Adaptive Fat Oxidation Is Coupled with Increased Lipid Storage in Adipose Tissue of Female Mice Fed High Dietary Fat and Sucrose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sourcing and Characterization of PMI5011 Extract
2.2. Experimental Animals
2.3. Glucose and Insulin Tolerance Tests, Blood Chemistry, and Hepatic Triglyceride Content
2.4. Immunohistochemistry
2.5. Fatty Acid Oxidation Assay
2.6. Analysis of Protein Expression
2.7. Analysis of Gene Expression
2.8. Ex Vivo Lipolysis Assay
2.9. Statistical Analysis
3. Results
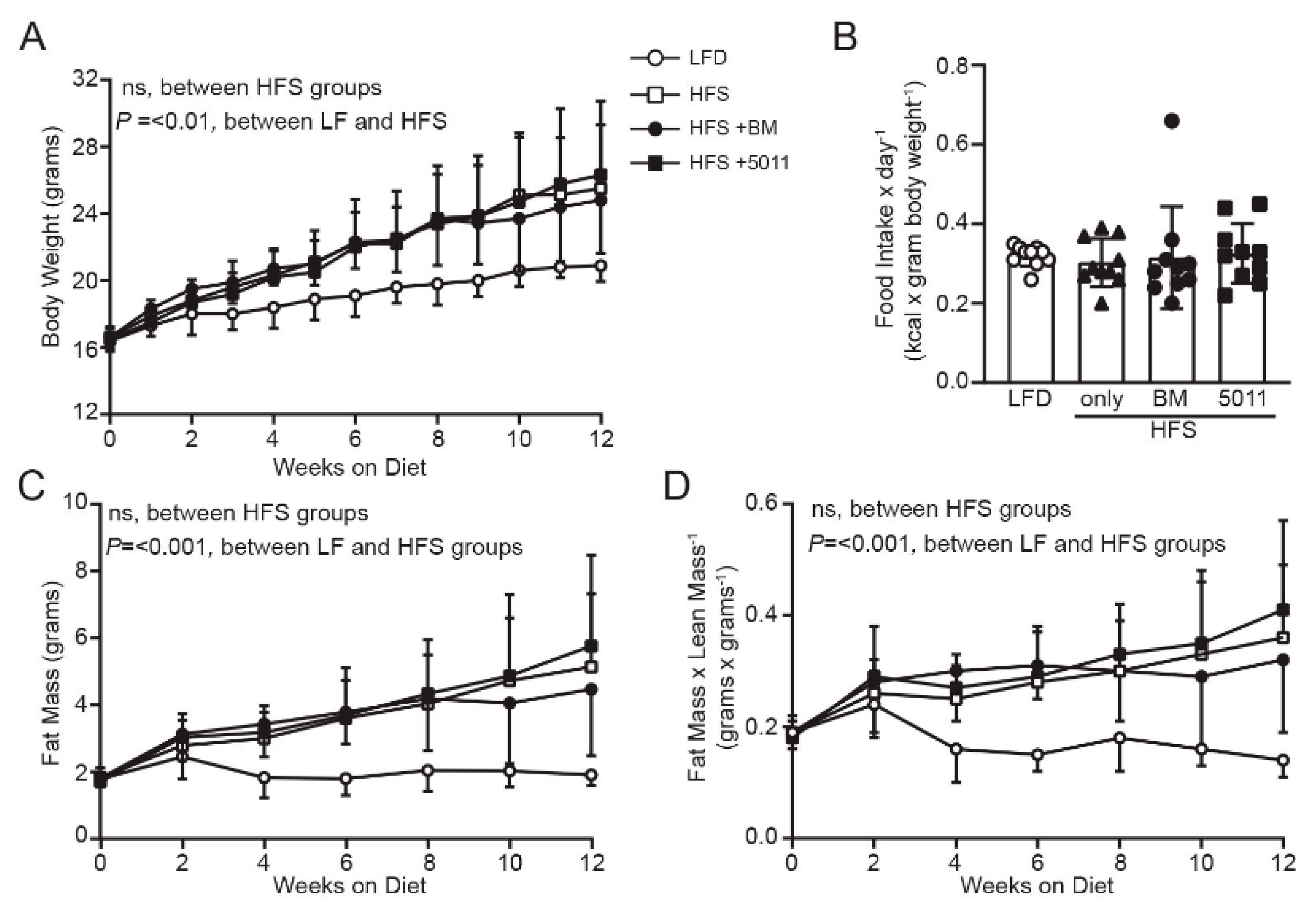
3.1. HFS Diet Induces Body Weight and Fat Mass Gain in Females That Is Not Reduced by Botanical Supplementation
3.2. Females Fed an HFS Diet Develop Glucose Intolerance and Insulin Resistance That Is Not Reversed by Botanical Supplementation
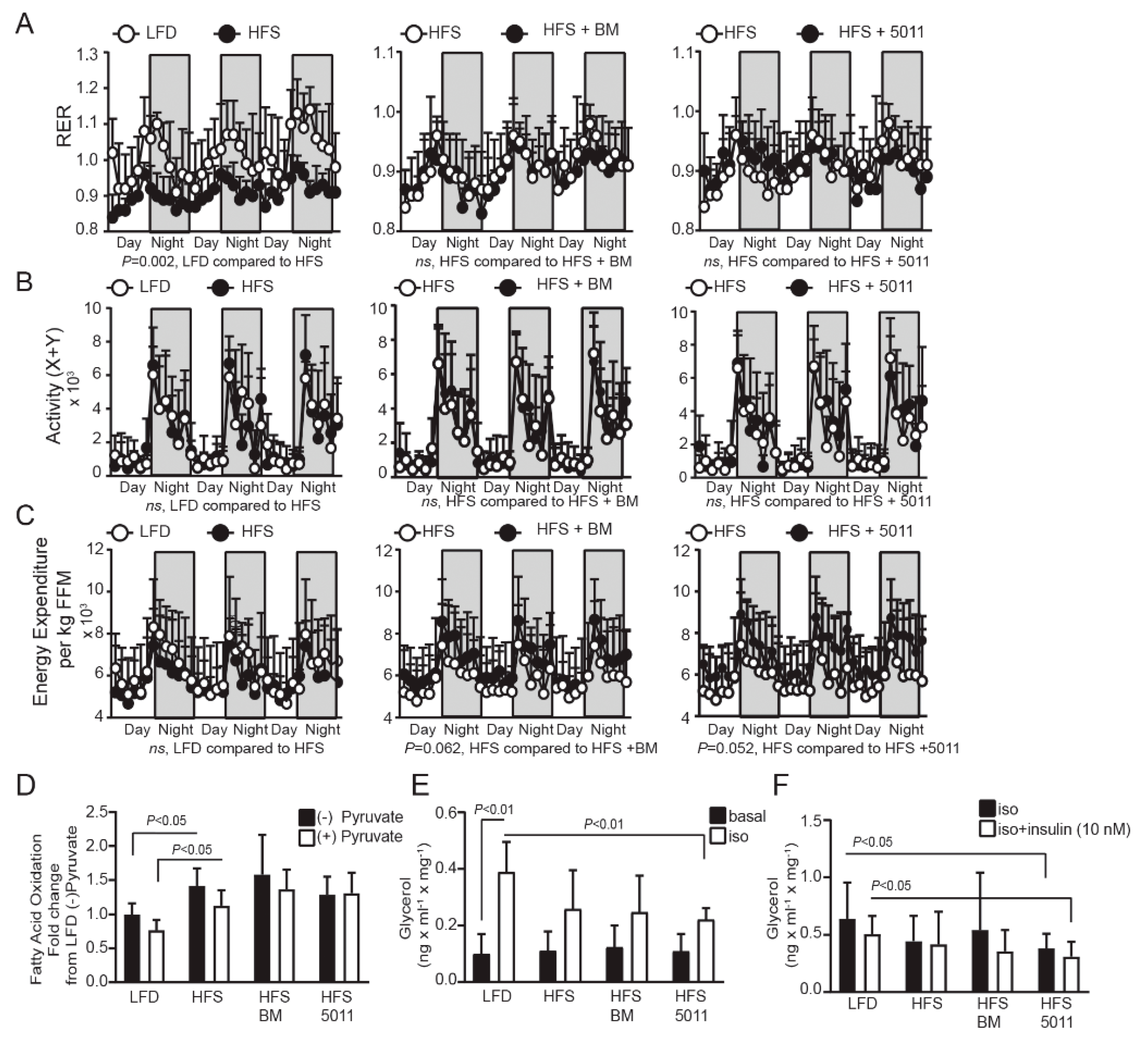
3.3. HFS Diet Increases Fatty Acid Oxidation as a Fuel Source but Reduces Skeletal Muscle Metabolic Flexibility—Botanical Supplementation Does Not Improve Substrate Switching Ability
3.4. Effect of the HFS Diet on Ex Vivo Measures of Lipolysis in Adipose Tissue
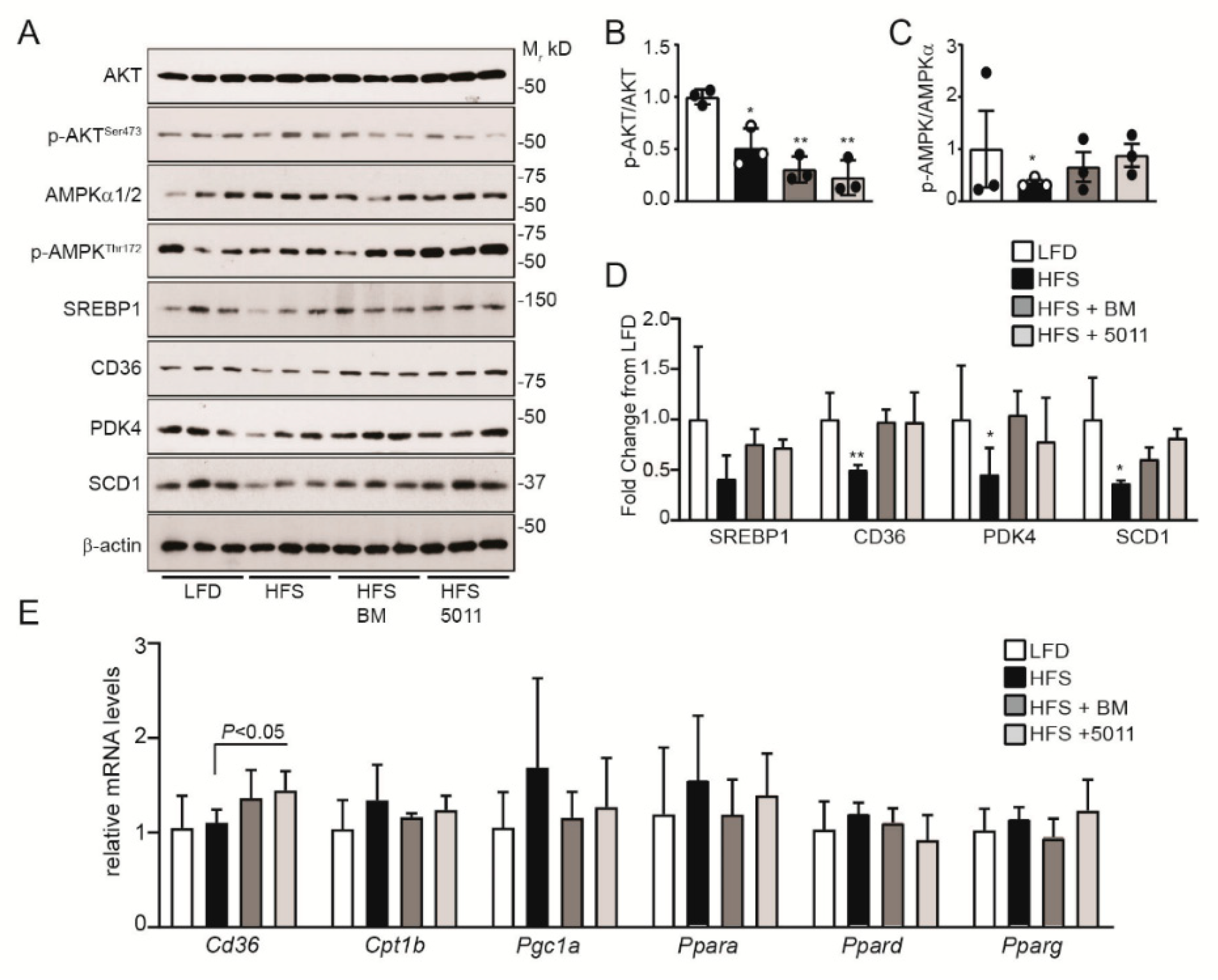
3.5. Effects of HFS Diet on Markers of Insulin Sensitivity and Fatty Acid Oxidation in Skeletal Muscle
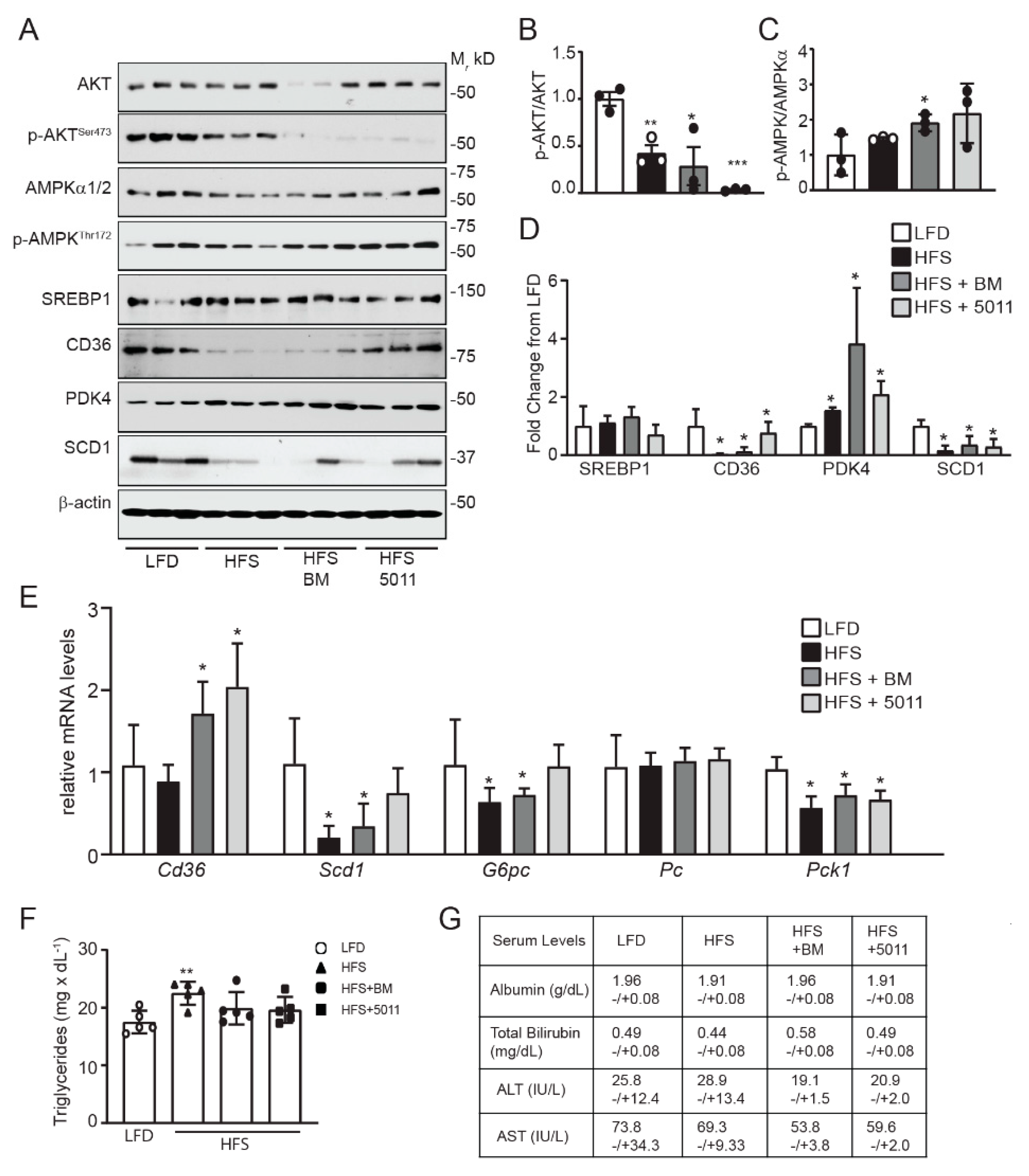
3.6. Effects of HFS Diet on Markers of Fatty Acid Oxidation and Insulin Sensitivity in the Liver
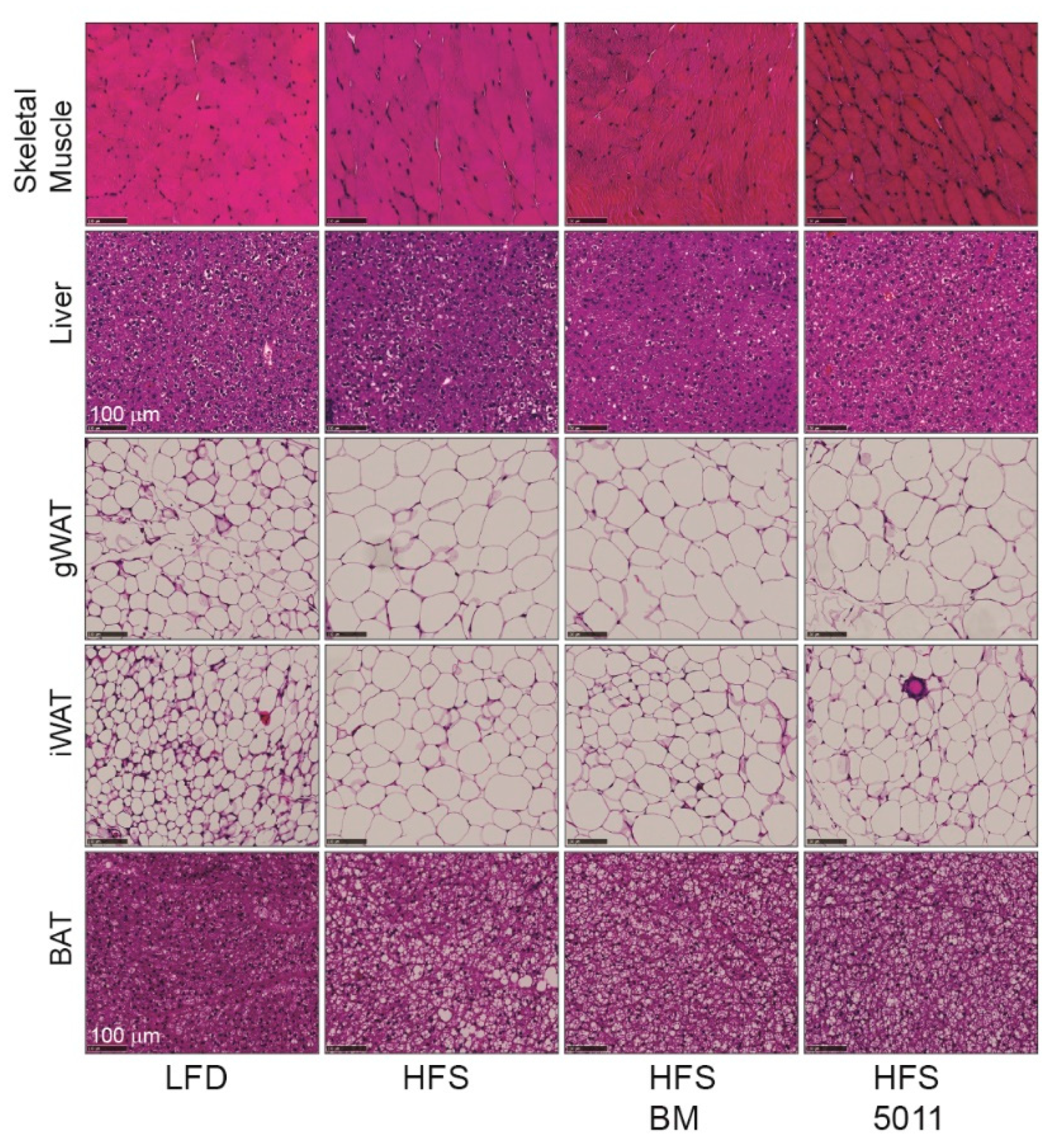
3.7. Effect of HFS Diet in Females on Lipid Accumulation in Skeletal Muscle, Liver, and Adipose Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary Intake and the Development of the Metabolic Syndrome. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Eckel Robert, H.; Grundy Scott, M.; Zimmet Paul, Z.; Cleeman James, I.; Donato Karen, A.; Fruchart, J.-C.; James, W.P.T.; Loria Catherine, M.; Smith Sidney, C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamiya, T.; Zaky, W.R.; Edmundowics, D.; Kadowaki, T.; Ueshima, H.; Kuller, L.H.; Sekikawa, A. World Health Organization-defined metabolic syndrome is a better predictor of coronary calcium than the adult treatment panel III criteria in American men aged 40–49 years. Diabetes Care 2004, 27, 2977–2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [Green Version]
- Sonnenberg, L.; Pencina, M.; Kimokoti, R.; Quatromoni, P.; Nam, B.H.; D’Agostino, R.; Meigs, J.B.; Ordovas, J.; Cobain, M.; Millen, B. Dietary patterns and the metabolic syndrome in obese and non-obese Framingham women. Obes. Res. 2005, 13, 153–162. [Google Scholar] [CrossRef]
- Wilson, P.W. Estimating cardiovascular disease risk and the metabolic syndrome: A Framingham view. Endocrinol. Metab. Clin. 2004, 33, 467–481. [Google Scholar] [CrossRef]
- Yang, Z.H.; Miyahara, H.; Takeo, J.; Katayama, M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol. Metab. Syndr. 2012, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Burchfield, J.G.; Kebede, M.A.; Meoli, C.C.; Stockli, J.; Whitworth, P.T.; Wright, A.L.; Hoffman, N.J.; Minard, A.Y.; Ma, X.; Krycer, J.R.; et al. High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice. J. Biol. Chem. 2018, 293, 5731–5745. [Google Scholar] [CrossRef] [Green Version]
- den Hartigh, L.J.; Wang, S.; Goodspeed, L.; Ding, Y.; Averill, M.; Subramanian, S.; Wietecha, T.; O’Brien, K.D.; Chait, A. Deletion of serum amyloid A3 improves high fat high sucrose diet-induced adipose tissue inflammation and hyperlipidemia in female mice. PLoS ONE 2014, 9, e108564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, R.L.; Gahche, J.J.; Miller, P.E.; Thomas, P.R.; Dwyer, J.T. Why US Adults Use Dietary SupplementsWhy US Adults Use Dietary Supplements. JAMA Internal Med. 2013, 173, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Obanda, D.N.; Ribnicky, D.M.; Raskin, I.; Cefalu, W.T. Bioactives of Artemisia dracunculus L. enhance insulin sensitivity by modulation of ceramide metabolism in rat skeletal muscle cells. Nutrition 2014, 30, S59–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheterpal, I.; Scherp, P.; Kelley, L.; Wang, Z.; Johnson, W.; Ribnicky, D.; Cefalu, W.T. Bioactives from Artemisia dracunculus L. enhance insulin sensitivity via modulation of skeletal muscle protein phosphorylation. Nutrition 2014, 30, S43–S51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Q.; Ribnicky, D.; Zhang, X.H.; Raskin, I.; Yu, Y.; Cefalu, W.T. Bioactives of Artemisia dracunculus L. enhance cellular insulin signaling in primary human skeletal muscle culture. Metabolism 2008, 57, S58–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Mendoza, T.; Ribnicky, D.; Poulev, A.; Noland, R.C.; Mynatt, R.L.; Raskin, I.; Cefalu, W.T.; Floyd, Z.E. An extract of Russian Tarragon Prevents Obesity-Related Ectopic Lipid Accumulation. Mol. Nutr. Food Res. 2018. [Google Scholar] [CrossRef]
- Han, J.H.; Tuan, N.Q.; Park, M.H.; Quan, K.T.; Oh, J.; Heo, K.S.; Na, M.; Myung, C.S. Cucurbitane Triterpenoids from the Fruits of Momordica Charantia Improve Insulin Sensitivity and Glucose Homeostasis in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2018, 62, e1700769. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Y.; Dong, Y. Bitter Melon Powder Protects against Obesity-associated Fatty Liver Disease by Improving Colonic Microenvironment in Rats with High-fat Diet-induced Obesity. Biomed. Environ. Sci. 2017, 30, 611–615. [Google Scholar] [CrossRef]
- Xu, J.; Cao, K.; Li, Y.; Zou, X.; Chen, C.; Szeto, I.M.; Dong, Z.; Zhao, Y.; Shi, Y.; Wang, J.; et al. Bitter gourd inhibits the development of obesity-associated fatty liver in C57BL/6 mice fed a high-fat diet. J. Nutr. 2014, 144, 475–483. [Google Scholar] [CrossRef]
- Shih, C.C.; Shlau, M.T.; Lin, C.H.; Wu, J.B. Momordica charantia ameliorates insulin resistance and dyslipidemia with altered hepatic glucose production and fatty acid synthesis and AMPK phosphorylation in high-fat-fed mice. Phytother. Res. 2014, 28, 363–371. [Google Scholar] [CrossRef]
- Matsui, S.; Yamane, T.; Takita, T.; Oishi, Y.; Kobayashi-Hattori, K. The hypocholesterolemic activity of Momordica charantia fruit is mediated by the altered cholesterol- and bile acid-regulating gene expression in rat liver. Nutr. Res. 2013, 33, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Yu, Y.; Mendoza, T.; Ribnicky, D.M.; Cefalu, W.T.; Floyd, Z.E. Potential adverse effects of botanical supplementation in high-fat-fed female mice. Biol. Sex Differ. 2018, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, B.; Ribnicky, D.M.; Poulev, A.; Logendra, S.; Cefalu, W.T.; Raskin, I. A natural history of botanical therapeutics. Metabolism 2008, 57, S3–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribnicky, D.M.; Poulev, A.; Watford, M.; Cefalu, W.T.; Raskin, I. Antihyperglycemic activity of Tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine 2006, 13, 550–557. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Ribnicky, D.; Zhang, X.H.; Zuberi, A.; Raskin, I.; Yu, Y.; Cefalu, W.T. An extract of Artemisia dracunculus L. enhances insulin receptor signaling and modulates gene expression in skeletal muscle in KK-A(y) mice. J. Nutr. Biochem. 2011, 22, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Zuberi, A.; Cefalu, W.T.; Raskin, I. Improved absorption and bioactivity of active compounds from an anti-diabetic extract of Artemisia dracunculus L. Int. J. Pharm. 2009, 370, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Zuberi, A.R. Strategies for assessment of botanical action on metabolic syndrome in the mouse and evidence for a genotype-specific effect of Russian tarragon in the regulation of insulin sensitivity. Metabolism 2008, 57, S10–S15. [Google Scholar] [CrossRef] [Green Version]
- Logendra, S.; Ribnicky, D.M.; Yang, H.; Poulev, A.; Ma, J.; Kennelly, E.J.; Raskin, I. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry 2006, 67, 1539–1546. [Google Scholar] [CrossRef]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef]
- Hulver, M.W.; Berggren, J.R.; Cortright, R.N.; Dudek, R.W.; Thompson, R.P.; Pories, W.J.; MacDonald, K.G.; Cline, G.W.; Shulman, G.I.; Dohm, G.L.; et al. Skeletal muscle lipid metabolism with obesity. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E741–E747. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mandarino, L.J. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.L.; Soeters, M.R.; Wust, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goris, A.H.; Westerterp, K.R. Postabsorptive respiratory quotient and food quotient-an analysis in lean and obese men and women. Eur. J. Clin. Nutr. 2000, 54, 546–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muoio, D.M.; Noland, R.C.; Kovalik, J.P.; Seiler, S.E.; Davies, M.N.; DeBalsi, K.L.; Ilkayeva, O.R.; Stevens, R.D.; Kheterpal, I.; Zhang, J.; et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012, 15, 764–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, J.M.; Azuma, K.; Kelley, C.; Pencek, R.; Radikova, Z.; Laymon, C.; Price, J.; Goodpaster, B.H.; Kelley, D.E. PET imaging reveals distinctive roles for different regional adipose tissue depots in systemic glucose metabolism in nonobese humans. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1134–E1141. [Google Scholar] [CrossRef] [Green Version]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-Function of CD36 and Importance of Fatty Acid Signal Transduction in Fat Metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, I.K.N.; Tusubira, D.; Ashrafi, H.; Dyrstad, S.E.; Hansen, L.; Liu, X.Z.; Nilsson, L.I.H.; Lovsletten, N.G.; Berge, K.; Wergedahl, H.; et al. Increased PDK4 mRNA expression is a sensitive marker of upregulated fatty acid oxidation. Mitochondrion 2019. [Google Scholar] [CrossRef]
- Zhang, S.; Hulver, M.W.; McMillan, R.P.; Cline, M.A.; Gilbert, E.R. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr. Metab. 2014, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- van der Heijden, R.A.; Sheedfar, F.; Morrison, M.C.; Hommelberg, P.P.; Kor, D.; Kloosterhuis, N.J.; Gruben, N.; Youssef, S.A.; de Bruin, A.; Hofker, M.H.; et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging (Albany NY) 2015, 7, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Terzo, S.; Caldara, G.F.; Ferrantelli, V.; Puleio, R.; Cassata, G.; Mule, F.; Amato, A. Pistachio Consumption Prevents and Improves Lipid Dysmetabolism by Reducing the Lipid Metabolizing Gene Expression in Diet-Induced Obese Mice. Nutrients 2018, 10, 1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flister, K.F.T.; Pinto, B.A.S.; Franca, L.M.; Coelho, C.F.F.; Dos Santos, P.C.; Vale, C.C.; Kajihara, D.; Debbas, V.; Laurindo, F.R.M.; Paes, A.M.A. Long-term exposure to high-sucrose diet down-regulates hepatic endoplasmic reticulum-stress adaptive pathways and potentiates de novo lipogenesis in weaned male mice. J. Nutr. Biochem. 2018, 62, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Dwyer, B.J.; Forbes, S.; Thiel, D.H.; Lewis, P.J.; Ramadori, G. Insulin Production and Resistance in Different Models of Diet-Induced Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2017, 18, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [Green Version]
- Bray, G.A. Fructose and risk of cardiometabolic disease. Curr. Atheroscler. Rep. 2012, 14, 570–578. [Google Scholar] [CrossRef]
- Halse, R.; Fryer, L.G.; McCormack, J.G.; Carling, D.; Yeaman, S.J. Regulation of glycogen synthase by glucose and glycogen: A possible role for AMP-activated protein kinase. Diabetes 2003, 52, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Choi, J.; Scafidi, S.; Wolfgang, M.J. Hepatic Fatty Acid Oxidation Restrains Systemic Catabolism during Starvation. Cell Rep. 2016, 16, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Hevener, A.L.; Clegg, D.J.; Mauvais-Jarvis, F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol. Cell. Endocrinol. 2015, 418 Pt 3, 306–321. [Google Scholar] [CrossRef] [Green Version]
- Hevener, A.L.; Zhou, Z.; Moore, T.M.; Drew, B.G.; Ribas, V. The impact of ERalpha action on muscle metabolism and insulin sensitivity—Strong enough for a man, made for a woman. Mol. Metab. 2018, 15, 20–34. [Google Scholar] [CrossRef]
- Paquette, A.; Chapados, N.A.; Bergeron, R.; Lavoie, J.M. Fatty acid oxidation is decreased in the liver of ovariectomized rats. Horm. Metab. Res. 2009, 41, 511–515. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, A.E.; Jun, S.; Gahche, J.J.; Tooze, J.A.; Dwyer, J.T.; Eicher-Miller, H.A.; Bhadra, A.; Guenther, P.M.; Potischman, N.; Dodd, K.W.; et al. Dietary Supplement Use Differs by Socioeconomic and Health-Related Characteristics among U.S. Adults, NHANES 2011–2014. Nutrients 2018, 10, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Giovannucci, E.; Rosner, B.; Willett, W.C.; Cho, E. Longitudinal and secular trends in dietary supplement use: Nurses’ Health Study and Health Professionals Follow-Up Study, 1986–2006. J. Acad. Nutr. Diet. 2014, 114, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Picciano, M.F.; McGuire, M.K. Use of dietary supplements by pregnant and lactating women in North America. Am. J. Clin. Nutr. 2009, 89, 663s–667s. [Google Scholar] [CrossRef] [Green Version]
- Kheterpal, I.; Coleman, L.; Ku, G.; Wang, Z.Q.; Ribnicky, D.; Cefalu, W.T. Regulation of insulin action by an extract of Artemisia dracunculus L. in primary human skeletal muscle culture: A proteomics approach. Phytother. Res. 2010, 24, 1278–1284. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, X.H.; Yu, Y.; Poulev, A.; Ribnicky, D.; Floyd, Z.E.; Cefalu, W.T. Bioactives from bitter melon enhance insulin signaling and modulate acyl carnitine content in skeletal muscle in high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 1064–1073. [Google Scholar] [CrossRef] [Green Version]
- Gollisch, K.S.; Brandauer, J.; Jessen, N.; Toyoda, T.; Nayer, A.; Hirshman, M.F.; Goodyear, L.J. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E495–E504. [Google Scholar] [CrossRef]
- Townsend, K.L.; Tseng, Y.H. Brown fat fuel utilization and thermogenesis. Trends Endocrinol. Metab. 2014, 25, 168–177. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuller, S.; Yu, Y.; Allerton, T.D.; Mendoza, T.; Ribnicky, D.M.; Floyd, Z.E. Adaptive Fat Oxidation Is Coupled with Increased Lipid Storage in Adipose Tissue of Female Mice Fed High Dietary Fat and Sucrose. Nutrients 2020, 12, 2233. https://doi.org/10.3390/nu12082233
Fuller S, Yu Y, Allerton TD, Mendoza T, Ribnicky DM, Floyd ZE. Adaptive Fat Oxidation Is Coupled with Increased Lipid Storage in Adipose Tissue of Female Mice Fed High Dietary Fat and Sucrose. Nutrients. 2020; 12(8):2233. https://doi.org/10.3390/nu12082233
Chicago/Turabian StyleFuller, Scott, Yongmei Yu, Timothy D. Allerton, Tamra Mendoza, David M. Ribnicky, and Z. Elizabeth Floyd. 2020. "Adaptive Fat Oxidation Is Coupled with Increased Lipid Storage in Adipose Tissue of Female Mice Fed High Dietary Fat and Sucrose" Nutrients 12, no. 8: 2233. https://doi.org/10.3390/nu12082233




