Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BI-RADS | Breast Imaging Reporting and Data System |
| BP | Blood pressure |
| CIs | Confidence intervals |
| CVD | Cardiovascular disease |
| HEPA | Health-enhancing physical activity |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| hsCRP | High sensitive C-reactive protein |
| LDL-C | Low-density lipoprotein cholesterol |
| PR | Prevalence ratio |
Appendix A
| Menopausal Stages | p for Trend | ||||
|---|---|---|---|---|---|
| Pre-Menopause | Early Transition | Late Transition | Post-Menopause | ||
| Never-smokers | |||||
| No. | 44,443 | 13,177 | 5997 | 13,742 | |
| Cases of dense breasts (%) | 47.7 | 45.1 | 34.4 | 13.3 | |
| Age-adjusted PR (95% CI) | reference | 0.96 (0.94–0.99) | 0.84 (0.81–0.87) | 0.48 (0.45–0.51) | <0.001 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.96 (0.94–0.97) | 0.89 (0.86–0.92) | 0.48 (0.45–0.50) | <0.001 |
| Former smokers | |||||
| No. | 1201 | 402 | 146 | 187 | |
| Cases of dense breasts (%) | 42.3 | 41.8 | 28.8 | 17.1 | |
| Age-adjusted PR (95% CI) | reference | 0.99 (0.87–1.13) | 0.73 (0.56–0.95) | 0.62 (0.41–0.93) | 0.005 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.93 (0.83–1.05) | 0.83 (0.65–1.06) | 0.59 (0.42–0.85) | 0.002 |
| Current smokers | |||||
| No. | 838 | 303 | 120 | 270 | |
| Cases of dense breasts (%) | 43.4 | 47.5 | 40.0 | 15.9 | |
| Age-adjusted PR (95% CI) | reference | 1.11 (0.96–1.28) | 1.03 (0.82–1.30) | 0.68 (0.48–0.96) | 0.25 |
| Multivariable-adjusted PR (95% CI) a | reference | 1.07 (0.94–1.22) | 1.09 (0.88–1.36) | 0.74 (0.54–1.01) | 0.158 |
References
- Boyd, N.; Martin, L.; Gunasekara, A.; Melnichouk, O.; Maudsley, G.; Peressotti, C.; Yaffe, M.; Minkin, S. Mammographic density and breast cancer risk: Evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol. Prev. Biomark. 2009, 18, 1754–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vachon, C.M.; Brandt, K.R.; Ghosh, K.; Scott, C.G.; Maloney, S.D.; Carston, M.J.; Pankratz, V.S.; Sellers, T.A. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol. Prev. Biomark. 2007, 16, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freer, P.E. Mammographic breast density: Impact on breast cancer risk and implications for screening. Radiographics 2015, 35, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.; Martin, L.; Stone, J.; Little, L.; Minkin, S.; Yaffe, M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol. Prev. Biomark. 2002, 11, 1048–1053. [Google Scholar]
- Martin, L.J.; Boyd, N.F. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: Hypotheses based on epidemiological evidence. Breast Cancer Res. 2008, 10, 201. [Google Scholar] [CrossRef]
- Boyd, N.F.; Lockwood, G.A.; Martin, L.J.; Knight, J.A.; Byng, J.W.; Yaffe, M.J.; Tritchler, D.L. Mammographic densities and breast cancer risk. Breast Dis. 1998, 10, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Vachon, C.M.; Sellers, T.A.; Vierkant, R.A.; Wu, F.F.; Brandt, K.R. Case-control study of increased mammographic breast density response to hormone replacement therapy. Cancer Epidemiol. Prev. Biomark. 2002, 11, 1382–1388. [Google Scholar]
- Lundstrom, E.; Christow, A.; Kersemaekers, W.; Svane, G.; Azavedo, E.; Soderqvist, G.; Mol-Arts, M.; Barkfeldt, J.; von Schoultz, B. Effects of tibolone and continuous combined hormone replacement therapy on mammographic breast density. Am. J. Obstet. Gynecol. 2002, 186, 717–722. [Google Scholar] [CrossRef]
- Persson, I.; Thurfjell, E.; Holmberg, L. Effect of estrogen and estrogen-progestin replacement regimens on mammographic breast parenchymal density. J. Clin. Oncol. 1997, 15, 3201–3207. [Google Scholar] [CrossRef]
- Cuzick, J.; Warwick, J.; Pinney, E.; Warren, R.M.; Duffy, S.W. Tamoxifen and breast density in women at increased risk of breast cancer. J. Natl. Cancer Inst. 2004, 96, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Checka, C.M.; Chun, J.E.; Schnabel, F.R.; Lee, J.; Toth, H. The relationship of mammographic density and age: Implications for breast cancer screening. AJR Am. J. Roentgenol. 2012, 198, W292–W295. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; Group, S.C. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef]
- Derby, C.A.; Crawford, S.L.; Pasternak, R.C.; Sowers, M.; Sternfeld, B.; Matthews, K.A. Lipid changes during the menopause transition in relation to age and weight: The Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2009, 169, 1352–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozakowski, J.; Gietka-Czernel, M.; Leszczynska, D.; Majos, A. Obesity in menopause—Our negligence or an unfortunate inevitability? Prz. Menopauzalny 2017, 16, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Kuni, C.C.; Anderson, K.; Anderson, V.E.; Sellers, T.A. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control 2000, 11, 653–662. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Dai, Z.; Wang, M.; Tian, T.; Liu, X.; Kang, H.; Guan, H.; Zhang, S.; Dai, Z. Association between body mass index and breast cancer risk: Evidence based on a dose-response meta-analysis. Cancer Manag. Res. 2018, 10, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Premenopausal Breast Cancer Collaborative, G.; Schoemaker, M.J.; Nichols, H.B.; Wright, L.B.; Brook, M.N.; Jones, M.E.; O'Brien, K.M.; Adami, H.O.; Baglietto, L.; Bernstein, L.; et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018, 4, e181771. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Zhou, Q.; Imam, M.U.; Cai, J.; Wang, Y.; Qi, M.; Sun, P.; Ping, Z.; Fu, X. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: A dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health 2017, 17, 936. [Google Scholar] [CrossRef]
- Chang, Y.; Ryu, S.; Choi, Y.; Zhang, Y.; Cho, J.; Kwon, M.J.; Hyun, Y.Y.; Lee, K.B.; Kim, H.; Jung, H.S.; et al. Metabolically Healthy Obesity and Development of Chronic Kidney Disease: A Cohort Study. Ann. Intern. Med. 2016, 164, 305–312. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malavolti, M.; Mussi, C.; Poli, M.; Fantuzzi, A.L.; Salvioli, G.; Battistini, N.; Bedogni, G. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann. Hum. Biol. 2003, 30, 380–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization; Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Lee, S.; Park, H.S.; Kim, S.M.; Kwon, H.S.; Kim, D.Y.; Kim, D.J.; Cho, G.J.; Han, J.H.; Kim, S.R.; Park, C.Y.; et al. Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Korean J. Obes. 2006, 15, 9. [Google Scholar]
- American College of Radiology; BI-RADS Committee. ACR BI-RADS® Atlas: Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Kang, E.Y.; Shin, J.H.; Kang, S.G.; Hwang, Y.N.; Cha, E.S.; Song, S.W. Relationship between Mammographic Dense Breast and Other Risk Factors of Breast Cancer in Korean Women. J. Korean Acad. Fam. Med. 2007, 28, 937–942. [Google Scholar]
- Mathiesen, U.L.; Franzen, L.E.; Aselius, H.; Resjo, M.; Jacobsson, L.; Foberg, U.; Fryden, A.; Bodemar, G. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig. Liver Dis. 2002, 34, 516–522. [Google Scholar] [CrossRef]
- Boyd, N.F.; Lockwood, G.A.; Byng, J.W.; Little, L.E.; Yaffe, M.J.; Tritchler, D.L. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br. J. Cancer 1998, 78, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- Burton, A.; Maskarinec, G.; Perez-Gomez, B.; Vachon, C.; Miao, H.; Lajous, M.; Lopez-Ridaura, R.; Rice, M.; Pereira, A.; Garmendia, M.L.; et al. Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLoS Med. 2017, 14, e1002335. [Google Scholar] [CrossRef] [Green Version]
- Su, H.I.; Freeman, E.W. Hormone changes associated with the menopausal transition. Minerva Ginecol. 2009, 61, 483–489. [Google Scholar]
- Sowers, M.R.; Zheng, H.; McConnell, D.; Nan, B.; Harlow, S.D.; Randolph, J.F., Jr. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J. Clin. Endocrinol. Metab. 2008, 93, 3847–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, L.M.; Gold, E.B.; Conroy, S.M.; Crandall, C.J.; Greendale, G.A.; Oestreicher, N.; Quesenberry, C.P., Jr.; Habel, L.A. Active, but not passive cigarette smoking was inversely associated with mammographic density. Cancer Causes Control. 2010, 21, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanko, L.B.; Christiansen, C. An update on the antiestrogenic effect of smoking: A literature review with implications for researchers and practitioners. Menopause 2004, 11, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, K.K.; Lynge, E.; Vejborg, I.; Tjonneland, A.; von Euler-Chelpin, M.; Andersen, Z.J. Cigarette smoking and mammographic density in the Danish Diet, Cancer and Health cohort. Cancer Causes Control 2016, 27, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Pagano, I.; Lurie, G.; Kolonel, L.N. A longitudinal investigation of mammographic density: The multiethnic cohort. Cancer Epidemiol. Prev. Biomark. 2006, 15, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Engmann, N.J.; Scott, C.; Jensen, M.R.; Winham, S.J.; Ma, L.; Brandt, K.R.; Mahmoudzadeh, A. Longitudinal Changes in Volumetric Breast Density in Healthy Women across the Menopausal Transition. Cancer Epidemiol. Prev. Biomark. 2019, 28, 1324–1330. [Google Scholar] [CrossRef]
- Hart, V.; Reeves, K.W.; Sturgeon, S.R.; Reich, N.G.; Sievert, L.L.; Kerlikowske, K.; Ma, L.; Shepherd, J.; Tice, J.A.; Mahmoudzadeh, A.P.; et al. The effect of change in body mass index on volumetric measures of mammographic density. Cancer Epidemiol. Prev. Biomark. 2015, 24, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Shieh, Y.; Scott, C.G.; Jensen, M.R.; Norman, A.D.; Bertrand, K.A.; Pankratz, V.S.; Brandt, K.R.; Visscher, D.W.; Shepherd, J.A.; Tamimi, R.M.; et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 2019, 21, 48. [Google Scholar] [CrossRef]
- Hudson, S.; Vik Hjerkind, K.; Vinnicombe, S.; Allen, S.; Trewin, C.; Ursin, G.; Dos-Santos-Silva, I.; De Stavola, B.L. Adjusting for BMI in analyses of volumetric mammographic density and breast cancer risk. Breast Cancer Res. 2018, 20, 156. [Google Scholar] [CrossRef]
- Baglietto, L.; Krishnan, K.; Stone, J.; Apicella, C.; Southey, M.C.; English, D.R.; Hopper, J.L.; Giles, G.G. Associations of mammographic dense and nondense areas and body mass index with risk of breast cancer. Am. J. Epidemiol. 2014, 179, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Soguel, L.; Durocher, F.; Tchernof, A.; Diorio, C. Adiposity, breast density, and breast cancer risk: Epidemiological and biological considerations. Eur. J. Cancer Prev. 2017, 26, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.Y.; Lobie, P.E. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes. Rev. 2000, 1, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, N.F.; Martin, L.J.; Bronskill, M.; Yaffe, M.J.; Duric, N.; Minkin, S. Breast tissue composition and susceptibility to breast cancer. J. Natl. Cancer Inst. 2010, 102, 1224–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, H.; Lang, K.; Rosso, A.; Borgquist, S.; Zackrisson, S.; Timberg, P. Measuring mammographic density: Comparing a fully automated volumetric assessment versus European radiologists’ qualitative classification. Eur. Radiol. 2016, 26, 4354–4360. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Dense Breasts | p-Value | |
|---|---|---|---|
| Absent | Present | ||
| Number | 50,080 | 32,597 | |
| Age (years) a | 45.8 (8.0) | 41.6 (5.0) | <0.001 |
| Height (m) a | 159.6 (5.3) | 160.7 (5.0) | <0.001 |
| BMI (kg/m2) a | 23.2 (3.2) | 20.9 (2.4) | <0.001 |
| BMI category (kg/m2) | <0.001 | ||
| <18.5 (underweight) | 3.0 | 13.0 | |
| 18.5–22.9 (normal weight) | 51.0 | 70.2 | |
| 23–24.9 (overweight) | 21.9 | 10.8 | |
| ≥25 (obesity) | 24.1 | 6.1 | |
| Body-fat percentage (n = 82,633) | 32.5 (5.7) | 28.0 (5.4) | <0.001 |
| Waist circumference (cm) (n = 82,659) | 78.7 (8.3) | 72.5 (6.5) | <0.001 |
| Fatty liver on ultrasound (%) (n = 82,267) | 23.8 | 5.9 | <0.001 |
| Smoking status (n = 80,826) | 0.369 | ||
| Never smokers, (%) | 95.6 | 95.8 | |
| Former smokers, (%) | 2.5 | 2.3 | |
| Current smokers, (%) | 1.9 | 1.9 | |
| Alcohol intake ≥10 g/day, (%) c (n = 77,407) | 12.3 | 12.2 | 0.796 |
| HEPA, (%) (n = 82,441) | 14.9 | 12.1 | <0.001 |
| Higher education, (%) d (n = 80,664) | 70.8 | 82.2 | <0.001 |
| Hypertension, (%) (n = 82,671) | 10.3 | 3.0 | <0.001 |
| Diabetes mellitus, (%) (n = 82,670) | 4.6 | 1.1 | <0.001 |
| Medication for hyperlipidemia, (%) | 5.1 | 1.0 | <0.001 |
| Family history of breast cancer (%) | 3.3 | 3.3 | 0.661 |
| Early menarche (%) e (n = 82,274) | 4.5 | 4.3 | 0.049 |
| Parity number (%) (n = 79,130) | <0.001 | ||
| 0 | 7.8 | 13.8 | |
| 1–2 | 78.1 | 79.7 | |
| ≥3 | 14.1 | 6.6 | |
| Female hormone medication (%) | 1.6 | 1.7 | 0.382 |
| Systolic BP (mmHg) a (n = 82,671) | 107.2 (12.8) | 102.1 (10.3) | <0.001 |
| Diastolic BP (mmHg) a (n = 82,671) | 68.1 (9.3) | 65.3 (8.1) | <0.001 |
| Glucose (mg/dL) a | 95.0 (15.1) | 91.4 (9.8) | <0.001 |
| Total cholesterol (mg/dL) a | 194.7 (34.7) | 186.2 (30.9) | <0.001 |
| LDL-C (mg/dL) a (n = 82,504) | 125.4 (33.4) | 113.9 (28.6) | <0.001 |
| HDL-C (mg/dL) a | 64.8 (16.0) | 70.0 (15.6) | <0.001 |
| Triglycerides (mg/dL) b | 81 (60–113) | 69 (54–91) | <0.001 |
| hsCRP (mg/L) b (n = 57,460) | 0.4 (0.3–0.8) | 0.3 (0.2–0.5) | <0.001 |
| HOMA-IR b (n = 81,649) | 1.32 (0.88–1.98) | 1.11 (0.76–1.58) | <0.001 |
| Total calorie intake (kcal/day) b (n = 47,947) | 1131 (812–1496) | 1092 (789–1444) | <0.001 |
| Menopausal Stages | p for Trend | ||||
|---|---|---|---|---|---|
| Pre-Menopause | Early Transition | Late Transition | Post-Menopause | ||
| Overall population | |||||
| No. | 46,532 | 13,896 | 6287 | 15,962 | |
| Cases of dense breasts (%) | 47.5 | 45.0 | 34.4 | 13.1 | |
| Age-adjusted PR (95% CI) | reference | 0.97 (0.95–0.99) | 0.84 (0.81–0.87) | 0.48 (0.46–0.51) | <0.001 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.96 (0.94–0.98) | 0.89 (0.86–0.92) | 0.48 (0.46–0.51) | <0.001 |
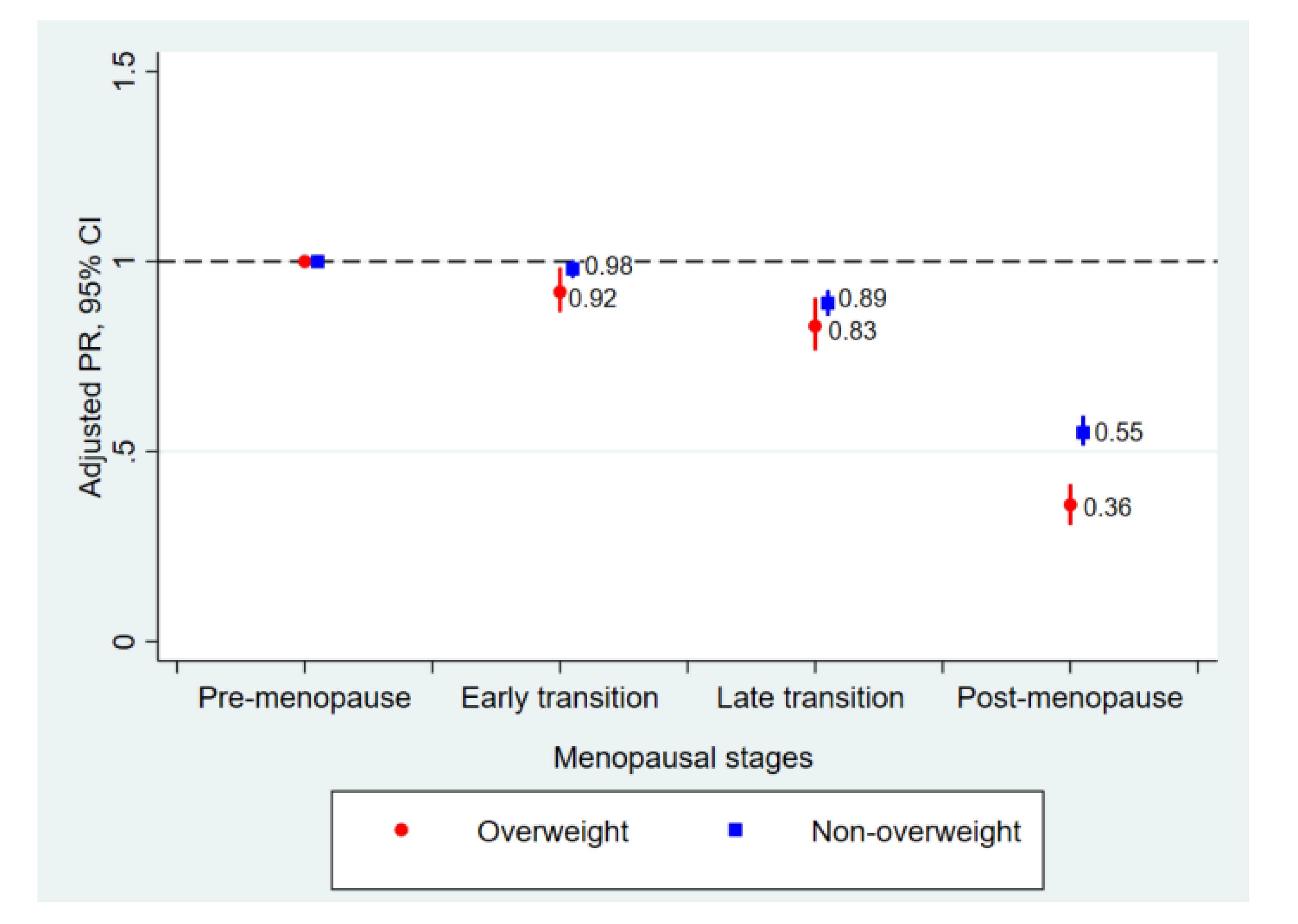
| Non-overweight (BMI < 23 kg/m2) | |||||
| No. | 32,772 | 9660 | 3690 | 8005 | |
| Cases of dense breasts (%) | 56.6 | 54.7 | 45.0 | 20.1 | |
| Age-adjusted PR (95% CI) | reference | 0.98 (0.96–1.00) | 0.89 (0.86–0.92) | 0.54 (0.51–0.57) | <0.001 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.98 (0.96–1.00) | 0.89 (0.86–0.92) | 0.55 (0.52–0.59) | <0.001 |
| Overweight (BMI ≥ 23 kg/m2) | |||||
| No. | 13,760 | 4236 | 2597 | 7957 | |
| Cases of dense breasts (%) | 25.8 | 23.0 | 19.3 | 6.1 | |
| Age-adjusted PR (95% CI) | reference | 0.90 (0.85–0.96) | 0.83 (0.76–0.90) | 0.35 (0.30–0.40) | <0.001 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.92 (0.87–0.98) | 0.83 (0.77–0.90) | 0.36 (0.31–0.41) | <0.001 |
| Subgroup | Menopausal Stages | p for Trend | p for Interaction | |||
|---|---|---|---|---|---|---|
| Pre-Menopause | Early Transition | Late Transition | Post-Menopause | |||
| Waist circumference | 0.001 | |||||
| <85 cm (n = 70,752) | reference | 0.98 (0.96–1.00) | 0.88 (0.85–0.91) | 0.53 (0.51–0.56) | <0.001 | |
| ≥85 cm (n = 11,907) | reference | 0.84 (0.74–0.95) | 0.84 (0.72–0.97) | 0.31 (0.24–0.41) | <0.001 | |
| Body-fat percentage | <0.001 | |||||
| <30% (n = 37,730) | reference | 0.97 (0.95–0.99) | 0.94 (0.90–0.98) | 0.63 (0.59–0.67) | <0.001 | |
| ≥30% (n = 44,903) | reference | 0.97 (0.93–1.00) | 0.83 (0.79–0.88) | 0.43 (0.40–0.48) | <0.001 | |
| Fatty liver on ultrasound | <0.001 | |||||
| No (n = 68,481) | reference | 0.97 (0.95–0.98) | 0.90 (0.87–0.93) | 0.52 (0.49–0.54) | <0.001 | |
| Yes (n = 13,786) | reference | 0. 87 (0.78–0.96) | 0.85 (0.75–0.97) | 0.38 (0.31–0.46) | <0.001 | |
| Smoking status | 0.258 | |||||
| Never smokers (n = 79,359) | reference | 0.96 (0.94–0.97) | 0.89 (0.86–0.92) | 0.48 (0.45–0.50) | <0.001 | |
| Former smokers (n = 1936) | reference | 0.93 (0.83–1.05) | 0.83 (0.65–1.06) | 0.59 (0.42–0.85) | 0.002 | |
| Current smokers (n = 1531) | reference | 1.07 (0.94–1.22) | 1.09 (0.88–1.36) | 0.74 (0.54–1.01) | 0.158 | |
| Alcohol intake | 0.347 | |||||
| <10 g /day (n = 67,913) | reference | 0.95 (0.93–0.97) | 0.89 (0.86–0.93) | 0.49 (0.46–0.52) | <0.001 | |
| ≥10 g/day (n = 9494) | reference | 0.98 (0.93–1.03) | 0.85 (0.77–0.94) | 0.50 (0.43–0.58) | <0.001 | |
| HEPA | 0.200 | |||||
| No (n = 71,072) | reference | 0.95 (0.93–0.97) | 0.88 (0.85–0.92) | 0.48 (0.45–0.51) | <0.001 | |
| Yes (n = 11,369) | reference | 1.01 (0.96–1.07) | 0.95 (0.86–1.05) | 0.52 (0.46–0.59) | <0.001 | |
| HOMA-IR | <0.001 | |||||
| <2.5 (n = 72,436) | reference | 0.96 (0.94–0.98) | 0.89 (0.86–0.92) | 0.50 (0.48–0.53) | <0.001 | |
| ≥2.5 (n = 9213) | reference | 0.91 (0.83–0.99) | 0.86 (0.75–0.98) | 0.34 (0.27–0.43) | <0.001 | |
| HsCRP | 0.354 | |||||
| <1.0 mg/L (n = 47,548) | reference | 0.96 (0.94–0.98) | 0.92 (0.88–0.95) | 0.50 (0.47–0.54) | <0.001 | |
| ≥1.0 mg/L (n = 9912) | reference | 1.01 (0.94–1.08) | 0.89 (0.80–1.00) | 0.48 (0.39–0.59) | <0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.Y.; Chang, Y.; Ahn, J.; Yun, J.-S.; Park, Y.L.; Park, C.H.; Shin, H.; Ryu, S. Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women. J. Clin. Med. 2020, 9, 2434. https://doi.org/10.3390/jcm9082434
Kim EY, Chang Y, Ahn J, Yun J-S, Park YL, Park CH, Shin H, Ryu S. Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women. Journal of Clinical Medicine. 2020; 9(8):2434. https://doi.org/10.3390/jcm9082434
Chicago/Turabian StyleKim, Eun Young, Yoosoo Chang, Jiin Ahn, Ji-Sup Yun, Yong Lai Park, Chan Heun Park, Hocheol Shin, and Seungho Ryu. 2020. "Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women" Journal of Clinical Medicine 9, no. 8: 2434. https://doi.org/10.3390/jcm9082434
APA StyleKim, E. Y., Chang, Y., Ahn, J., Yun, J.-S., Park, Y. L., Park, C. H., Shin, H., & Ryu, S. (2020). Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women. Journal of Clinical Medicine, 9(8), 2434. https://doi.org/10.3390/jcm9082434





