, Rai V, Kaushal K, Omar BJ, Pandey A, Jain N. Immune Dysfunction and Multiple Treatment Modalities for the SARS-CoV-2 Pandemic: Races of Uncontrolled Running Sweat? Biology. 2020; 9(9):243. https://doi.org/10.3390/biology9090243
Chicago/Turabian StyleKothari, Ashish, Vanya Singh, Uttam Kumar Nath, Sandeep Kumar, Vineeta Rai, Karanvir Kaushal, Balram Ji Omar, Atul Pandey, and Neeraj Jain. 2020. "Immune Dysfunction and Multiple Treatment Modalities for the SARS-CoV-2 Pandemic: Races of Uncontrolled Running Sweat?" Biology 9, no. 9: 243. https://doi.org/10.3390/biology9090243
APA StyleKothari, A., Singh, V., Nath, U. K., Kumar, S., Rai, V., Kaushal, K., Omar, B. J., Pandey, A., & Jain, N. (2020). Immune Dysfunction and Multiple Treatment Modalities for the SARS-CoV-2 Pandemic: Races of Uncontrolled Running Sweat? Biology, 9(9), 243. https://doi.org/10.3390/biology9090243







