Controlling Avian Influenza Virus in Bangladesh: Challenges and Recommendations
Abstract
:1. Introduction
2. Ecology and Epidemiology of AIVs in Bangladesh
2.1. Geographical and Ecological Frameworks
2.2. Poultry Rearing Systems and Trading Chains
2.3. Virus Transmission and Risk Factors
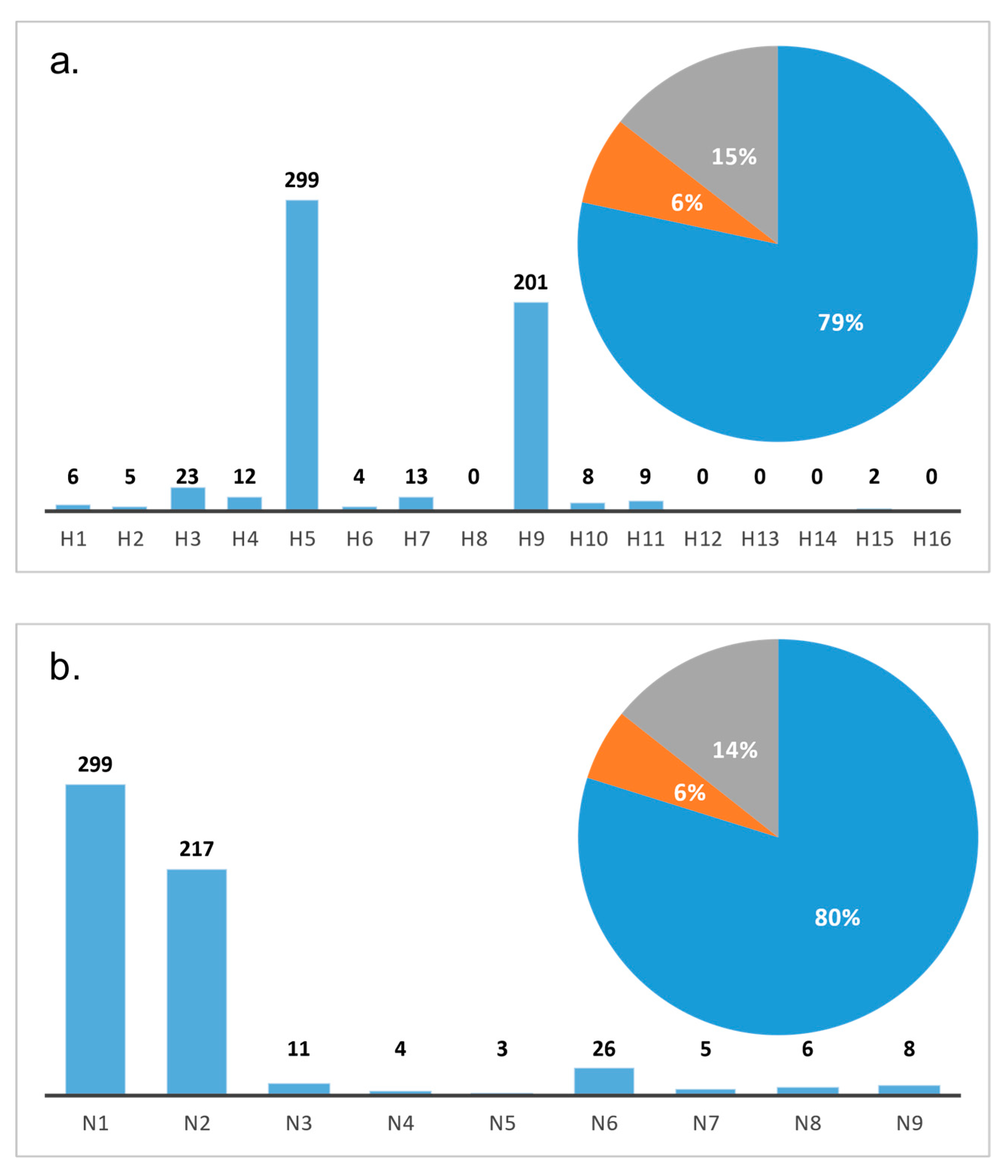
3. Overview of AIV Sub- and Pathotypes in Bangladesh
3.1. HPAI Viruses
3.2. LPAI Viruses
3.3. Zoonotic Transmission
4. Towards Effective Control and Prevention
- Improving biosecurity:
- -
- Biosecurity and compliance levels must be harmonized, monitored, and enforced.
- -
- Training and awareness for all level of stakeholders, including managers, farmers, and traders; training courses on biosecurity, rapid outbreak response, communication programs.
- -
- Regulations for small scale commercial and backyard poultry under avian influenza vaccination, including community engagement in biosecurity programs, e.g., households have to be encouraged to keep backyard chickens and ducks in separate night shelters.
- Tightening of trading control and biosecurity at LBMs:
- -
- Reduce inter-district/inter-regional poultry trading through promotion of regional trading.
- -
- Avoid mixing of birds from different sources and days. Birds of different species have to be kept separately and isolated.
- -
- Enhanced disease control at the sources, including upgrading poultry wholesale markets and slaughterhouses and further processing of poultry and selling in the supermarkets, which may help in the gradual phasing out of LBMs.
- -
- Encourage to wear personal protective equipment when working at LBMs, regular cleaning and disinfection (C&D) of the market area, consider decreeing market closure days once per week for thorough C&D.
- -
- Avoid mixing of wild migratory birds and domestic ducks during winter season and spring.
- General:
- -
- Monitoring both H5N1 and H9N2 vaccination across the country by the government regulatory authority through their field service network.
- -
- Updating response policy to H5N1 outbreaks in the context of vaccination programs.
- -
- Increasing medium- and long-term capacities of the veterinary and public health systems to strengthen the emergency response to disease outbreaks.
- -
- Promoting the “One Health” approach to ensure cross sector coordination in control of AI outbreaks.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Knipe, D.M.; Howley, P.M.; Fields, B.N. Fields Virology, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Chapter 47; pp. 1647–1689. [Google Scholar]
- Alexander, D.J. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis. 2007, 51, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Yuen, K.Y. Avian influenza virus infections in humans. Chest 2006, 129, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Herfst, S.; Imai, M.; Kawaoka, Y.; Fouchier, R.A. Avian influenza virus transmission to mammals. Curr. Top. Microbiol. Immunol. 2014, 385, 137–155. [Google Scholar] [CrossRef]
- Ghedin, E.; Sengamalay, N.A.; Shumway, M.; Zaborsky, J.; Feldblyum, T.; Subbu, V.; Spiro, D.J.; Sitz, J.; Koo, H.; Bolotov, P.; et al. Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature 2005, 437, 1162–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Jagger, B.W.; Wise, H.M.; Kash, J.C.; Walters, K.A.; Wills, N.M.; Xiao, Y.L.; Dunfee, R.L.; Schwartzman, L.M.; Ozinsky, A.; Bell, G.L.; et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 2012, 337, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Fouchier, R.A.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef] [Green Version]
- OIE. Influenza A Cleavage Site; World Organization for Animal Health (OIE): Paris, France, 2014; Available online: https://www.oie.int/doc/ged/D13484.PDF (accessed on 2 May 2020).
- Böttcher-Friebertshäuser, E.; Garten, W.; Matrosovich, M.; Klenk, H.D. The hemagglutinin: A determinant of pathogenicity. Curr. Top. Microbiol. 2014, 385, 3–34. [Google Scholar] [CrossRef]
- Alexander, D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000, 74, 3–13. [Google Scholar] [CrossRef]
- Guo, Y.J.; Krauss, S.; Senne, D.A.; Mo, I.P.; Lo, K.S.; Xiong, X.P.; Norwood, M.; Shortridge, K.F.; Webster, R.G.; Guan, Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology 2000, 267, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Webster, R.G. Molecular characterization of H9N2 influenza viruses: Were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 1999, 96, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Suarez, D.L.; Perdue, M.L.; Cox, N.; Rowe, T.; Bender, C.; Huang, J.; Swayne, D.E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J. Virol. 1998, 72, 6678–6688. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.T.; Wang, J.; Shen, Y.; Zhou, B.; Duan, L.; Cheung, C.L.; Ma, C.; Lycett, S.J.; Leung, C.Y.; Chen, X.; et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013, 502, 241–244. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.J.; Donis, R.O. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- Pusch, E.A.; Suarez, D.L. The Multifaceted Zoonotic Risk of H9N2 Avian Influenza. Vet. Sci. 2018, 5, 82. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Q.; Wang, S.; Liu, S.; Hou, G.; Li, J.; Jiang, W.; Wang, K.; Peng, C.; Liu, D.; Guo, A.; et al. Diversity and distribution of type A influenza viruses: An updated panorama analysis based on protein sequences. Virol. J. 2019, 16, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.S.; Ersbøll, A.K.; Biswas, P.K.; Christensen, J.P.; Hannan, A.S.; Toft, N. Ecological determinants of highly pathogenic avian influenza (H5N1) outbreaks in Bangladesh. PLoS ONE 2012, 7, e33938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Barua, H.; Das, A.; Rahman, M.H.; Giasuddin, M.; Hannan, A.S.; Habib, M.A.; Ahad, A.; et al. Avian influenza outbreaks in chickens, Bangladesh. Emerg. Infect. Dis. 2008, 14, 1909–1912. [Google Scholar] [CrossRef]
- Barman, S.; Marinova-Petkova, A.; Hasan, M.K.; Akhtar, S.; El-Shesheny, R.; Turner, J.C.; Franks, J.; Walker, D.; Seiler, J.; Friedman, K.; et al. Role of domestic ducks in the emergence of a new genotype of highly pathogenic H5N1 avian influenza A viruses in Bangladesh. Emerg. Microbes Infect. 2017, 6, e72. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Chowdury, S.; Hodges, E.; Rahman, M.Z.; Jang, Y.; Hossain, M.E.; Jones, J.; Stark, T.J.; Di, H.; Cook, P.W.; et al. Detection of highly pathogenic avian influenza A(H5N6) viruses in waterfowl in Bangladesh. Virology 2019, 534, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jannat, N.; Chowdhury, E.H.; Parvin, R.; Begum, J.A.; Khan, M.A.H.N.A.; Islam, M.R. Investigation of an outbreak of low pathogenic avian influenza in poultry in Bangladesh. Int. J. Livest. Res. 2013, 3, 21–32. [Google Scholar]
- Parvin, R.; Heenemann, K.; Halami, M.Y.; Chowdhury, E.H.; Islam, M.R.; Vahlenkamp, T.W. Full-genome analysis of avian influenza virus H9N2 from Bangladesh reveals internal gene reassortments with two distinct highly pathogenic avian influenza viruses. Arch. Virol. 2014, 159, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Sarker, R.D.; Giasuddin, M.; Chowdhury, E.H.; Islam, M.R. Serological and virological surveillance of avian influenza virus in domestic ducks of the north-east region of Bangladesh. BMC Vet. Res. 2017, 13, 180. [Google Scholar] [CrossRef] [Green Version]
- Rimi, N.A.; Hassan, M.Z.; Chowdhury, S.; Rahman, M.; Sultana, R.; Biswas, P.K.; Debnath, N.C.; Islam, S.S.; Ross, A.G. A Decade of Avian Influenza in Bangladesh: Where Are We Now? Trop. Med. Infect. Dis. 2019, 4, 119. [Google Scholar] [CrossRef] [Green Version]
- Khatun, A.; Giasuddin, M.; Islam, K.M.; Khanom, S.; Samad, M.A.; Islam, M.R.; Noor, M.; Bhuiyan, J.U.; Kim, W.I.; Eo, S.K.; et al. Surveillance of avian influenza virus type A in semi-scavenging ducks in Bangladesh. BMC Vet. Res. 2013, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Parvin, R.; Kabiraj, C.K.; Mumu, T.T.; Chowdhury, E.H.; Islam, M.R.; Beer, M.; Harder, T. Active virological surveillance in backyard ducks in Bangladesh: Detection of avian influenza and gammacoronaviruses. Avian Pathol. 2020, 1–8. [Google Scholar] [CrossRef]
- Parvin, R.; Kamal, A.H.; Haque, M.E.; Chowdhury, E.H.; Giasuddin, M.; Islam, M.R.; Vahlenkamp, T.W. Genetic characterization of highly pathogenic H5N1 avian influenza virus from live migratory birds in Bangladesh. Virus Genes 2014, 49, 438–448. [Google Scholar] [CrossRef]
- Takekawa, J.Y.; Prosser, D.J.; Collins, B.M.; Douglas, D.C.; Perry, W.M.; Yan, B.; Ze, L.; Hou, Y.; Lei, F.; Li, T.; et al. Movements of wild ruddy shelducks in the Central Asian Flyway and their spatial relationship to outbreaks of highly pathogenic avian influenza H5N1. Viruses 2013, 5, 2129–2152. [Google Scholar] [CrossRef]
- Palm, E.C.; Newman, S.H.; Prosser, D.J.; Xiao, X.; Ze, L.; Batbayar, N.; Balachandran, S.; Takekawa, J.Y. Mapping migratory flyways in Asia using dynamic Brownian bridge movement models. Mov. Ecol. 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Lepage, D. Avibase. 2015. Available online: http://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=bd&list=clements (accessed on 12 May 2020).
- Marinova-Petkova, A.; Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Hasan, M.K.; Akhtar, S.; Turner, J.; Walker, D.; Seiler, P.; Franks, J.; et al. The Continuing Evolution of H5N1 and H9N2 Influenza Viruses in Bangladesh Between 2013 and 2014. Avian Dis. 2016, 60, 108–117. [Google Scholar] [CrossRef]
- Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Smith, G.J.; Fourment, M.; Walker, D.; McClenaghan, L.; Alam, S.M.; Hasan, M.K.; Seiler, P.; et al. Antigenic and molecular characterization of avian influenza A(H9N2) viruses, Bangladesh. Emerg. Infect. Dis. 2013, 19, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, N.A.; Khan, S.U.; Zanders, N.; Balish, A.; Haider, N.; Islam, A.; Chowdhury, S.; Rahman, M.Z.; Haque, A.; Hosseini, P.; et al. Genetically Diverse Low Pathogenicity Avian Influenza A Virus Subtypes Co-Circulate among Poultry in Bangladesh. PLoS ONE 2016, 11, e0152131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.U.; Gurley, E.S.; Gerloff, N. Avian influenza surveillance in domestic waterfowl and environment of live bird markets in Bangladesh, 2007–2012. Sci. Rep. 2018, 8, 9396. [Google Scholar] [CrossRef] [PubMed]
- Dolberg, F. Poultry Sector Country Overview: Bangladesh. FAO Animal Production and Health Division, 2008. Available online: http://www.fao.org/3/a-ai319e.pdf (accessed on 2 May 2020).
- The World Bank. Agriculture & Rural Development. 2013. Available online: http://data.worldbank.org/topic/agriculture-and-rural-development?display=graph (accessed on 12 April 2020).
- Sultana, R.; Nahar, N.; Rimi, N.A.; Azad, S.; Islam, M.S.; Gurley, E.S.; Luby, S.P. Backyard poultry raising in Bangladesh: A valued resource for the villagers and a setting for zoonotic transmission of avian influenza. A qualitative study. Rural Remote Health 2012, 12, 1927. [Google Scholar] [PubMed]
- FAO. Comparative Performance of Sonali Chickens, Commercial Broilers, Layersand Local Non-descript (deshi) Chickens in Selected Areas of Bangladesh; Food and Agriculture Organization (FAO): Rome, Italy, 2015; Available online: www.fao.org/3/a-i4725e.pdf (accessed on 10 April 2020).
- Bhuiyan, A.K.F.H.; Bhuiyan, M.S.A.; Deb, G.K. Indigenous chicken genetic resources in Bangladesh: Current status and future outlook. Anim. Genet. Resour. Inf. 2005, 36, 73–84. [Google Scholar] [CrossRef]
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Barua, H.; Das, A.; Rahman, M.H.; Giasuddin, M.; Hannan, A.S.; Habib, A.M.; Debnath, N.C. Risk factors for infection with highly pathogenic influenza A virus (H5N1) in commercial chickens in Bangladesh. Vet. Rec. 2009, 164, 743–746. [Google Scholar] [CrossRef]
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Das, A.; Rahman, M.H.; Barua, H.; Giasuddin, M.; Hannan, A.S.; Habib, M.A.; Debnath, N.C. Risk for infection with highly pathogenic avian influenza virus (H5N1) in backyard chickens, Bangladesh. Emerg. Infect. Dis. 2009, 15, 1931–1936. [Google Scholar] [CrossRef]
- Huque, K.; Khan, M. Socio-geographic distribution of livestock and poultry in Bangladesh—A review. Bangladesh J. Anim. Sci. 2017, 46, 65–81. [Google Scholar] [CrossRef]
- DLS. Livestock Economy at a Glance, Government of Bangladesh. Department of Livestock Services (DLS), Bangladesh. 2018. Available online: http://dls.portal.gov.bd/sites/default/files/files/dls.portal.gov.bd/page/ee5f4621_fa3a_40ac_8bd9_898fb8ee4700/Livestock%20Economy%20at%20a%20glance%20%20%282017–2018%29.pdf (accessed on 24 April 2020).
- Parvin, R.; Begum, J.A.; Nooruzzaman, M.; Chowdhury, E.H.; Islam, M.R.; Vahlenkamp, T.W. Review analysis and impact of co-circulating H5N1 and H9N2 avian influenza viruses in Bangladesh. Epidemiol. Infect. 2018, 146, 1259–1266. [Google Scholar] [CrossRef]
- Alam, J.; Giasuddin, M.; Samad, M.A.; Taimur, M.J.F.A. Recent evidence of Avian Influenza in Bangladesh: A review. World Poult. Sci. J. 2010, 66, 455–464. [Google Scholar] [CrossRef]
- Kim, S.H. Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt. Viruses 2018, 10, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.C.; Feeroz, M.M.; Hasan, M.K.; Akhtar, S.; Walker, D.; Seiler, P.; Barman, S.; Franks, J.; Jones-Engel, L.; McKenzie, P.; et al. Insight into live bird markets of Bangladesh: An overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg. Microbes Infect. 2017, 6, e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyen, N.; Ahmed, G.; Gupta, S.; Tenzin, T.; Khan, R.; Khan, T.; Debnath, N.; Yamage, M.; Pfeiffer, D.U.; Fournie, G. A large-scale study of a poultry trading network in Bangladesh: Implications for control and surveillance of avian influenza viruses. BMC Vet. Res. 2018, 14, 12. [Google Scholar] [CrossRef] [Green Version]
- Nasreen, S.; Khan, S.U.; Luby, S.P.; Gurley, E.S.; Abedin, J.; Zaman, R.U.; Sohel, B.M.; Rahman, M.; Hancock, K.; Levine, M.Z.; et al. Highly pathogenic Avian Influenza A(H5N1) virus infection among workers at live bird markets, Bangladesh, 2009–2010. Emerg. Infect. Dis. 2015, 21, 629–637. [Google Scholar] [CrossRef]
- FAO. Approaches to Controlling, Preventing and Eliminating H5N1 Highly Pathogenic Avian Influenza in Endemic Countries. 2011. Available online: http://www.fao.org/3/i2150e/i2150e00.htm (accessed on 12 March 2020).
- Killingley, B.; Nguyen-Van-Tam, J. Routes of influenza transmission. Influenza Other Respir. Viruses 2013, 7, 42–51. [Google Scholar] [CrossRef]
- Peacock, T.H.P.; James, J.; Sealy, J.E.; Iqbal, M. A Global Perspective on H9N2 Avian Influenza Virus. Viruses 2019, 11, 620. [Google Scholar] [CrossRef] [Green Version]
- Parvin, R.; Begum, J.A.; Chowdhury, E.H.; Islam, M.R. Co-subsistence of avian influenza virus subtypes of low and high pathogenicity in Bangladesh: Challenges for diagnosis, risk assessment and control. Sci. Rep. 2019, 9, 8306. [Google Scholar] [CrossRef] [Green Version]
- Rimi, N.A.; Sultana, R.; Muhsina, M.; Uddin, B.; Haider, N.; Nahar, N.; Zeidner, N.; Sturm-Ramirez, K.; Luby, S.P. Biosecurity Conditions in Small Commercial Chicken Farms, Bangladesh 2011–2012. Ecohealth 2017, 14, 244–258. [Google Scholar] [CrossRef]
- Sarker, B.C.; Alam, M.A.; Rahman, M.M.; Islam, A.F.M.T.; Chowdhury, M.G.F. Waste management of commercial poultry farms in Bangladesh. J. Innov. Dev. Strateg. 2009, 3, 34–37. [Google Scholar]
- Biswas, P.K.; Giasuddin, M.; Nath, B.K.; Islam, M.Z.; Debnath, N.C.; Yamage, M. Biosecurity and Circulation of Influenza A (H5N1) Virus in Live-Bird Markets in Bangladesh, 2012. Transbound. Emerg. Dis. 2017, 64, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Berman, L.; Haider, N.; Gerloff, N.; Rahman, M.Z.; Shu, B.; Rahman, M.; Dey, T.K.; Davis, T.C.; Das, B.C.; et al. Investigating a crow die-off in January-February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Arch. Virol. 2014, 159, 509–518. [Google Scholar] [CrossRef] [PubMed]
- SAPPLPP. Combating Bird Flu through Bio-security Measures at Farm and Community Level: Evidence from Bangladesh; Good Practice Note: Delhi, India, 2010; Available online: http://sapplpp.org/publications/good-practice-notes-briefs/small-holder-poultry/BDGP03-combating-bird-flu-through-bio-security-measures.html#.XvRGsigzbIU (accessed on 24 March 2020).
- Sultana, R.; Rimi, N.A.; Azad, S.; Islam, M.S.; Khan, M.S.; Gurley, E.S.; Nahar, N.; Luby, S.P. Bangladeshi backyard poultry raisers’ perceptions and practices related to zoonotic transmission of avian influenza. J. Infect. Dev. Countr. 2012, 6, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Rimi, N.A.; Sultana, R.; Ishtiak-Ahmed, K.; Rahman, M.Z.; Hasin, M.; Islam, M.S.; Azziz-Baumgartner, E.; Nahar, N.; Gurley, E.S.; Luby, S.P. Understanding the failure of a behavior change intervention to reduce risk behaviors for avian influenza transmission among backyard poultry raisers in rural Bangladesh: A focused ethnography. BMC Public Health 2016, 16, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popy, F.Y.; Chowdhury, Q.; Alam, S.; Roy, S.; Dipta, P.M.; Ahmed, J. Backyard Poultry Management and Production System at Barlekha Upazila, Moulvibazar, Bangladesh. Int. J. Sci. Bus. 2018, 2, 90–100. [Google Scholar]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Shanta, I.S.; Hasnat, M.A.; Zeidner, N.; Gurley, E.S.; Azziz-Baumgartner, E.; Sharker, M.A.Y.; Hossain, K.; Khan, S.U.; Haider, N. Raising Backyard Poultry in Rural Bangladesh: Financial and Nutritional Benefits, but Persistent Risky Practices. Transbound. Emerg. Dis. 2017, 64, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Rimi, N.A.; Sultana, R.; Ishtiak-Ahmed, K.; Haider, N.; Azziz-Baumgartner, E.; Nahar, N.; Luby, S.P. Where backyard poultry raisers seek care for sick poultry: Implications for avian influenza prevention in Bangladesh. BMC Public Health 2018, 18, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, N.; Akhter, M.; Mamun, S.; Chowdhury, E.H.; Das, P.M. Bio-security in small scale poultry farms against avian influenza: Knowledge, attitude and practices. Asian J. Med. Biol. Res. 2016, 1, 670–676. [Google Scholar] [CrossRef]
- Islam, M.; Huque, Q. Practices of bio-security in small-scale broiler farms. Bangladesh Vet. 2007, 24, 72–78. [Google Scholar]
- Osmani, M.G.; Ward, M.P.; Giasuddin, M.; Islam, M.R.; Kalam, A. The spread of highly pathogenic avian influenza (subtype H5N1) clades in Bangladesh, 2010 and 2011. Prev. Vet. Med. 2014, 114, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.P.; Tardif-Douglin, D.; Ryan-Silva, R.; Magnani, R. Controlling highly pathogenic avian influenza, Bangladesh. Emerg. Infect. Dis. 2012, 18, 2083–2085. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.A.; Smallwood, C.; Imam, T.; Mahmud, R.; Hasan, R.B.; Hasan, M.; Anwer, M.S.; Rashid, M.H.; Hoque, M.A. Assessment of hygienic conditions of live bird markets on avian influenza in Chittagong metro, Bangladesh. Prev. Vet. Med. 2017, 142, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Nooruzzaman, M.; Haque, M.E.; Chowdhury, E.H.; Islam, M.R. Pathology of clade 2.3.2.1 avian influenza virus (H5N1) infection in quails and ducks in Bangladesh. Avian Pathol. 2019, 48, 73–79. [Google Scholar] [CrossRef]
- Nooruzzaman, M.; Mumu, T.T.; Hasnat, A.; Akter, M.N.; Rasel, M.S.U.; Rahman, M.M.; Parvin, R.; Begum, J.A.; Chowdhury, E.H.; Islam, M.R. A new reassortant clade 2.3.2.1a H5N1 highly pathogenic avian influenza virus causing recent outbreaks in ducks, geese, chickens and turkeys in Bangladesh. Transbound. Emerg. Dis. 2019, 66, 2120–2133. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.E.; Giasuddin, M.; Chowdhury, E.H.; Islam, M.R. Molecular evolution of H5N1 highly pathogenic avian influenza viruses in Bangladesh between 2007 and 2012. Avian Pathol. 2014, 43, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Barman, S.; Turner, J.C.M.; Hasan, M.K.; Akhtar, S.; El-Shesheny, R.; Franks, J.; Walker, D.; Seiler, P.; Friedman, K.; Kercher, L.; et al. Continuing evolution of highly pathogenic H5N1 viruses in Bangladeshi live poultry markets. Emerg. Microbes Infect. 2019, 8, 650–661. [Google Scholar] [CrossRef]
- Xu, X.; Subbarao; Cox, N.J.; Guo, Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999, 261, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Sonnberg, S.; Webby, R.J.; Webster, R.G. Natural history of highly pathogenic avian influenza H5N1. Virus Res. 2013, 178, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Smith, G.J.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. [Google Scholar] [CrossRef] [Green Version]
- OIE. OIE Situation Report for Avian Influenza. 2018. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/OIE_AI_situation_report/OIE_SituationReport_AI_31052018.pdf (accessed on 20 April 2020).
- Islam, M.R.; Baqi, M.A.; Giasuddin, M.; Samad, M.A. Molecular characterization and phylogenetic analysis of highly pathogenic H5N1 avian influenza virus of chickens of Bangladesh. In Proceedings of the Bangkok International Conference on Avian Influenza 2008: Integration from Knowledge to Control, Bangkok, Thailand, 23–25 January 2008. [Google Scholar]
- Chakraborty, A.; Rahman, M.; Hossain, M.J.; Khan, S.U.; Haider, M.S.; Sultana, R.; Ali Rimi, N.; Islam, M.S.; Haider, N.; Islam, A.; et al. Mild Respiratory Illness Among Young Children Caused by Highly Pathogenic Avian Influenza A (H5N1) Virus Infection in Dhaka, Bangladesh, 2011. J. Infect. Dis. 2017, 216, S520–S528. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Haque, M.E.; Giasuddin, M.; Chowdhury, E.H.; Samad, M.A.; Parvin, R.; Nooruzzaman, M.; Rahman, M.M.; Monoura, P. New introduction of clade 2.3.2.1 avian influenza virus (H5N1) into Bangladesh. Transbound. Emerg. Dis. 2012, 59, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.P.; Balasuriya, U.B.; Yamage, M. Genetic diversity and phylogenetic analysis of highly pathogenic avian influenza (HPAI) H5N1 viruses circulating in Bangladesh from 2007-2011. Transbound. Emerg. Dis. 2013, 60, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Marinova-Petkova, A.; Feeroz, M.M.; Rabiul Alam, S.M.; Kamrul Hasan, M.; Akhtar, S.; Jones-Engel, L.; Walker, D.; McClenaghan, L.; Rubrum, A.; Franks, J.; et al. Multiple introductions of highly pathogenic avian influenza H5N1 viruses into Bangladesh. Emerg. Microbes Infect. 2014, 3, e11. [Google Scholar] [CrossRef] [PubMed]
- ICDDRB. The First Fatal Human Infection with Highly Pathogenic Avian Influenza A (H5N1) Virus Detected in Bangladesh. Health and Science Bulletin; International Centre for Diarrhoeal Disease Research (ICDDRB): Dhaka, Bangladesh, 2013; Volume 11, Available online: http://dspace.icddrb.org/jspui/bitstream/123456789/4890/1/2013-ICDDRBHealthScienceBulletin-Vol11%283%29-English.pdf (accessed on 20 April 2020).
- Monne, I.; Yamage, M.; Dauphin, G.; Claes, F.; Ahmed, G.; Giasuddin, M.; Salviato, A.; Ormelli, S.; Bonfante, F.; Schivo, A.; et al. Reassortant avian influenza A(H5N1) viruses with H9N2-PB1 gene in poultry, Bangladesh. Emerg. Infect. Dis. 2013, 19, 1630–1634. [Google Scholar] [CrossRef]
- ICDDRB. First Confirmed Human Infection with Avian Influenza A (H5N1) Virus in Bangladesh. Health and Science Bulletin; International Centre for Diarrhoeal Disease Research (ICDDRB): Dhaka, Bangladesh, 2008; Volume 6, Available online: http://dspace.icddrb.org/jspui/bitstream/123456789/4862/1/2008-ICDDRBHealthScienceBulletin-Vol6%282%29-English.pdf (accessed on 24 March 2020).
- WHO. Influenza at the Human-Animal Interface, Summary and Assessment, 5th March 2012; World Health Organization (WHO): Geneva, Switzerland, 2012; Available online: https://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_05March12.pdf (accessed on 18 March 2020).
- WHO. Influenza at the Human-Animal Interface, Summary and Assessment, 20th January 2016; World Health Organization (WHO): Geneva, Switzerland, 2016; Available online: https://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_20_Jan_2016.pdf (accessed on 17 March 2020).
- ICDDRB. Outbreak of Mild Respiratory Disease Caused by H5N1 and H9N2 Infections among Young Children in Dhaka, Bangladesh, 2011; Health and Science Bulletin; International Centre for Diarrhoeal Disease Research (ICDDRB): Dhaka, Bangladesh, 2011; Volume 9, pp. 5–12. Available online: http://dspace.icddrb.org/jspui/bitstream/123456789/4874/1/2011-ICDDRBHealthScienceBulletin-Vol9%282%29-English.pdf (accessed on 24 March 2020).
- WHO. Influenza at the human-Animal Interface, Summary and Assessment, 4th September 2015; World Health Organization (WHO): Geneva, Switzerland, 2015; Available online: https://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_04_September_2015.pdf (accessed on 17 March 2020).
- Giasuddin, M.; Samad, M.A.; Karim, M.R.; Ali, M.Z.; Pramanik, P.; Hasan, M.; Sufian, A. Emergence of novel H5N6 avian influenza virus in Bangladesh. In Proceedings of the Bangladesh Society of Veterinary Education and Research (BSVER), ASConXXIV, Bangladesh Agricultural University, Mymensingh, Bangladesh, 24–25 March 2018. [Google Scholar]
- Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Walker, D.; Alam, S.; Hasan, M.; McKenzie, P.; Krauss, S.; Webby, R.J.; Webster, R.G. Genesis of avian influenza H9N2 in Bangladesh. Emerg. Microbes Infect. 2014, 3, e88. [Google Scholar] [CrossRef]
- Iqbal, M.; Yaqub, T.; Reddy, K.; McCauley, J.W. Novel genotypes of H9N2 influenza A viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 viruses. PLoS ONE 2009, 4, e5788. [Google Scholar] [CrossRef] [Green Version]
- Parvin, R.; Schinkoethe, J.; Grund, C.; Ulrich, R.; Bönte, F.; Behr, K.P.; Voss, M.; Samad, M.A.; Hassan, K.E.; Luttermann, C.; et al. Comparison of pathogenicity of subtype H9 avian influenza wild-type viruses from a wide geographic origin expressing mono-, di-, or tri-basic hemagglutinin cleavage sites. Vet. Res. 2020, 51, 48. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuen, K.Y.; Chan, P.K.; Peiris, M.; Tsang, D.N.; Que, T.L.; Shortridge, K.F.; Cheung, P.T.; To, W.K.; Ho, E.T.; Sung, R.; et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef]
- Peiris, M.; Yuen, K.Y.; Leung, C.W.; Chan, K.H.; Ip, P.L.; Lai, R.W.; Orr, W.K.; Shortridge, K.F. Human infection with influenza H9N2. Lancet (Lond. Engl.) 1999, 354, 916–917. [Google Scholar] [CrossRef]
- Parry, J. H7N9 avian flu infects humans for the first time. BMJ 2013, 346, f2151. [Google Scholar] [CrossRef]
- Mostafa, A.; Abdelwhab, E.M. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef] [Green Version]
- WHO. Cumulative Number of Confirmed Human Cases for Avian Influenza A (H5N1) Reported to WHO, 2003–2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/influenza/human_animal_interface/2020_01_20_tableH5N1.pdf?ua=1 (accessed on 20 March 2020).
- WHO. Human Infection with Avian Influenza A(H7N9) Virus—China; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/csr/don/20-february-2017-ah7n9-china/en/ (accessed on 24 March 2020).
- Joseph, U.; Su, Y.C.; Vijaykrishna, D.; Smith, G.J. The ecology and adaptive evolution of influenza A interspecies transmission. Influenza Other Respir. Viruses 2017, 11, 74–84. [Google Scholar] [CrossRef]
- Lee, D.H.; Song, C.S. H9N2 avian influenza virus in Korea: Evolution and vaccination. Clin. Exp. Vaccine Res. 2013, 2, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Awuni, J.A.; Bianco, A.; Dogbey, O.J.; Fusaro, A.; Yingar, D.T.; Salviato, A.; Ababio, P.T.; Milani, A.; Bonfante, F.; Monne, I. Avian influenza H9N2 subtype in Ghana: Virus characterization and evidence of co-infection. Avian Pathol. 2019, 48, 470–476. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, Y.; Liu, X.; Peng, D.; Liu, W.; Liu, H.; Lu, S.; Liu, X. Characterization of H9N2 influenza viruses isolated from vaccinated flocks in an integrated broiler chicken operation in eastern China during a 5 year period (1998–2002). J. Gen. Virol. 2008, 89, 3102–3112. [Google Scholar] [CrossRef] [PubMed]
- El Houadfi, M.; Fellahi, S.; Nassik, S.; Guérin, J.L.; Ducatez, M.F. First outbreaks and phylogenetic analyses of avian influenza H9N2 viruses isolated from poultry flocks in Morocco. Virol. J. 2016, 13, 140. [Google Scholar] [CrossRef] [Green Version]
- Naeem, K.; Siddique, N. Use of strategic vaccination for the control of avian influenza in Pakistan. Dev. Biol. 2006, 124, 145–150. [Google Scholar]
- Swayne, D.E.; Pavade, G.; Hamilton, K.; Vallat, B.; Miyagishima, K. Assessment of national strategies for control of high-pathogenicity avian influenza and low-pathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Rev. Sci. Tech. 2011, 30, 839–870. [Google Scholar] [CrossRef] [PubMed]
- Sims, L.D. Intervention strategies to reduce the risk of zoonotic infection with avian influenza viruses: Scientific basis, challenges and knowledge gaps. Influenza Other Respir. Viruses 2013, 7, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; Dauphin, G.; Rushton, J.; McGrane, J.; Lubroth, J.; Tripodi, A.; Gilbert, J.; Sims, L.D. Experiences with vaccination in countries endemically infected with highly pathogenic avian influenza: The Food and Agriculture Organization perspective. Rev. Sci. Tech. 2009, 28, 293–305. [Google Scholar] [CrossRef]
- PoultryMed. Bangladesh: Avian Influenza Immunization Program. 2012. Available online: http://www.poultrymed.com/Poultrymed/Templates/showpage.asp?DBID=1&LNGID=1&TMID=178&FID=1585&PID=0&IID=3418 (accessed on 11 May 2020).
- Government of Bangladesh. Introduce Bird Flu Vaccines for Poultry Farms from Mid-December; Dhaka Herald, 3 December 2013. Available online: http://www.dhakaherald.com/news/local/govt-to-introduce-bird-flu-vaccines-for-poultry-farms-from-mid-dec/ (accessed on 24 March 2020).
- Sarker, S.; Talukder, S.; Chowdhury, E.H.; Das, P.M. Knowledge, attitudes and practices on biosecurity of workers in live bird markets at Mymensingh, Bangladesh. Arpn J. Agric. Biol. Sci. 2011, 6, 12–17. [Google Scholar]


| Sectors | Possible Risk Factors | References |
|---|---|---|
| Backyard poultry |
| [27,40,43,61,62,63,64,65,66,67] |
| Commercial poultry | ||
| Small and medium enterprises |
| [21,39,43,57,68,69] |
| Large holding (specifically, commercial layer farms) |
| [21,70] |
| Live bird markets (LBM) |
| [21,27,39,57,59,61,71,72] |
| AIV Subtypes/ Clade/ Lineage | Year | Patient | Clinical Signs | Poultry Exposure | Case Fatality | References |
|---|---|---|---|---|---|---|
| HPAI H5N1 | ||||||
| N/A | 2008 | 15-month-old male | Fever and difficulty in breathing | Exposure to slaughtered chicken | Recovered | [88] |
| 2.2.2 | 2011 | 13-month-old female | Fever, cough, and loose stool | Close proximity to well-appearing, sick, or dead birds | Recovered | [82] |
| 2.2.2 | 2011 | 31-month-old male | Fever, cough, runny nose, conjunctivitis, vomiting, and diarrhea | Close proximity to backyard poultry, history of visiting live bird market, and handling bird before onset of infection | Recovered | [82] |
| N/A | 2012 | 26-year-old male | Cough | Exposure to live bird market | Recovered | [89] |
| N/A | 2012 | 18-year-old male | Cough | Exposure to live bird market | Recovered | [89] |
| N/A | 2012 | 40-year-old male | Mild illness | Exposure to live bird market | Recovered | [89] |
| 2.3.2.1 | 2013 | 23-month-old male | severe pneumonia, meningitis, and disseminated intravascular coagulation | Close proximity to backyard sick chicken | Fatal | [86] |
| N/A | 2015 | 60-year-old male | Severe acute respiratory signs | Exposure to live backyard poultry | Recovered | [90] |
| LPAI H9N2 | ||||||
| G1 | 2011 | 51-month-old female | Fever, headache, runny nose, cough, and sneezing | Close exposure to sick bird | Recovered | [91] |
| G1 | 2015 | 42-month-old female | Mild illness | Close contact with poultry, including sick quails | Recovered | [92] |
| G1 | 2015 | 46-year-old male | Fever | Poultry worker, regular exposure to bird | Recovered | [90] |
| Genotype | Clade 2.2.2 | Clade 2.3.4.2 | Clade 2.3.2.1c | Clade 2.3.2.1a (Old) | Clade 2.3.2.1a (Old) with H9N2-like PB1 | Clade 2.3.2.1a (New) R1 | Clade 2.3.2.1a (New) R2 |
|---|---|---|---|---|---|---|---|
| Gene constellation |  |  |  |  |  |  |  |
| Features | All eight gene segments are H5N1 HA clade 2.2.2-like. | All gene segments are H5N1 HA clade 2.3.4.2-like. | All gene segments except M, are H5N1 HA clade 2.3.2.1c-like. M gene resembling Chinese H9N2 subtype. | All gene segments are H5N1 HA clade 2.3.2.1a-like. | All gene segments except PB1 are H5N1 HA clade 2.3.2.1a-like. PB1 gene resembling Bangladeshi H9N2 viruses under G1-Western lineage. | H5N1 HA clade 2.3.2.1a-like HA, NA and M genes. LPAI-like PB2, PB1, PA, NP, and NS genes. PA gene resembling H5N1 clade 2.3.2.1a (new) viruses. | H5N1 HA clade 2.3.2.1a-like HA, NA, and M genes. LPAI-like PB2, PB1, PA, NP, and NS genes. PA gene resembling H3N8-like BD LPAI viruses. |
| Timeline | 2007–2011 | 2011 | 2012 | 2011–2015 | 2011–2015 | 2013–continuing | 2017–continuing |
| Complete genome sequences available: Year (No.) | Total: 12 2007 (1), 2010 (8), 2011 (3) | Total: 2 2011 (2) | Total: 2 2012 (2) | Total: 84 2011 (27), 2012 (32), 2013 (4), 2014 (15), 2015 (6) | Total: 8 2011 (2), 2012 (2), 2013 (3), 2015 (1) | Total: 98 2013 (4), 2015 (11), 2016 (25), 2017 (41), 2018 (17) | Total: 14 2017 (9), 2018 (5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, R.; Nooruzzaman, M.; Kabiraj, C.K.; Begum, J.A.; Chowdhury, E.H.; Islam, M.R.; Harder, T. Controlling Avian Influenza Virus in Bangladesh: Challenges and Recommendations. Viruses 2020, 12, 751. https://doi.org/10.3390/v12070751
Parvin R, Nooruzzaman M, Kabiraj CK, Begum JA, Chowdhury EH, Islam MR, Harder T. Controlling Avian Influenza Virus in Bangladesh: Challenges and Recommendations. Viruses. 2020; 12(7):751. https://doi.org/10.3390/v12070751
Chicago/Turabian StyleParvin, Rokshana, Mohammed Nooruzzaman, Congriev Kumar Kabiraj, Jahan Ara Begum, Emdadul Haque Chowdhury, Mohammad Rafiqul Islam, and Timm Harder. 2020. "Controlling Avian Influenza Virus in Bangladesh: Challenges and Recommendations" Viruses 12, no. 7: 751. https://doi.org/10.3390/v12070751







