Postnatal Overnutrition Induces Changes in Synaptic Transmission to Leptin Receptor-Expressing Neurons in the Arcuate Nucleus of Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Voltage-Clamp Recordings
2.4. ARH mRNA Expression
2.5. Statistical Analysis
3. Results
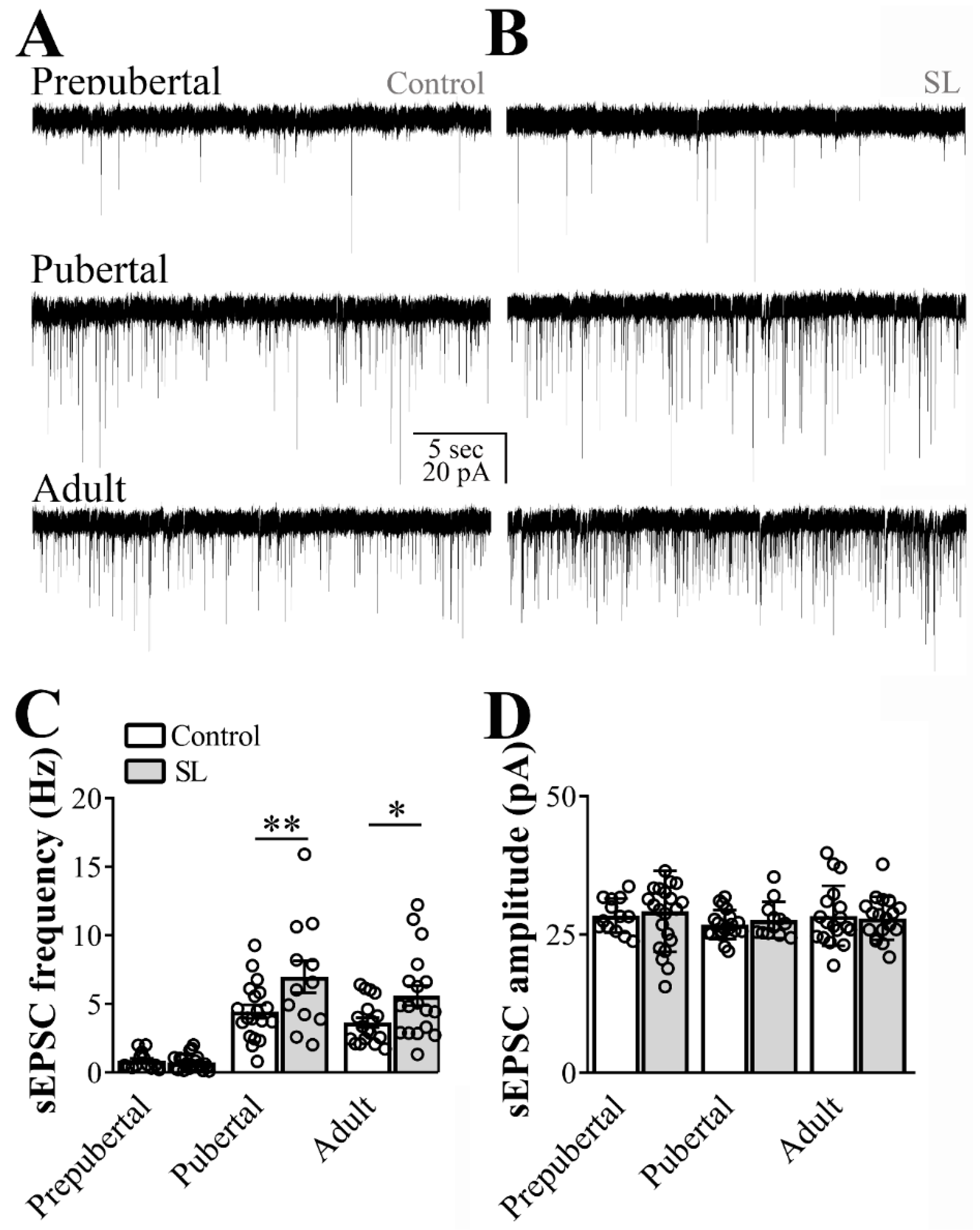
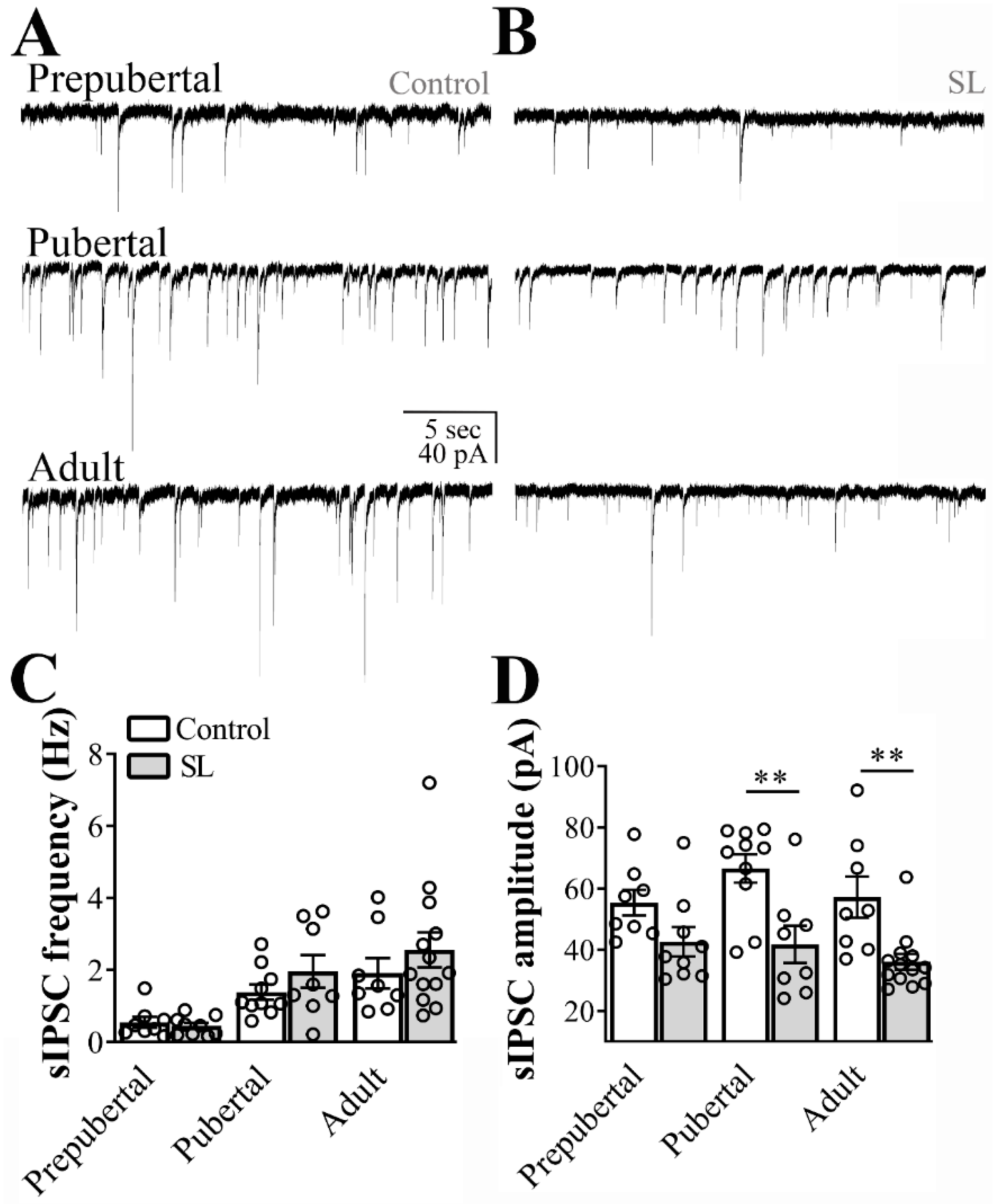
3.1. Excitatory and Inhibitory Synaptic Transmission to ARH LepR-Expressing Cells Increase at Pubertal Stage
3.2. Metabolic Consequences of the SL Model
3.3. Postnatal Overnutrition Amplifies the Excitatory Synaptic Transmission to ARH LepR-Expressing Neurons Starting at Puberty
3.4. Inhibitory Transmission to ARH Neurons is Attenuated in SL Mice
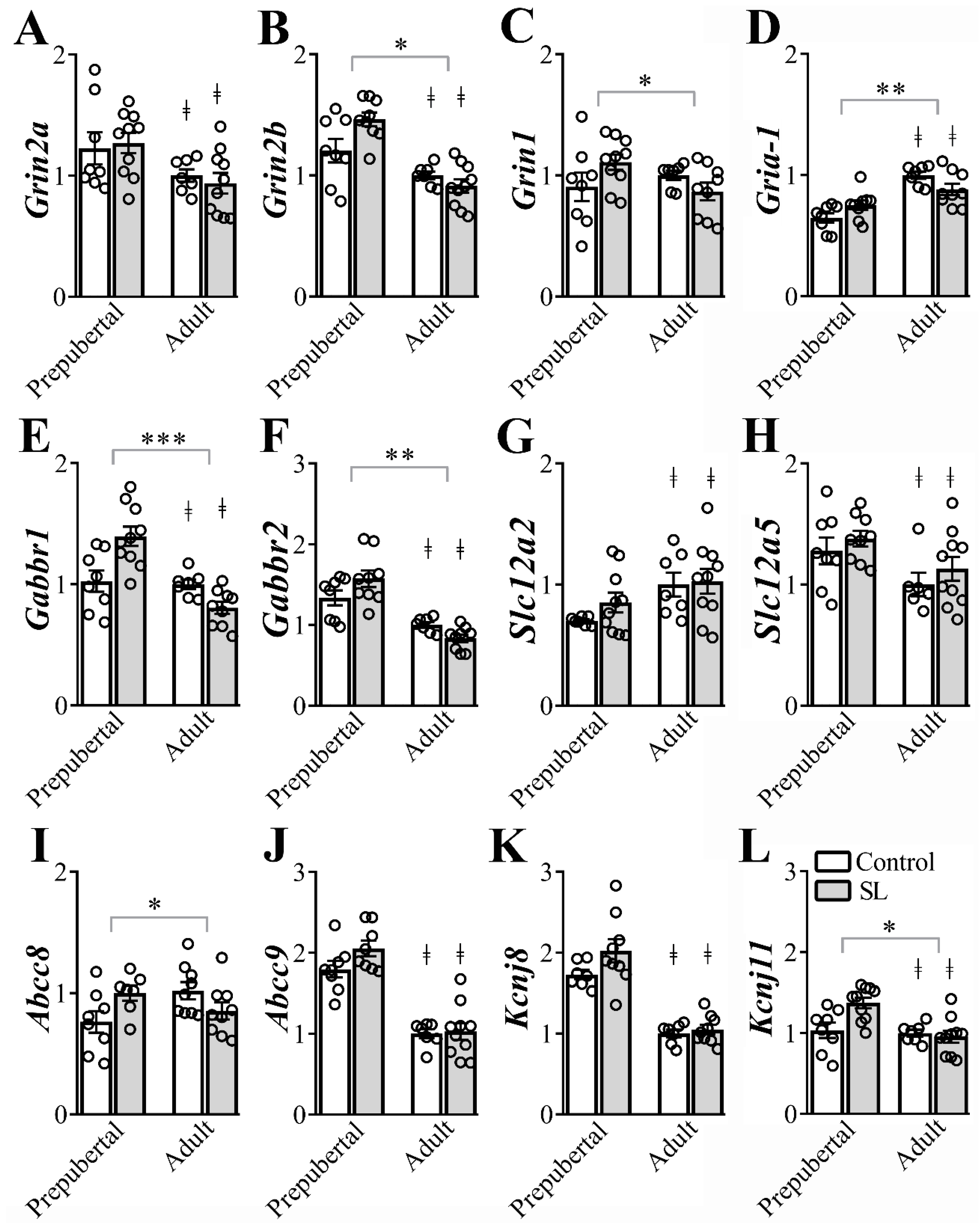
3.5. The Gene Expression of Several Ion Channel Receptors in the ARH is Modulated by Postnatal Overnutrition
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gali Ramamoorthy, T.; Begum, G.; Harno, E.; White, A. Developmental programming of hypothalamic neuronal circuits: Impact on energy balance control. Front. Neurosci. 2015, 9, 126. [Google Scholar] [CrossRef]
- Plagemann, A.; Heidrich, I.; Gotz, F.; Rohde, W.; Dorner, G. Obesity and enhanced diabetes and cardiovascular risk in adult-rats due to early postnatal overfeeding. Exp. Clin. Endocrinol. 1992, 99, 154–158. [Google Scholar] [CrossRef]
- Glavas, M.M.; Kirigiti, M.A.; Xiao, X.Q.; Enriori, P.J.; Fisher, S.K.; Evans, A.E.; Grayson, B.E.; Cowley, M.A.; Smith, M.S.; Grove, K.L. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology 2010, 151, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G. Organizational actions of metabolic hormones. Front. Neuroendocrinol. 2013, 34, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.M.; Swick, A.; Romsos, D.R. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am. J. Physiol. 1999, 277, R742–R747. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef]
- Mantzoros, C.S. Leptin and the hypothalamus: Neuroendocrine regulation of food intake. Mol. Psychiatry 1999, 4, 8–12. [Google Scholar] [CrossRef][Green Version]
- Park, H.-K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metab. Clin. Exp. 2015, 64, 24–34. [Google Scholar] [CrossRef]
- Evans, M.C.; Anderson, G.M. Neuroendocrine integration of nutritional signals on reproduction. J. Mol. Endocrinol. 2017, 58, R107–R128. [Google Scholar] [CrossRef]
- Elmquist, J.K.; Elias, C.F.; Saper, C.B. From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron 1999, 22, 221–232. [Google Scholar] [CrossRef]
- Elias, C.F.; Kelly, J.F.; Lee, C.E.; Ahima, R.S.; Drucker, D.J.; Saper, C.B.; Elmquist, J.K. Chemical characterization of leptin-activated neurons in the rat brain. J. Comp. Neurol. 2000, 423, 261–281. [Google Scholar] [CrossRef]
- Cravo, R.M.; Margatho, L.O.; Osborne-Lawrence, S.; Donato, J., Jr.; Atkin, S.; Bookout, A.L.; Rovinsky, S.; Frazao, R.; Lee, C.E.; Gautron, L.; et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011, 173, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, A.; Bouyer, K.; Wang, C.H.; Simerly, R. A critical period for the trophic actions of leptin on AgRP neurons in the arcuate nucleus of the hypothalamus. J. Comp. Neurol. 2018, 526, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Baquero, A.F.; de Solis, A.J.; Lindsley, S.R.; Kirigiti, M.A.; Smith, M.S.; Cowley, M.A.; Zeltser, L.M.; Grove, K.L. Developmental switch of leptin signaling in arcuate nucleus neurons. J. Neurosci. 2014, 34, 9982–9994. [Google Scholar] [CrossRef] [PubMed]
- Baquero, A.F.; Kirigiti, M.A.; Baquero, K.C.; Lee, S.J.; Smith, M.S.; Grove, K.L. Developmental changes in synaptic distribution in arcuate nucleus neurons. J. Neurosci. 2015, 35, 8558–8569. [Google Scholar] [CrossRef]
- Newton, A.J.; Hess, S.; Paeger, L.; Vogt, M.C.; Fleming Lascano, J.; Nillni, E.A.; Brüning, J.C.; Kloppenburg, P.; Xu, A.W. AgRP innervation onto pomc neurons increases with age and is accelerated with chronic high-fat feeding in male mice. Endocrinology 2013, 154, 172–183. [Google Scholar] [CrossRef]
- Pinto, S.; Roseberry, A.G.; Liu, H.; Diano, S.; Shanabrough, M.; Cai, X.; Friedman, J.M.; Horvath, T.L. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 2004, 304, 110–115. [Google Scholar] [CrossRef]
- Yang, Y.; Atasoy, D.; Su, H.H.; Sternson, S.M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 2011, 146, 992–1003. [Google Scholar] [CrossRef]
- Liu, T.; Kong, D.; Shah, B.P.; Ye, C.; Koda, S.; Saunders, A.; Ding, J.B.; Yang, Z.; Sabatini, B.L.; Lowell, B.B. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 2012, 73, 511–522. [Google Scholar] [CrossRef]
- Roberts, B.L.; Bennett, C.M.; Carroll, J.M.; Lindsley, S.R.; Kievit, P. Early overnutrition alters synaptic signaling and induces leptin resistance in arcuate proopiomelanocortin neurons. Physiol. Behav. 2019, 206, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.; Silveira, M.A.; Lima, L.B.; Furigo, I.C.; Zampieri, T.T.; Ramos-Lobo, A.M.; Buonfiglio, D.C.; Teixeira, P.D.; Frazao, R.; Donato, J., Jr. Changes in leptin signaling by SOCS3 modulate fasting-induced hyperphagia and weight regain in mice. Endocrinology 2016, 157, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- Juan De Solis, A.; Baquero, A.F.; Bennett, C.M.; Grove, K.L.; Zeltser, L.M. Postnatal undernutrition delays a key step in the maturation of hypothalamic feeding circuits. Mol. Metab. 2016, 5, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Nagaishi, V.S.; Cardinali, L.I.; Zampieri, T.T.; Furigo, I.C.; Metzger, M.; Donato, J., Jr. Possible crosstalk between leptin and prolactin during pregnancy. Neuroscience 2014, 259, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, T.M.; Silveira, M.A.; Zampieri, T.T.; Frazao, R.; Donato, J., Jr. Fatness rather than leptin sensitivity determines the timing of puberty in female mice. Mol. Cell Endocrinol. 2016, 423, 11–21. [Google Scholar] [CrossRef]
- Castellano, J.M.; Bentsen, A.H.; Sánchez-Garrido, M.A.; Ruiz-Pino, F.; Romero, M.; Garcia-Galiano, D.; Aguilar, E.; Pinilla, L.; Diéguez, C.; Mikkelsen, J.D.; et al. Early metabolic programming of puberty onset: Impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology 2011, 152, 3396–3408. [Google Scholar] [CrossRef]
- Nelson, J.F.; Karelus, K.; Felicio, L.S.; Johnson, T.E. Genetic influences on the timing of puberty in mice. Biol. Reprod. 1990, 42, 649–655. [Google Scholar] [CrossRef]
- Bohlen, T.M.; Silveira, M.A.; Buonfiglio, D.D.C.; Ferreira-Neto, H.C.; Cipolla-Neto, J.; Donato, J.; Frazao, R. A short-day photoperiod delays the timing of puberty in female mice. Front. Endocrinol. 2018, 9, 44. [Google Scholar] [CrossRef]
- Silveira, M.A.; Furigo, I.C.; Zampieri, T.T.; Bohlen, T.M.; de Paula, D.G.; Franci, C.R.; Donato, J., Jr.; Frazao, R. STAT5 signaling in kisspeptin cells regulates the timing of puberty. Mol. Cell Endocrinol. 2017, 448, 55–65. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Castellano, J.M.; Ruiz-Pino, F.; Garcia-Galiano, D.; Manfredi-Lozano, M.; Leon, S.; Romero-Ruiz, A.; Dieguez, C.; Pinilla, L.; Tena-Sempere, M. Metabolic programming of puberty: Sexually dimorphic responses to early nutritional challenges. Endocrinology 2013, 154, 3387–3400. [Google Scholar] [CrossRef]
- Davis, A.M.; Penschuck, S.; Fritschy, J.-M.; McCarthy, M.M. Developmental switch in the expression of GABAA receptor subunits α1 and α2 in the hypothalamus and limbic system of the rat. Dev. Brain Res. 2000, 119, 127–138. [Google Scholar] [CrossRef]
- Magnusson, K.R. Aging of the NMDA receptor: From a mouse’s point of view. Future Neurol. 2012, 7, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fukuda, A. Development and regulation of chloride homeostasis in the central nervous system. Front. Cell. Neurosci. 2015, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Melnick, I.; Pronchuk, N.; Cowley, M.A.; Grove, K.L.; Colmers, W.F. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron 2007, 56, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Frazao, R.; Cravo, R.M.; Donato, J., Jr.; Ratra, D.V.; Clegg, D.J.; Elmquist, J.K.; Zigman, J.M.; Williams, K.W.; Elias, C.F. Shift in Kiss1 cell activity requires estrogen receptor alpha. J. Neurosci. 2013, 33, 2807–2820. [Google Scholar] [CrossRef] [PubMed]
- Cravo, R.M.; Frazao, R.; Perello, M.; Osborne-Lawrence, S.; Williams, K.W.; Zigman, J.M.; Vianna, C.; Elias, C.F. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS ONE 2013, 8, e58698. [Google Scholar] [CrossRef]
- Vong, L.; Ye, C.; Yang, Z.; Choi, B.; Chua, S., Jr.; Lowell, B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011, 71, 142–154. [Google Scholar] [CrossRef]
- Krashes, M.J.; Shah, B.P.; Madara, J.C.; Olson, D.P.; Strochlic, D.E.; Garfield, A.S.; Vong, L.; Pei, H.; Watabe-Uchida, M.; Uchida, N.; et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 2014, 507, 238–242. [Google Scholar] [CrossRef]
- Sternson, S.M.; Shepherd, G.M.; Friedman, J.M. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat. Neurosci. 2005, 8, 1356–1363. [Google Scholar] [CrossRef]
- Garfield, A.S.; Shah, B.P.; Burgess, C.R.; Li, M.M.; Li, C.; Steger, J.S.; Madara, J.C.; Campbell, J.N.; Kroeger, D.; Scammell, T.E.; et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 2016, 19, 1628–1635. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, D.K.; Jo, Y.H. Cholinergic neurons in the dorsomedial hypothalamus regulate food intake. Mol. Metab. 2017, 6, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Rau, A.R.; Hentges, S.T. GABAergic inputs to pomc neurons originating from the dorsomedial hypothalamus are regulated by energy state. J. Neurosci. 2019, 39, 6449–6459. [Google Scholar] [CrossRef] [PubMed]
- Uner, A.; Goncalves, G.H.; Li, W.; Porceban, M.; Caron, N.; Schonke, M.; Delpire, E.; Sakimura, K.; Bjorbaek, C. The role of GluN2A and GluN2B NMDA receptor subunits in AgRP and POMC neurons on body weight and glucose homeostasis. Mol. Metab. 2015, 4, 678–691. [Google Scholar] [CrossRef]
- Stanley, B.G.; Ha, L.H.; Spears, L.C.; Dee, M.G. Lateral hypothalamic injections of glutamate, kainic acid,d,l-α-amino-3-hydroxy-5-methyl-isoxazole propionic acid or N-methyl-d-aspartic acid rapidly elicit intense transient eating in rats. Brain Res. 1993, 613, 88–95. [Google Scholar] [CrossRef]
- Clarkson, J.; Herbison, A.E. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol. Cell Endocrinol. 2006, 254–255, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Moenter, S.M.; Chu, Z.; Christian, C.A. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J. Neuroendocrinol. 2009, 21, 327–333. [Google Scholar] [CrossRef]
- Manfredi-Lozano, M.; Roa, J.; Ruiz-Pino, F.; Piet, R.; Garcia-Galiano, D.; Pineda, R.; Zamora, A.; Leon, S.; Sanchez-Garrido, M.A.; Romero-Ruiz, A.; et al. Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty. Mol. Metab. 2016, 5, 844–857. [Google Scholar] [CrossRef]
- Padilla, S.L.; Qiu, J.; Nestor, C.C.; Zhang, C.; Smith, A.W.; Whiddon, B.B.; Ronnekleiv, O.K.; Kelly, M.J.; Palmiter, R.D. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl. Acad Sci. USA 2017, 114, 2413–2418. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Lomniczi, A.; Mastronardi, C.; Heger, S.; Roth, C.; Parent, A.S.; Matagne, V.; Mungenast, A.E. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology 2006, 147, 1166–1174. [Google Scholar] [CrossRef]
- Davies, C.H.; Starkey, S.J.; Pozza, M.F.; Collingridge, G.L. GABAB autoreceptors regulate the induction of LTP. Nature 1991, 349, 609–611. [Google Scholar] [CrossRef]
- Ahima, R.S.; Dushay, J.; Flier, S.N.; Prabakaran, D.; Flier, J.S. Leptin accelerates the onset of puberty in normal female mice. J. Clin. Investig. 1997, 99, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Chehab, F.F.; Mounzih, K.; Lu, R.; Lim, M.E. Early onset of reproductive function in normal female mice treated with leptin. Science 1997, 275, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Galvez, M.P.; Greenspan, L.C.; Succop, P.A.; Vangeepuram, N.; Pinney, S.M.; Teitelbaum, S.; Windham, G.C.; Kushi, L.H.; Wolff, M.S. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 2010, 126, e583–e590. [Google Scholar] [CrossRef] [PubMed]
- Hillman, J.B.; Huang, B.; Pinney, S.M.; Biro, F.M. Early pubertal development and insulin sensitivity among school-aged girls: Mediation via adiposity. J. Pediatr. Adolesc. Gynecol. 2013, 26, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Spanswick, D.; Smith, M.A.; Groppi, V.E.; Logan, S.D.; Ashford, M.L. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 1997, 390, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Bosch, M.A.; Smart, J.L.; Qiu, J.; Rubinstein, M.; Ronnekleiv, O.K.; Low, M.J.; Kelly, M.J. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 2003, 144, 1331–1340. [Google Scholar] [CrossRef]
- Thomzig, A.; Laube, G.; Prüss, H.; Veh, R.W. Pore-forming subunits of K-ATP channels, Kir6.1 and Kir6.2, display prominent differences in regional and cellular distribution in the rat brain. J. Comp. Neurol. 2005, 484, 313–330. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Abcc8 | 5′-gtcctgaggcaatacctggg | gtcttctggaacagcagcct |
| Gabbr1 | atactgcacgccgttctgag | tcccggagcatctgtagtca |
| Gabbr2 | gttgtactcgccgaccttca | agtcacgggtcaagttgtgtt |
| Gabra1 | gtccagcagtcggtccaaaa | agcacactgtcgggaagaag |
| Gapdh | gggtcccagcttaggttcat | tacggccaaatccgttcaca |
| Gria-1 | ccaatcccagccctccaatc | cggaagtaaggacaagaccagt |
| Grin1 | cccggtgctcgtgtcttt | cgtgaacgtgtggaggaaga |
| Grin2a | ccatctcaccgtcaccaacaa | aattgctctgcagaagggctc |
| Grin2b | accaaatcgctttgccgatg | Ttacaaccggtgcctagctg |
| Kcnj8 | cgcagacgtgaatgacctga | catggagaagagtggcctgg |
| Kcnj11 | cctcctctctcgagtacggt | gctgtcccgaaagggcatta |
| Slc12a2 | cagaaagatgaggaagaggatggc | ggcacaatagggcctttggat |
| Slcl2a5 | gaataaaggccccagtcccg | aagttttcccactccggctt |
| Body Weight (g) | Prepubertal | Pubertal | Adult | p-Value |
|---|---|---|---|---|
| Control | 5.0 ± 0.2 (n = 15) | 14.7 ± 0.4 (n = 12) | 18.6 ± 0.3 (n = 14) | age: F(2, 59) = 790.0, p < 0.0001 |
| SL | 8.2 ± 0.3 (n = 8) **** | 17.3 ± 0.3 (n = 8) **** | 19.3 ± 0.3 (n = 8) | litter size: F(1, 59) = 62.5, p < 0.0001 |
| Interaction: F(2, 59) = 7.7, p = 0.001 | ||||
| Fat Pad (g) | ||||
| Control | 0.02 ± 0.002 (n = 8) | 0.05 ± 0.003 (n = 7) | 0.06 ± 0.007 (n = 4) | age: F(2, 37) = 34.9, p < 0.0001 |
| SL | 0.05 ± 0.002 (n = 8) **** | 0.06 ± 0.004 (n = 7) | 0.06 ± 0.002 (n = 9) | litter size: F(1, 37) = 31.1, p < 0.0001 |
| Interaction: F(2, 37) = 8.9, p = 0.007 | ||||
| Serum Leptin (pg/mL) | ||||
| Control | 1.3 ± 0.2 (n = 8) | 0.4 ± 0.04 (n = 8) | 0.2 ± 0.02 (n = 8) | age: F(2, 42) = 54.7, p < 0.0001 |
| SL | 5.0 ± 0.7 (n = 8) **** | 0.3 ± 0.04 (n = 8) | 0.3 ± 0.02 (n = 8) | litter size: F(1, 42) = 21.8, p < 0.0001 |
| Interaction: F(2, 42) = 22.3, p < 0.001 | ||||
| Sexual Maturation | Vaginal Opening (days) | First Estrus (days) | ||
| Control | 33.7 ± 1.3 (n = 14) | 43.4 ± 1.6 (n = 11) | ||
| SL | 29.8 ± 0.5 (n = 18) ** | 37.3 ± 1.0 (n = 12) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampieri, T.T.; Bohlen, T.M.; Silveira, M.A.; Lana, L.C.; de Paula, D.G.; Donato, J., Jr.; Frazao, R. Postnatal Overnutrition Induces Changes in Synaptic Transmission to Leptin Receptor-Expressing Neurons in the Arcuate Nucleus of Female Mice. Nutrients 2020, 12, 2425. https://doi.org/10.3390/nu12082425
Zampieri TT, Bohlen TM, Silveira MA, Lana LC, de Paula DG, Donato J Jr., Frazao R. Postnatal Overnutrition Induces Changes in Synaptic Transmission to Leptin Receptor-Expressing Neurons in the Arcuate Nucleus of Female Mice. Nutrients. 2020; 12(8):2425. https://doi.org/10.3390/nu12082425
Chicago/Turabian StyleZampieri, Thais Tessari, Tabata Mariz Bohlen, Marina Augusto Silveira, Larissa Campista Lana, Daniella G. de Paula, Jose Donato, Jr., and Renata Frazao. 2020. "Postnatal Overnutrition Induces Changes in Synaptic Transmission to Leptin Receptor-Expressing Neurons in the Arcuate Nucleus of Female Mice" Nutrients 12, no. 8: 2425. https://doi.org/10.3390/nu12082425
APA StyleZampieri, T. T., Bohlen, T. M., Silveira, M. A., Lana, L. C., de Paula, D. G., Donato, J., Jr., & Frazao, R. (2020). Postnatal Overnutrition Induces Changes in Synaptic Transmission to Leptin Receptor-Expressing Neurons in the Arcuate Nucleus of Female Mice. Nutrients, 12(8), 2425. https://doi.org/10.3390/nu12082425






