Exosomal MicroRNAs in Pregnancy Provides Insight into a Possible Cure for Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Participant Clinical Characteristics
2.2. Isolation and Characterisation of Exosomes in Maternal Circulation
2.3. Exosomal miRNA Expression in Pregnancy and Their Links to Cancer Metastasis and Immunosuppression
2.3.1. Exosomal hsa-miR-451a
2.3.2. Exosomal hsa-miR-302d-3p
2.3.3. Exosomal hsa-miR-223-3p
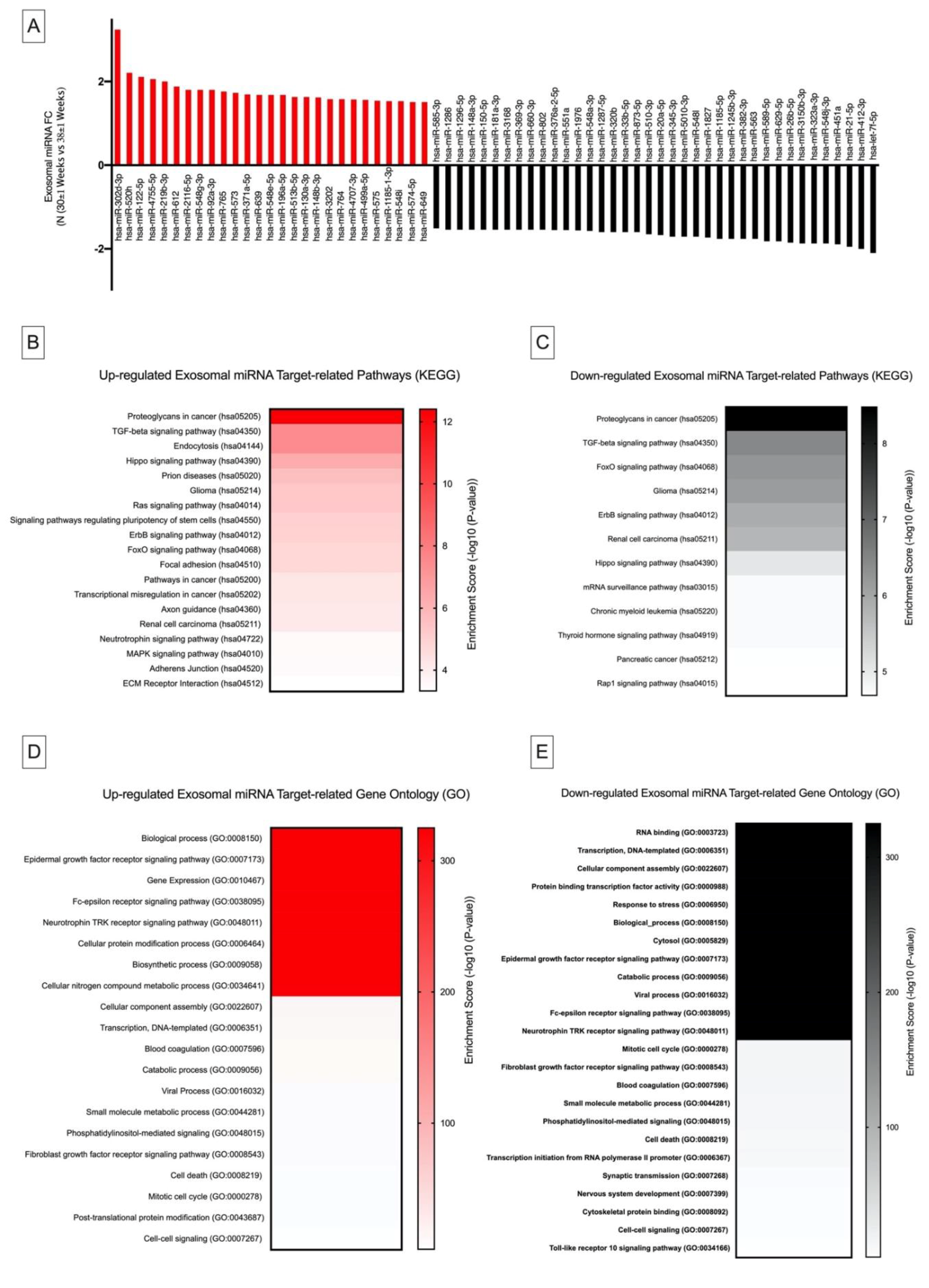
2.4. Differential Exosomal miRNA Expression in Pregnancy and Their Links to Cancer Metastasis and Immunosuppression
2.4.1. Kyoto Encyclopedia of Genes and Genomes Pathway Analysis
2.4.2. Gene Ontology Analysis
2.5. Future Experimental Framework for the Repurposing of Exosomal miRNA Identified in Pregnancy towards a Cure for Cancer
3. Materials and Methods
3.1. Ethics Statement
3.2. Study Group
3.3. Exosome Isolation
3.4. Exosomal RNA Isolation
3.5. Exosome Characterisation
3.5.1. Nanoparticle Tracking Analysis
3.5.2. Western Blotting
3.5.3. Transmission Electron Microscopy
3.6. Quantification of Total and Placenta-Derived Exosomes
3.7. NanoString nCounter System miRNA Assay
3.8. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tai, Y.-L.; Chen, K.-C.; Hsieh, J.-T.; Shen, T.-L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Menon, R. Placental exosomes: A proxy to understand pregnancy complications. Am. J. Reprod. Immunol. 2017, 79, e12788. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.; Stapleton, J.T.; Okeoma, C.M. Vehicles of intercellular communication: Exosomes and HIV-1. J. Gen. Virol. 2019, 100, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Lasser, C. Exosomal RNA as Biomarkers and the Therapeutic Potential of Exosome Vectors. Expert Opin. Biol. Ther. 2012, 12, S189–S197. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Wohl, D.; Yagel, S. Regulation of trophoblast invasion: From normal implantation to pre-eclampsia. Mol. Cell. Endocrinol. 2002, 187, 233–238. [Google Scholar] [CrossRef]
- Costanzo, V.; Bardelli, A.; Siena, S.; Abrignani, S. Exploring the links between cancer and placenta development. Open Boil. 2018, 8, 180081. [Google Scholar] [CrossRef]
- Holtan, S.G.; Creedon, D.J.; Haluska, P.; Markovic, S.N. Cancer and pregnancy: Parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin. Proc. 2009, 84, 985–1000. [Google Scholar] [CrossRef]
- Ferretti, C.; Bruni, L.; Dangles-Marie, V.; Pecking, A.; Bellet, D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum. Reprod. Updat. 2006, 13, 121–141. [Google Scholar] [CrossRef]
- Mullen, C.A. Review: Analogies between Trophoblastic and Malignant Cells. Am. J. Reprod. Immunol. 1998, 39, 41–49. [Google Scholar] [CrossRef]
- D’Souza, A.W.; Wagner, G.P. Malignant cancer and invasive placentation. Evol. Med. Public Health 2014, 2014, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Manzo, G. Similarities between Embryo Development and Cancer Process Suggest New Strategies for Research and Therapy of Tumors: A New Point of View. Front. Cell Dev. Boil. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, G.; Hu, W.; Yao, Y.; Yu, X.-F. Emerging roles and therapeutic value of exosomes in cancer metastasis. Mol. Cancer 2019, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Romero, K.S.; Perez, A.; Illanes, S.; Mitchell, M.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Sabapatha, A.; Gercel-Taylor, C.; Taylor, D.D. Specific Isolation of Placenta-Derived Exosomes from the Circulation of Pregnant Women and Their Immunoregulatory Consequences. Am. J. Reprod. Immunol. 2006, 56, 345–355. [Google Scholar] [CrossRef]
- Pillay, P.; Maharaj, N.; Moodley, J.; Mackraj, I. Placental exosomes and pre-eclampsia: Maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta 2016, 46, 18–25. [Google Scholar] [CrossRef]
- Pillay, P.; Moodley, K.; Moodley, J.; Mackraj, I. Placenta-derived exosomes: Potential biomarkers of preeclampsia. Int. J. Nanomed. 2017, 12, 8009–8023. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-Derived Exosomes and Syncytiotrophoblast Microparticles and their Role in Human Reproduction: Immune Modulation for Pregnancy Success. Am. J. Reprod. Immunol. 2014, 72, 440–457. [Google Scholar] [CrossRef]
- Pillay, P.; Vatish, M.; Duarte, R.; Moodley, J.; Mackraj, I. Exosomal microRNA profiling in early and late onset preeclamptic pregnant women reflects pathophysiology. Int. J. Nanomed. 2019, 14, 5637–5657. [Google Scholar] [CrossRef]
- Minna, E.; Romeo, P.; Dugo, M.; De Cecco, L.; Todoerti, K.; Pilotti, S.; Perrone, F.; Seregni, E.; Agnelli, L.; Neri, A.; et al. Correction: miR-451a is underexpressed and targets AKT/mTOR pathway in papillary thyroid carcinoma. Oncotarget 2018, 9, 12534. [Google Scholar] [CrossRef]
- Diring, J.; Camuzeaux, B.; Donzeau, M.; Vigneron, M.; Rosa-Calatrava, M.; Kédinger, C.; Chatton, B. A Cytoplasmic Negative Regulator Isoform of ATF7 Impairs ATF7 and ATF2 Phosphorylation and Transcriptional Activity. PLoS ONE 2011, 6, e23351. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Europe-Finner, G.N. Identification of human myometrial target genes of the c-Jun NH2-terminal kinase (JNK) pathway: The role of activating transcription factor 2 (ATF2) and a novel spliced isoform ATF2-small. J. Mol. Endocrinol. 2005, 34, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Phillips, R.J.; Pollard, A.J.; Gillmore, K.; Robson, S.C.; Europe-Finner, G.N. Characterization and Functional Analysis of cAMP Response Element Modulator Protein and Activating Transcription Factor 2 (ATF2) Isoforms in the Human Myometrium during Pregnancy and Labor: Identification of a Novel ATF2 Species with Potent Transactivation Properties. J. Clin. Endocrinol. Metab. 2002, 87, 1717–1728. [Google Scholar] [PubMed]
- Bhoumik, A.; Ronai, Z. ATF2: A transcription factor that elicits oncogenic or tumor suppressor activities. Cell Cycle 2008, 7, 2341–2345. [Google Scholar] [CrossRef]
- Li, Q.; Gao, W.-Q.; Dai, W.-Y.; Yu, C.; Zhu, R.-Y.; Jin, J. ATF2 translation is induced under chemotherapeutic drug-mediated cellular stress via an IRES-dependent mechanism in human hepatic cancer Bel7402 cells. Oncol. Lett. 2016, 12, 4795–4802. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, J.; Zhang, G.; Wang, Q.; Wu, C.; Zhang, Q.; Wang, H.; Sun, P.; Xiang, R.; Yang, S. Exosomal miR-451a Functions as a Tumor Suppressor in Hepatocellular Carcinoma by Targeting LPIN1. Cell. Physiol. Biochem. 2019, 53, 19–35. [Google Scholar]
- Iozzo, R.V.; Sanderson, R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011, 15, 1013–1031. [Google Scholar] [CrossRef]
- Hjelm, A.M.; Barchan, K.; Malmström, A.; Ekman-Ordeberg, G.E. Changes of the uterine proteoglycan distribution at term pregnancy and during labour. Eur. J. Obstet. Gynecol. Reprod. Boil. 2002, 100, 146–151. [Google Scholar] [CrossRef]
- Özler, S.; Demircan, K. The investigation of the role of proteoglycans and ADAMTS levels in fetal membranes in physiopathological process of gestational diabetes. Med. Hypotheses 2017, 104, 182–184. [Google Scholar] [CrossRef]
- Van Sinderen, M.; Cuman, C.; Winship, A.; Menkhorst, E.M.; Dimitriadis, E. The chrondroitin sulfate proteoglycan (CSPG4) regulates human trophoblast function. Placenta 2013, 34, 907–912. [Google Scholar] [CrossRef]
- Wegrowski, Y.; Maquart, F.-X. Involvement of stromal proteoglycans in tumour progression. Crit. Rev. Oncol. 2004, 49, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J. Immunological relationship between the mother and the fetus. Int. Rev. Immunol. 2002, 21, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Jarnicki, A.G.; Lysaght, J.; Todryk, S.M.; Mills, K.H. Suppression of Antitumor Immunity by IL-10 and TGF-β-Producing T Cells Infiltrating the Growing Tumor: Influence of Tumor Environment on the Induction of CD4+ and CD8+ Regulatory T Cells. J. Immunol. 2006, 177, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, C.; Pingitore, J.; Rothé, F.; Marchio, C.; Clatot, F.; Rouas, G.; Richard, F.; Bertucci, F.; Mariani, O.; Galant, C.; et al. ESR1 mutations in metastatic lobular breast cancer patients. NPJ Breast Cancer 2019, 5, 9. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.-M.; Vats, P.; Su, F.; Lonigro, R.J.; Cao, X.; Kalyana-Sundaram, S.; Wang, R.; Ning, Y.; Hodges, L.; et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013, 45, 1446–1451. [Google Scholar] [CrossRef]
- Saha Roy, S.; Vadlamudi, R.K. Role of estrogen receptor signaling in breast cancer metastasis. Int. J. Breast Cancer 2012, 2012, 654698. [Google Scholar] [CrossRef]
- Condon, J.C.; Kyathanahalli, C.; Anamthathmakula, P.; Jeyasuria, P. Estrogen/estrogen receptor action and the pregnant myometrium. Curr. Opin. Physiol. 2020, 13, 135–140. [Google Scholar] [CrossRef]
- Phung, J.; Paul, J.; Smith, R. Maintenance of Pregnancy and Parturition. In Maternal-Fetal and Neonatal Endocrinology; Christopher, S., Cheri, K., Deal, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 13; pp. 169–187. [Google Scholar]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Mizuuchi, M.; Cindrova-Davies, T.; Olovsson, M.; Charnock-Jones, D.S.; Burton, G.J.; Yung, H.W. Placental endoplasmic reticulum stress negatively regulates transcription of placental growth factor via ATF4 and ATF6β: Implications for the pathophysiology of human pregnancy complications. J. Pathol. 2016, 238, 550–561. [Google Scholar] [CrossRef]
- Sisinni, L.; Pietrafesa, M.; Lepore, S.; Maddalena, F.; Condelli, V.; Esposito, F.; Landriscina, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Breast Cancer: The Balance between Apoptosis and Autophagy and Its Role in Drug Resistance. Int. J. Mol. Sci. 2019, 20, 857. [Google Scholar] [CrossRef]
- Oslowski, C.M.; Urano, F. Measuring ER Stress and the Unfolded Protein Response Using Mammalian Tissue Culture System. Unfolded Protein Response Cell. Stress Part B 2011, 490, 71–92. [Google Scholar]
- Rapacz-Leonard, A.; Dąbrowska, M.; Janowski, T. Major Histocompatibility Complex I Mediates Immunological Tolerance of the Trophoblast during Pregnancy and May Mediate Rejection during Parturition. Mediat. Inflamm. 2014, 2014, 1–11. [Google Scholar]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Sui, C.; Huang, K.; Wang, L.; Hu, D.; Xiong, T.; Wang, R.; Zhang, H. MicroRNA-223-3p suppresses leukemia inhibitory factor expression and pinopodes formation during embryo implantation in mice. Am. J. Transl. Res. 2016, 8, 1155–1163. [Google Scholar] [PubMed]
- Zhang, R.; Zhang, L.-J.; Yang, M.-L.; Huang, L.-S.; Chen, G.; Feng, Z.-B. Potential role of microRNA-223-3p in the tumorigenesis of hepatocellular carcinoma: A comprehensive study based on data mining and bioinformatics. Mol. Med. Rep. 2017, 17, 2211–2228. [Google Scholar] [CrossRef] [PubMed]
- Pascual-García, M.; Teixidor, E.B.; Planas-Rigol, E.; Rubio-Perez, C.; Iurlaro, R.; Arias, A.; Cuartas, I.; Sala-Hojman, A.; Escudero, L.; Martinez-Ricarte, F.; et al. LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+ T cell tumor-infiltration impairing anti-PD1 therapy. Nat. Commun. 2019, 10, 2416. [Google Scholar] [CrossRef]
- Kang, I.; Chang, M.Y.; Wight, T.N.; Frevert, C.W. Proteoglycans as Immunomodulators of the Innate Immune Response to Lung Infection. J. Histochem. Cytochem. 2018, 66, 241–259. [Google Scholar] [CrossRef]
- Frey, H.; Schroeder, N.; Manon-Jensen, T.; Iozzo, R.V.; Schaefer, L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013, 280, 2165–2179. [Google Scholar] [CrossRef]
- Large, M.J.; Wetendorf, M.; Lanz, R.B.; Hartig, S.M.; Creighton, C.J.; Mancini, M.A.; Kovanci, E.; Lee, K.-F.; Threadgill, D.W.; Lydon, J.P.; et al. The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy. PLoS Genet. 2014, 10, e1004451. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Krikun, G.; Runic, R.; Schwartz, L.B.; Mesia, A.F.; Schatz, F. Progestin-Epidermal Growth Factor Regulation of Tissue Factor Expression during Decidualization of Human Endometrial Stromal Cells. J. Clin. Endocrinol. Metab. 2000, 85, 297–301. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2017, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Burton, O.T.; Oettgen, H.C. Beyond immediate hypersensitivity: Evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol. Rev. 2011, 242, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Ekström, E.S.; Nilsson, C.; Holmlund, U.; Van Der Ploeg, I.; Sandstedt, B.; Lilja, G.; Scheynius, A. IgE is expressed on, but not produced by, fetal cells in the human placenta irrespective of maternal atopy. Clin. Exp. Immunol. 2002, 127, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Bundhoo, A.; Paveglio, S.; Rafti, E.; Dhongade, A.; Blumberg, R.S.; Matson, A. Evidence that FcRn mediates the transplacental passage of maternal IgE in the form of IgG anti-IgE/IgE immune complexes. Clin. Exp. Allergy 2015, 45, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Stingl, G.; Maurer, D. IgE-Mediated Allergen Presentation via Fc Epsilon Rl on Antigen-Presenting Cells. Int. Arch. Allergy Immunol. 1997, 113, 24–29. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Josephs, D.H.; Spicer, J.F.; Karagiannis, P.; Gould, H.J.; Karagiannis, S.N. IgE Immunotherapy: A novel concept with promise for the treatment of cancer. mAbs 2014, 6, 54–72. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genome Res. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Salamonsen, L.; Evans, J.; Nguyen, H.P.; Edgell, T.A. The Microenvironment of Human Implantation: Determinant of Reproductive Success. Am. J. Reprod. Immunol. 2015, 75, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Sandovici, I.; Hoelle, K.; Angiolini, E.; Constancia, M. Placental adaptations to the maternal–fetal environment: Implications for fetal growth and developmental programming. Reprod. Biomed. Online 2012, 25, 68–89. [Google Scholar] [CrossRef] [PubMed]
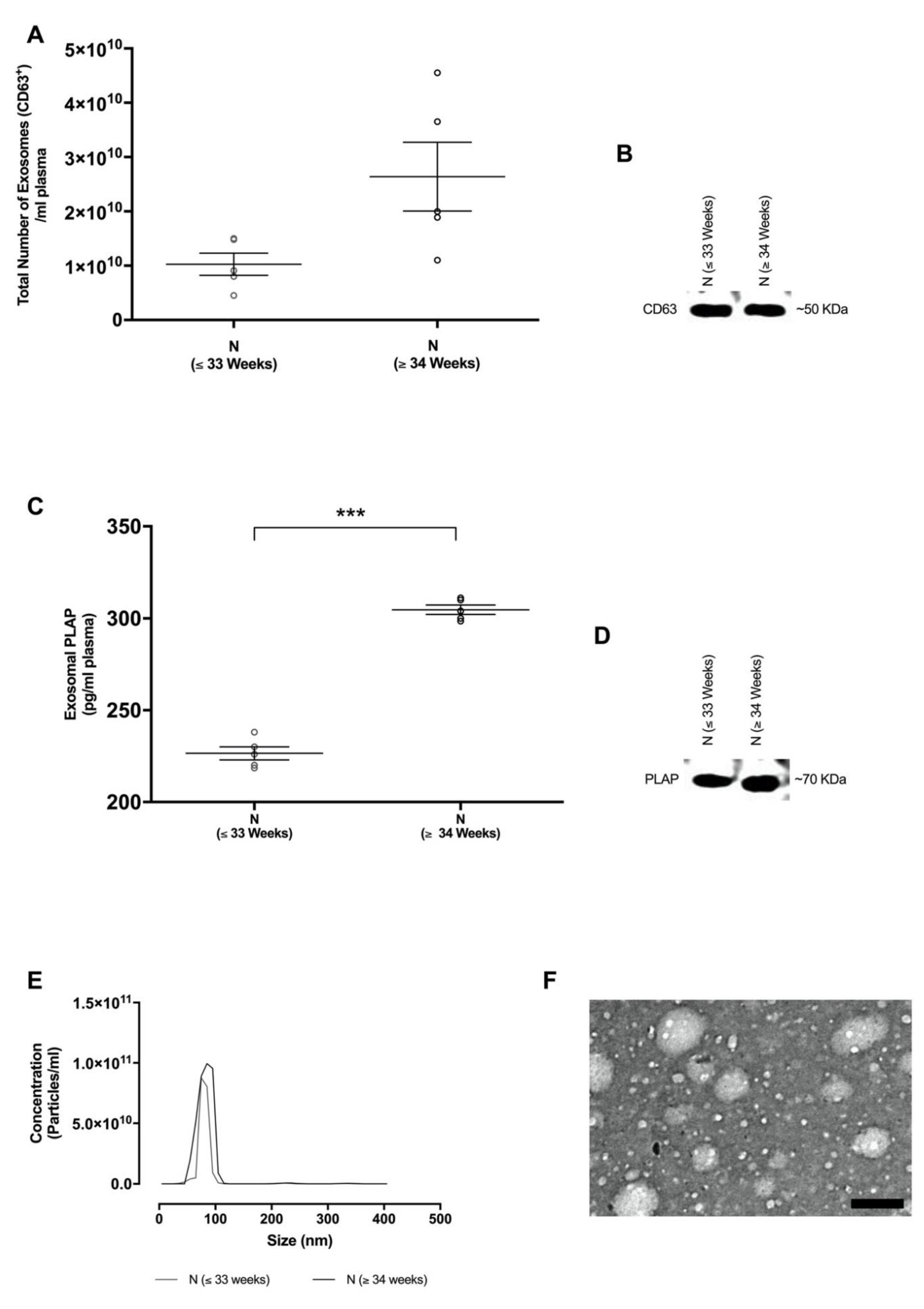

| Variables | Normotensive Pregnant Women | |
|---|---|---|
| ≤33 Weeks (n = 5) | ≥34 Weeks (n = 5) | |
| Age (years) | 30.43 ± 1.1 | 33.12 ± 1.1 |
| Weight (kg) | 65.88 ± 3.25 | 67.57 ± 2.22 |
| Height (cm) | 155.77 ± 4.12 | 157.66 ± 4.55 |
| BMI | 25.98 ± 2.14 | 26.95 ± 2.99 |
| Gestational Age (weeks) | 30.11 ± 1.56 (26–33) | 38 ± 1.9 (34–38) |
| Systolic/diastolic blood pressure (mm Hg) | 123/81 ± 2.22/1.88(90–120/50–80) | 119/79 ± 2.1/4.3 (90–120/50–80) |
| Urine Protein (mg/dL) | ND | ND |
| Exosomal miRNA Identified | Mean Expression Values (Log2) | Kyoto Encyclopedia of Genes and Genomes Analysis (p ≤ 0.05) | ||||
|---|---|---|---|---|---|---|
| Normotensives (30 ± 1 Weeks) | Normotensives (38 ± 1 Weeks) | Computational DIANA miRPath microT-CDS Target Pathway | p Value | Target Genes | Target Physiological Function | |
| hsa-miR-451a | 11.06 ± 0.03 | 9.95 ± 0.01 | Estrogen signaling (hsa04915) | 3.0 × 10−3 | ATF2 | Cell cycle regulation, Proapoptotic protein regulation |
| hsa-miR-302d-3p | 7.54 ± 0.21 | 9.24 ± 0.81 | Estrogen signaling (hsa04915) | 8.9 × 10−5 | ESR1, ATF6B, CREB5, CREB1, SOS1, KCNJ6, SHC4, PIK3CA | Cell cycle regulation, Proapoptotic protein regulation |
| Proteoglycans in cancer (hsa05205) | 1.0 × 10−3 | ESR1, PDCD4, SMAD2, SDC1, PTCH1, ROCK2, RDX, ERBB3, FZD3, ANK2, TFAP4, SOS1, PIK3CA, CD44 | Cell migration and Invasion, Cell growth and survival | |||
| hsa-miR-223-3p | 4.95 ± 0.1 | 5.5 ± 0.03 | Mucin-type O-Glycan biosynthesis (hsa00512) | 2.5 × 10−6 | GALNT7, GALNT18 | Protein secretion, stability, processing and function |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillay, P.; Moodley, K.; Vatish, M.; Moodley, J. Exosomal MicroRNAs in Pregnancy Provides Insight into a Possible Cure for Cancer. Int. J. Mol. Sci. 2020, 21, 5384. https://doi.org/10.3390/ijms21155384
Pillay P, Moodley K, Vatish M, Moodley J. Exosomal MicroRNAs in Pregnancy Provides Insight into a Possible Cure for Cancer. International Journal of Molecular Sciences. 2020; 21(15):5384. https://doi.org/10.3390/ijms21155384
Chicago/Turabian StylePillay, Preenan, Kogi Moodley, Manu Vatish, and Jagidesa Moodley. 2020. "Exosomal MicroRNAs in Pregnancy Provides Insight into a Possible Cure for Cancer" International Journal of Molecular Sciences 21, no. 15: 5384. https://doi.org/10.3390/ijms21155384
APA StylePillay, P., Moodley, K., Vatish, M., & Moodley, J. (2020). Exosomal MicroRNAs in Pregnancy Provides Insight into a Possible Cure for Cancer. International Journal of Molecular Sciences, 21(15), 5384. https://doi.org/10.3390/ijms21155384





