Assessment of Diet Quality and Adherence to Dietary Guidelines in Gastrointestinal Cancer Survivors: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
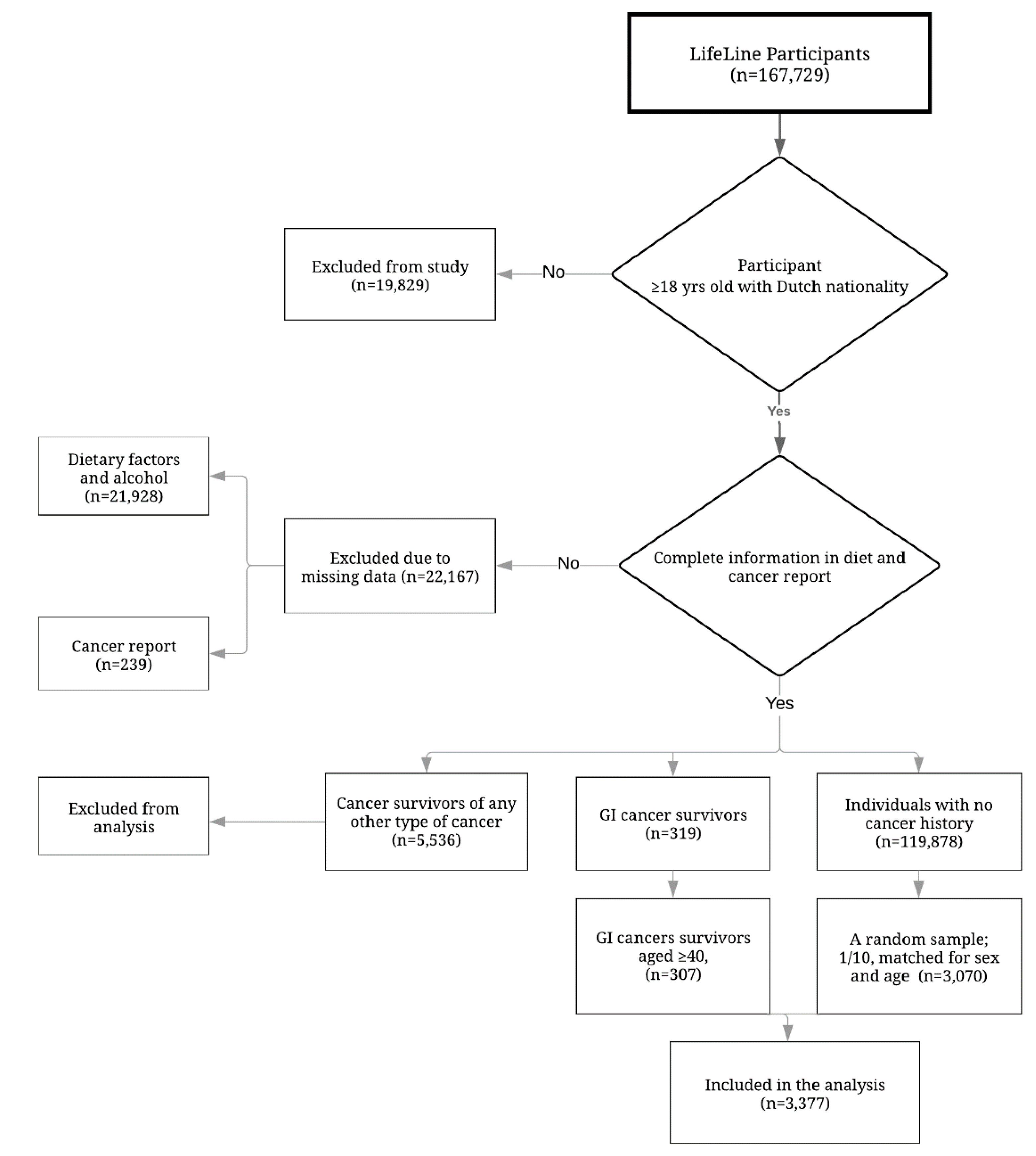
2.2. Study Population
2.3. Food Intake Assessment
Diet Quality Assessment
2.4. Covariates
2.5. Data Analysis
3. Results
4. Discussion
5. Study Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Missel, M.; Hansen, M.; Jackson, R.; Siemsen, M.; Schonau, M.N. Re-embodying eating after surgery for oesophageal cancer: Patients’ lived experiences of participating in an education and counselling nutritional intervention. J. Clin. Nurs. 2018, 27, 1420–1430. [Google Scholar] [CrossRef]
- Kotronoulas, G.; Papadopoulou, C.; Burns-Cunningham, K.; Simpson, M.; Maguire, R. A systematic review of the supportive care needs of people living with and beyond cancer of the colon and/or rectum. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2017, 29, 60–70. [Google Scholar] [CrossRef]
- Mehra, K.; Berkowitz, A.; Sanft, T. Diet, Physical Activity, and Body Weight in Cancer Survivorship. Med. Clin. N. Am. 2017, 101, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.A.; Berkowitz, Z.; Rodriguez, J.L. Awareness of Dietary and Alcohol Guidelines Among Colorectal Cancer Survivors. Am. J. Prev. Med. 2015, 49, S509–S517. [Google Scholar] [CrossRef] [PubMed]
- Sun, V.; Grant, M.; Wendel, C.S.; McMullen, C.K.; Bulkley, J.E.; Altschuler, A.; Ramirez, M.; Baldwin, C.M.; Herrinton, L.J.; Hornbrook, M.C.; et al. Dietary and Behavioral Adjustments to Manage Bowel Dysfunction After Surgery in Long-Term Colorectal Cancer Survivors. Ann. Surg. Oncol. 2015, 22, 4317–4324. [Google Scholar] [CrossRef] [PubMed]
- Mosher, C.E.; Sloane, R.; Morey, M.C.; Snyder, D.C.; Cohen, H.J.; Miller, P.E.; Demark-Wahnefried, W. Associations between lifestyle factors and quality of life among older long-term breast, prostate, and colorectal cancer survivors. Cancer 2009, 115, 4001–4009. [Google Scholar] [CrossRef] [PubMed]
- Vinke, P.C.; Corpeleijn, E.; Dekker, L.H.; Jacobs, D.R., Jr.; Navis, G.; Kromhout, D. Development of the food-based Lifelines Diet Score (LLDS) and its application in 129,369 Lifelines participants. Eur. J. Clin. Nutr. 2018, 72, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- LeMasters, T.J.; Madhavan, S.S.; Sambamoorthi, U.; Kurian, S. Health behaviors among breast, prostate, and colorectal cancer survivors: A US population-based case-control study, with comparisons by cancer type and gender. J. Cancer Surviv. Res. Pract. 2014, 8, 336–348. [Google Scholar] [CrossRef]
- Hawkes, A.L.; Lynch, B.M.; Youlden, D.R.; Owen, N.; Aitken, J.F. Health behaviors of Australian colorectal cancer survivors, compared with noncancer population controls. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2008, 16, 1097–1104. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, J.Y.; Meyerhardt, J.A. Diet and lifestyle in survivors of colorectal cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 1–27. [Google Scholar] [CrossRef]
- Kim, M.; Choi, K.S.; Suh, M.; Jun, J.K.; Chuck, K.W.; Park, B. Risky Lifestyle Behaviors among Gastric Cancer Survivors Compared with Matched Non-cancer Controls: Results from Baseline Result of Community Based Cohort Study. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2018, 50, 738–747. [Google Scholar] [CrossRef]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.; Dotinga, A.; Vonk, J.M.; van Dijk, F.; van Zon, S.K.; Wijmenga, C.; Wolffenbuttel, B.H.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015, 44, 1172–1180. [Google Scholar] [CrossRef]
- Molag, M.L.; de Vries, J.H.; Duif, N.; Ocke, M.C.; Dagnelie, P.C.; Goldbohm, R.A.; van’t Veer, P. Selecting informative food items for compiling food-frequency questionnaires: Comparison of procedures. Br. J. Nutr. 2010, 104, 446–456. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Elmadfa, I.; Djazayery, A.; Golalipour, M.J.; Sadighi, J.; Salehi, F.; Sadeghian Sharif, S. Efficacy of flour fortification with folic acid in women of childbearing age in Iran. Ann. Nutr. Metab. 2011, 58, 188–196. [Google Scholar] [CrossRef]
- Kromhout, D.; Spaaij, C.J.; de Goede, J.; Weggemans, R.M. The 2015 Dutch food-based dietary guidelines. Eur. J. Clin. Nutr. 2016, 70, 869–878. [Google Scholar] [CrossRef]
- Gopalakrishna, A.; Chang, A.; Longo, T.A.; Fantony, J.J.; Harrison, M.R.; Wischmeyer, P.E.; Inman, B.A. Dietary patterns and health-related quality of life in bladder cancer survivors. Urol. Oncol. 2018, 36, 469.e21–469.e29. [Google Scholar] [CrossRef]
- Zhang, F.F.; Liu, S.; John, E.M.; Must, A.; Demark-Wahnefried, W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer 2015, 121, 4212–4221. [Google Scholar] [CrossRef]
- Park, B.; Lee, J.; Kim, J. Imbalanced Nutrient Intake in Cancer Survivors from the Examination from the Nationwide Health Examination Center-Based Cohort. Nutrients 2018, 10, 212. [Google Scholar] [CrossRef]
- Guinter, M.A.; McCullough, M.L.; Gapstur, S.M.; Campbell, P.T. Associations of Pre- and Postdiagnosis Diet Quality with Risk of Mortality among Men and Women with Colorectal Cancer. J. Clin. Oncol. 2018. [Google Scholar] [CrossRef]
- Voortman, T.; Kiefte-de Jong, J.C.; Ikram, M.A.; Stricker, B.H.; van Rooij, F.J.A.; Lahousse, L.; Tiemeier, H.; Brusselle, G.G.; Franco, O.H.; Schoufour, J.D. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur. J. Epidemiol. 2017, 32, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Neuhouser, M.L.; Hedderson, M.M.; Schwartz, S.M.; Standish, L.J.; Bowen, D.J. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J. Am. Diet. Assoc. 2003, 103, 323–328. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Neuhouser, M.L.; Mayne, S.T.; Irwin, M.L.; Albanes, D.; Gail, M.H.; Alfano, C.M.; Bernstein, L.; McTiernan, A.; Reedy, J.; et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol. Biomark. 2010, 19, 2220–2228. [Google Scholar] [CrossRef]
- Tantoy, I.Y.; Cooper, B.A.; Dhruva, A.; Cataldo, J.; Paul, S.M.; Conley, Y.P.; Hammer, M.; Wright, F.; Dunn, L.B.; Levine, J.D.; et al. Changes in the Occurrence, Severity, and Distress of Symptoms in Patients with Gastrointestinal Cancers Receiving Chemotherapy. J. Pain Symptom Manag. 2018, 55, 808–834. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.; Black, A.E. Markers of the validity of reported energy intake. J. Nutr. 2003, 133, 895S–920S. [Google Scholar] [CrossRef] [PubMed]

| Characteristics 2 | Reference Population (n = 3070) | GI Cancer Survivors (n = 307) | ||
|---|---|---|---|---|
| n (%) | LLD Score Mean (SE) | n (%) | LLD Score Mean (SE) | |
| Age at inclusion (years) | ||||
| <55 | 878 (28.60) | 24.31 (0.25) | 76 (24.80) | 24.39 (0.68) |
| ≥55 | 2192 (71.40) | 26.56 (0.10) | 231 (75.20) | 26.15 (0.38) |
| p-value | <0.001 | <0.01 | ||
| Sex | ||||
| Men | 1560 (50.80) | 24.75 (0.14) | 155 (50.80) | 24.85 (0.44) |
| Women | 1510 (49.20) | 27.13 (0.15) | 151 (49.20) | 26.60 (0.50) |
| p-value | <0.001 | <0.01 | ||
| Comorbid chronic disease 3 | ||||
| No | 875 (28.50) | 25.92 (0.20) | 77 (25.10) | 25.79 (0.65) |
| Yes | 2195 (71.50) | 25.92 (0.12) | 230 (74.90) | 25.69 (0.39) |
| p-value | 0.98 | >0.05 | ||
| Educational level | ||||
| Low | 1472 (48.50) | 25.80 (0.15) | 162 (53.10) | 25.09 (0.47) |
| Medium | 829 (27.30) | 25.37 (0.21) | 61 (20.00) | 25.80 (0.71) |
| High | 736 (24.20) | 26.72 (0.21) | 85 (26.90) | 27.10 (0.63) |
| p-value | <0.001 | <0.05 | ||
| Smoking | ||||
| Never | 1115 (35.90) | 26.32 (0.17) | 91 (29.80) | 25.80 (0.63) |
| Former | 1510 (50.20) | 26.37 (0.14) | 175 (57.40) | 25.82 (0.44) |
| Current | 426 (13.90) | 23.31 (0.30) | 39 (12.80) | 24.97 (0.98) |
| p-value | <0.001 | >0.05 | ||
| Alcohol consumption | ||||
| <10 g/day | 789 (25.70) | 26.04 (0.21) | 90 (23.90) | 27.06 (0.62) |
| ≥10 g/da | 2281 (74.30) | 25.88 (0.12) | 217 (70.70) | 25.15 (0.39) |
| p-value | 0.06 | <0.01 | ||
| Categorized BMI (kg/m2) | ||||
| <25 | 1028 (35.40) | 25.60 (0.18) | 104 (13.70) | 26.24 (0.57) |
| 25 ≤ BMI < 30 | 1486 (48.50) | 26.45 (0.14) | 139 (36.80) | 25.67 (0.48) |
| ≥30 | 553 (18.00) | 25.42 (0.26) | 64 (49.50) | 24.95 (0.79) |
| p-value | <0.001 | >0.05 | ||
| Physical activity (min/week) | ||||
| <150 | 1259 (41.00) | 24.85 (0.17) | 124 (40.40) | 25.13 (0.55) |
| ≥150 | 1811 (59.00) | 26.66 (0.13) | 183 (59.60) | 26.10 (0.42) |
| p-value | <0.001 | >0.05 | ||
| Sedentary Behavior 4 | ||||
| No | 2452 (79.90) | 25.70 (0.19) | 60 (19.50) | 26.53 (0.70) |
| Yes | 618 (20.10) | 26.76 (0.23) | 247 (80.50) | 25.51 (0.39) |
| p-value | <0.001 | >0.05 | ||
| Study Population | Individuals with High Diet Quality (%) | Unadjusted Analysis | Adjusted Analysis |
|---|---|---|---|
| OR 2 (95% CI) | OR (95% CI) | ||
| Reference population | 60.10 | ref | ref |
| GI cancer survivors | 59.60 | 0.98 (0.77 to 1.24) | 0.97 (0.73 to 1.23) |
| Reference population | 60.10 | ref | ref |
| GI survivors (stratified by time since diagnosis) | |||
| ≤4 years | 61.40 | 1.05 (0.74 to 1.50) | 1.12 (0.77 to 1.61) |
| 5–9 years | 55.20 | 0.82 (0.53 to 1.25) | 0.81 (0.52 to 1.26) |
| ≥10 years | 61.40 | 1.05 (0.68 to 1.62) | 0.93 (0.60 to 1.46) |
| Reference population | 60.10 | ref | ref |
| GI cancer survivors (stratified by tumor site) | |||
| CRC | 58.30 | 1.04 (0.80 to 1.36) | 1.05 (0.80 to 1.38) |
| Other GI tract cancer | 61.50 | 0.80 (0.48 to 1.29) | 0.71 (0.42 to 1.18) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moazzen, S.; Cortés-Ibañez, F.O.; van Leeuwen, B.L.; Alizadeh, B.Z.; de Bock, G.H. Assessment of Diet Quality and Adherence to Dietary Guidelines in Gastrointestinal Cancer Survivors: A Cross-Sectional Study. Nutrients 2020, 12, 2232. https://doi.org/10.3390/nu12082232
Moazzen S, Cortés-Ibañez FO, van Leeuwen BL, Alizadeh BZ, de Bock GH. Assessment of Diet Quality and Adherence to Dietary Guidelines in Gastrointestinal Cancer Survivors: A Cross-Sectional Study. Nutrients. 2020; 12(8):2232. https://doi.org/10.3390/nu12082232
Chicago/Turabian StyleMoazzen, Sara, Francisco O. Cortés-Ibañez, Barbara L. van Leeuwen, Behrooz Z. Alizadeh, and Geertruida H. de Bock. 2020. "Assessment of Diet Quality and Adherence to Dietary Guidelines in Gastrointestinal Cancer Survivors: A Cross-Sectional Study" Nutrients 12, no. 8: 2232. https://doi.org/10.3390/nu12082232
APA StyleMoazzen, S., Cortés-Ibañez, F. O., van Leeuwen, B. L., Alizadeh, B. Z., & de Bock, G. H. (2020). Assessment of Diet Quality and Adherence to Dietary Guidelines in Gastrointestinal Cancer Survivors: A Cross-Sectional Study. Nutrients, 12(8), 2232. https://doi.org/10.3390/nu12082232





