Serosurveillance and Molecular Investigation of Wild Deer in Australia Reveals Seroprevalence of Pestivirus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Serological Methods
2.3. RNA Extraction and RT-PCR
2.4. Statistical Analysis
3. Results
3.1. Deer Sampling and Distribution
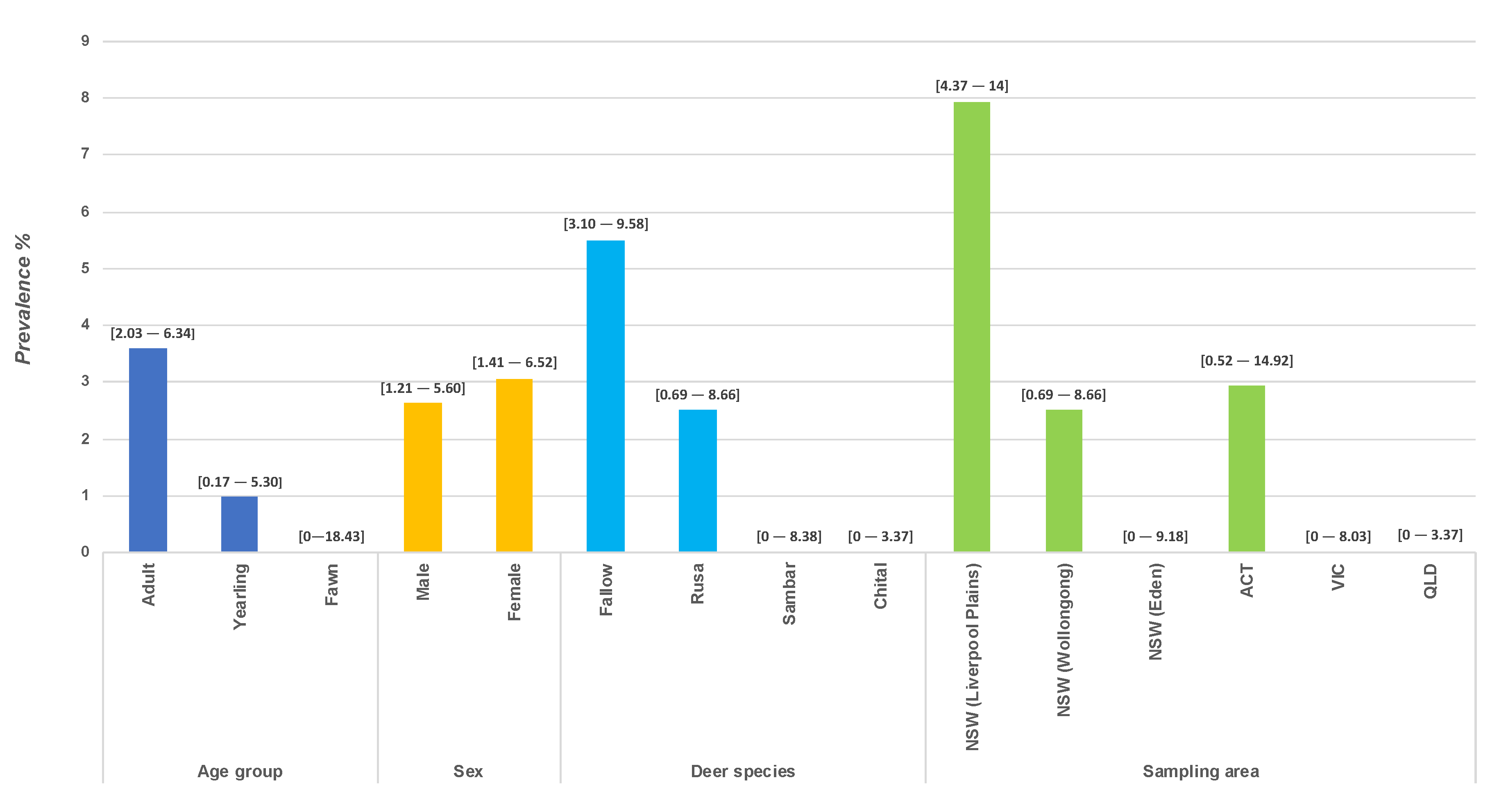
3.2. ELISA Testing
3.3. RT-PCR Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, N.E.; Bennett, A.; Forsyth, D.M.; Bowman, D.M.J.S.; Lefroy, E.C.; Wood, S.W.; Woolnough, A.P.; West, P.; Hampton, J.O.; Johnson, C.N. A systematic review of the impacts and management of introduced deer (family Cervidae) in Australia. Wildl. Res. 2016, 43, 515–532. [Google Scholar] [CrossRef]
- Cripps, J.K.; Pacioni, C.; Scroggie, M.P.; Woolnough, A.P.; Ramsey, D.S.L. Introduced deer and their potential role in disease transmission to livestock in Australia. Mammal Rev. 2019, 49, 60–77. [Google Scholar] [CrossRef]
- Casaubon, J.; Vogt, H.R.; Stalder, H.; Hug, C.; Ryser-Degiorgis, M.P. Bovine viral diarrhea virus in free-ranging wild ruminants in Switzerland: Low prevalence of infection despite regular interactions with domestic livestock. BMC Vet. Res. 2012, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.; Backus, L.; Lynn, T.; Powers, B.; Salman, M. Passive, opportunistic wildlife disease surveillance in the Rocky Mountain Region, USA. Transbound. Emerg. Dis. 2008, 55, 308–314. [Google Scholar] [CrossRef]
- Graham, D.A.; Gallagher, C.; Carden, R.F.; Lozano, J.M.; Moriarty, J.; O’Neill, R. A survey of free-ranging deer in Ireland for serological evidence of exposure to bovine viral diarrhoea virus, bovine herpes virus-1, bluetongue virus and Schmallenberg virus. Irel. Vet. J. 2017, 70, 13. [Google Scholar] [CrossRef]
- Roug, A.; Swift, P.; Torres, S.; Jones, K.; Johnson, C.K. Serosurveillance for livestock pathogens in free-ranging mule deer (Odocoileus hemionus). PLoS ONE 2012, 7, e50600. [Google Scholar] [CrossRef]
- Munday, B.L. A serological study of some infectious diseases of Tasmanian wildlife. J. Wildl. Dis. 1972, 8, 169–175. [Google Scholar] [CrossRef]
- English, A.W. Serological survey of wild fallow deer (Dama dama) in New South Wales, Australia. Vet. Rec. 1982, 110, 153–154. [Google Scholar] [CrossRef]
- McKenzie, R.A.; Green, P.E.; Thornton, A.M.; Chung, Y.S.; MacKenzie, A.R.; Cybinski, D.H.; St George, T.D. Diseases of deer in south eastern Queensland. Aust. Vet. J. 1985, 62, 424. [Google Scholar] [CrossRef]
- Moriarty, A. Ecology and Enviromental Impact of Javan Rusa Deer (Cervus timorensis russa) in the Royal National Park. Ph.D. Thesis, University of Western Sydney, Sydney, NSW, Australia, 2004. [Google Scholar]
- Vilcek, S.; Herring, A.J.; Herring, J.A.; Nettleton, P.F.; Lowings, J.P.; Paton, D.J. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 1994, 136, 309–323. [Google Scholar] [CrossRef]
- Golender, N.; Bumbarov, V.Y.; Erster, O.; Beer, M.; Khinich, Y.; Wernike, K. Development and validation of a universal S-segment-based real-time RT-PCR assay for the detection of Simbu serogroup viruses. J. Virol. Methods 2018, 261, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Aizawa, M.; Takayoshi, K.; Kokuba, T.; Yanase, T.; Shirafuji, H.; Tsuda, T.; Yamakawa, M. Phylogenetic relationships of the G gene sequence of bovine ephemeral fever virus isolated in Japan, Taiwan and Australia. Vet. Microbiol. 2009, 137, 217–223. [Google Scholar] [CrossRef]
- Ohashi, S.; Yoshida, K.; Yanase, T.; Kato, T.; Tsuda, T. Simultaneous detection of bovine arboviruses using single-tube multiplex reverse transcription-polymerase chain reaction. J. Virol. Methods 2004, 120, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Huaman, J.L.; Pacioni, C.; Forsyth, D.M.; Pople, A.; Hampton, J.O.; Helbig, K.J.; Carvalho, T. Screening of Blood Parasites in Australian Wild Deer. Authorea 2020. [Google Scholar] [CrossRef]
- Scharnbock, B.; Roch, F.F.; Richter, V.; Funke, C.; Firth, C.L.; Obritzhauser, W.; Baumgartner, W.; Kasbohrer, A.; Pinior, B. A meta-analysis of bovine viral diarrhoea virus (BVDV) prevalences in the global cattle population. Sci. Rep. 2018, 8, 14420. [Google Scholar] [CrossRef]
- Van Dyck, S.; Strahan, R. The Mammals of Australia; Reed New Holland: Sydney, NSW, Australia, 2008. [Google Scholar]
- Aguirre, A.A.; Hansen, D.E.; Starkey, E.E.; Mclean, R.G. Serologic Survey of Wild Cervids for Potential Disease Agents in Selected National-Parks in the United-States. Prev. Vet. Med. 1995, 21, 313–322. [Google Scholar] [CrossRef]
- Rodriguez-Prieto, V.; Kukielka, D.; Rivera-Arroyo, B.; Martinez-Lopez, B.; de las Heras, A.I.; Sanchez-Vizcaino, J.M.; Vicente, J. Evidence of shared bovine viral diarrhea infections between red deer and extensively raised cattle in south-central Spain. BMC Vet. Res. 2016, 12. [Google Scholar] [CrossRef]
- Fernandez-Aguilar, X.; Lopez-Olvera, J.R.; Marco, I.; Rosell, R.; Colom-Cadena, A.; Soto-Heras, S.; Lavin, S.; Cabezon, O. Pestivirus in alpine wild ruminants and sympatric livestock from the Cantabrian Mountains, Spain. Vet. Rec. 2016, 178, U557–U586. [Google Scholar] [CrossRef] [PubMed]
- Frolich, K. Bovine Virus Diarrhea and Mucosal Disease in Free-Ranging and Captive Deer (Cervidae) in Germany. J. Wildl. Dis. 1995, 31, 247–250. [Google Scholar] [CrossRef]
- Duncan, C.; Van Campen, H.; Soto, S.; LeVan, I.K.; Baeten, L.A.; Miller, M.W. Persistent Bovine viral diarrhea virus infection in wild cervids of Colorado. J. Vet. Diagn. Investig. 2008, 20, 650–653. [Google Scholar] [CrossRef]
- Passler, T.; Walz, P.H.; Ditchkoff, S.S.; Walz, H.L.; Givens, M.D.; Brock, K.V. Evaluation of hunter-harvested white-tailed deer for evidence of bovine viral diarrhea virus infection in Alabama. J. Vet. Diagn. Investig. 2008, 20, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.; Ridpath, J.; Palmer, M.V.; Driskell, E.; Spraker, T. Histopathologic and immunohistochemical findings in two white-tailed deer fawns persistently infected with Bovine viral diarrhea virus. J. Vet. Diagn. Investig. 2008, 20, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Passler, T.; Walz, P.H.; Ditchkoff, S.S.; Givens, M.D.; Maxwell, H.S.; Brock, K.V. Experimental persistent infection with bovine viral diarrhea virus in white-tailed deer. Vet. Microbiol. 2007, 122, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.E.; Heaney, J.; Thomas, C.J.; Brownlie, J. Infectivity of pestivirus following persistence of acute infection. Vet. Microbiol. 2009, 138, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J. Immunological responses to bovine virus diarrhoea virus infections. Rev. Sci. Tech. 1990, 9, 95–103. [Google Scholar] [CrossRef]
- Van Campen, H.; Williams, E.S.; Edwards, J.; Cook, W.; Stout, G. Experimental infection of deer with bovine viral diarrhea virus. J. Wildl. Dis. 1997, 33, 567–573. [Google Scholar] [CrossRef]
- Tessaro, S.V.; Carman, P.S.; Deregt, D. Viremia and virus shedding in elk infected with type 1 and virulent type 2 bovine viral diarrhea virus. J. Wildl. Dis. 1999, 35, 671–677. [Google Scholar] [CrossRef]
- Fredriksen, B.; Sandvik, T.; Loken, T.; Odegaard, S.A. Level and duration of serum antibodies in cattle infected experimentally and naturally with bovine virus diarrhoea virus. Vet. Rec. 1999, 144, 111–114. [Google Scholar] [CrossRef]
- Hanon, J.B.; De Baere, M.; De la Ferte, C.; Roelandt, S.; Van der Stede, Y.; Cay, B. Evaluation of 16 commercial antibody ELISAs for the detection of bovine viral diarrhea virus-specific antibodies in serum and milk using well-characterized sample panels. J. Vet. Diagn. Investig. 2017, 29, 833–843. [Google Scholar] [CrossRef]
- Morrondo, M.P.; Perez-Creo, A.; Prieto, A.; Cabanelas, E.; Diaz-Cao, J.M.; Arias, M.S.; Fernandez, P.D.; Pajares, G.; Remesar, S.; Lopez-Sandez, C.M.; et al. Prevalence and distribution of infectious and parasitic agents in roe deer from Spain and their possible role as reservoirs. Ital. J. Anim. Sci. 2017, 16, 266–274. [Google Scholar] [CrossRef]
- Loeffen, W.L.; van Beuningen, A.; Quak, S.; Elbers, A.R. Seroprevalence and risk factors for the presence of ruminant pestiviruses in the Dutch swine population. Vet. Microbiol. 2009, 136, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.A.; Lanyon, S.R.; Reichel, M.P. Investigation of AGID and two commercial ELISAs for the detection of Bovine viral diarrhea virus-specific antibodies in sheep serum. J. Vet. Diagn. Investig. 2017, 29, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Cherpes, T.L.; Meyn, L.A.; Hillier, S.L. Plasma versus serum for detection of herpes simplex virus type 2-specific immunoglobulin G antibodies with a glycoprotein G2-based enzyme immunoassay. J. Clin. Microbiol. 2003, 41, 2758–2759. [Google Scholar] [CrossRef]
- Parra-Álvarez, S.; Coronel-Ruiz, C.; Castilla, M.G.; Velandia-Romero, M.L.; Castellanos, J.E. Alta correlación en la detección de anticuerpos y antígenos de virus del dengue en muestras de suero y plasma. Rev. Fac. Med. 2015, 63, 687–693. [Google Scholar] [CrossRef]
- Lin, H.T.; Hsu, C.H.; Tsai, H.J.; Lin, C.H.; Lo, P.Y.; Wang, S.L.; Wang, L.C. Influenza A plasma and serum virus antibody detection comparison in dogs using blocking enzyme-linked immunosorbent assay. Vet. World 2015, 8, 580–583. [Google Scholar] [CrossRef][Green Version]
- Tavernier, P.; Sys, S.U.; De Clercq, K.; De Leeuw, I.; Caij, A.B.; De Baere, M.; De Regge, N.; Fretin, D.; Roupie, V.; Govaerts, M.; et al. Serologic screening for 13 infectious agents in roe deer (Capreolus capreolus) in Flanders. Infect. Ecol. Epidemiol. 2015, 5, 29862. [Google Scholar] [CrossRef]
- Krametter, R.; Nielsen, S.S.; Loitsch, A.; Froetscher, W.; Benetka, V.; Moestl, K.; Baumgartner, W. Pestivirus exposure in free-living and captive deer in Austria. J. Wildl. Dis. 2004, 40, 791–795. [Google Scholar] [CrossRef]
- Cuteri, V.; Diverio, S.; Carnieletto, P.; Turilli, C.; Valente, C. Serological survey for antibodies against selected infectious agents among fallow deer (Dama dama) in central Italy. Zentralbl Veterinarmed B 1999, 46, 545–549. [Google Scholar] [CrossRef]
- Boadella, M.; Carta, T.; Oleaga, A.; Pajares, G.; Munoz, M.; Gortazar, C. Serosurvey for selected pathogens in Iberian roe deer. BMC Vet. Res. 2010, 6, 51. [Google Scholar] [CrossRef]
- Decaro, N.; Lucente, M.S.; Lanave, G.; Gargano, P.; Larocca, V.; Losurdo, M.; Ciambrone, L.; Marino, P.A.; Parisi, A.; Casalinuovo, F.; et al. Evidence for Circulation of Bovine Viral Diarrhoea Virus Type 2c in Ruminants in Southern Italy. Transbound. Emerg. Dis. 2017, 64, 1935–1944. [Google Scholar] [CrossRef]
- Fernandez-Sirera, L.; Cabezon, O.; Dematteis, A.; Rossi, L.; Meneguz, P.G.; Gennero, M.S.; Allepuz, A.; Rosell, R.; Lavin, S.; Marco, I. Survey of Pestivirus infection in wild and domestic ungulates from south-western Italian Alps. Eur. J. Wildlife Res. 2012, 58, 425–431. [Google Scholar] [CrossRef]
- Bianchi, M.V.; Silveira, S.; Mosena, A.C.S.; de Souza, S.O.; Konradt, G.; Canal, C.W.; Driemeier, D.; Pavarini, S.P. Pathological and virological features of skin lesions caused by BVDV in cattle. Braz. J. Microbiol. 2019, 50, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Grooms, D.L.; Brock, K.V.; Ward, L.A. Detection of bovine viral diarrhea virus in the ovaries of cattle acutely infected with bovine viral diarrhea virus. J. Vet. Diagn. Investig. 1998, 10, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Givens, M.D.; Heath, A.M.; Brock, K.V.; Brodersen, B.W.; Carson, R.L.; Stringfellow, D.A. Detection of bovine viral diarrhea virus in semen obtained after inoculation of seronegative postpubertal bulls. Am. J. Vet. Res. 2003, 64, 428–434. [Google Scholar] [CrossRef]
- Ohmann, H.B. Pathogenesis of bovine viral diarrhoea-mucosal disease: Distribution and significance of BVDV antigen in diseased calves. Res. Vet. Sci. 1983, 34, 5–10. [Google Scholar] [CrossRef]
- Thiry, J.; Keuser, V.; Muylkens, B.; Meurens, F.; Gogev, S.; Vanderplasschen, A.; Thiry, E. Ruminant alphaherpesviruses related to bovine herpesvirus 1. Vet. Res. 2006, 37, 169–190. [Google Scholar] [CrossRef]
- Gu, X.; Kirkland, P.D. Infectious Bovine Rhinotracheitis. In Australian and New Zealand Standard Diagnostic Procedures; The Department of Agriculture and Water: Canberra, ACT, Australia, 2008; pp. 1–18. [Google Scholar]
- Lillehaug, A.; Vikoren, T.; Larsen, I.L.; Akerstedt, J.; Tharaldsen, J.; Handeland, K. Antibodies to ruminant alpha-herpesviruses and pestiviruses in Norwegian cervids. J. Wildl. Dis. 2003, 39, 779–786. [Google Scholar] [CrossRef]
- Inglis, D.M.; Bowie, J.M.; Allan, M.J.; Nettleton, P.F. Ocular disease in red deer calves associated with a herpesvirus infection. Vet. Rec. 1983, 113, 182–183. [Google Scholar] [CrossRef]
- Ek-Kommonen, C.; Pelkonen, S.; Nettleton, P.F. Isolation of a herpesvirus serologically related to bovine herpesvirus 1 from a reindeer (Rangifer tarandus). Acta Vet. Scand. 1986, 27, 299–301. [Google Scholar]
- Lyaku, J.R.S.; Nettleton, P.F.; Marsden, H. A Comparison of Serological Relationships among 5 Ruminant Alphaherpesviruses by Elisa. Arch. Virol. 1992, 124, 333–341. [Google Scholar] [CrossRef]
- Martin, W.B.; Castrucci, G.; Frigeri, F.; Ferrari, M. A Serological Comparison of Some Animal Herpesviruses. Comp. Immunol. Microb. 1990, 13, 75–84. [Google Scholar] [CrossRef]
- Nixon, P.; Edwards, S.; White, H. Serological Comparisons of Antigenically Related Herpesviruses in Cattle, Red Deer and Goats. Vet. Res. Commun. 1988, 12, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Klement, E. Epidemiology and control of bovine ephemeral fever. Vet. Res. 2015, 46, 124. [Google Scholar] [CrossRef] [PubMed]
- Weir, R.P.; Agnihotri, K. Epizootic Haemorrhagic Disease. In Australian and New Zealand Standard Diagnostic Procedures; The Department of Agriculture and Water: Canberra, ACT, Australia, 2014; pp. 1–14. [Google Scholar]
- Australia Animal Health. National Arbovirus Monitoring Program 2015–2016 Report. Available online: https://www.animalhealthaustralia.com.au/wp-content/uploads/2015/09/NAMP-Annual-Report_FA_print.pdf (accessed on 1 April 2020).
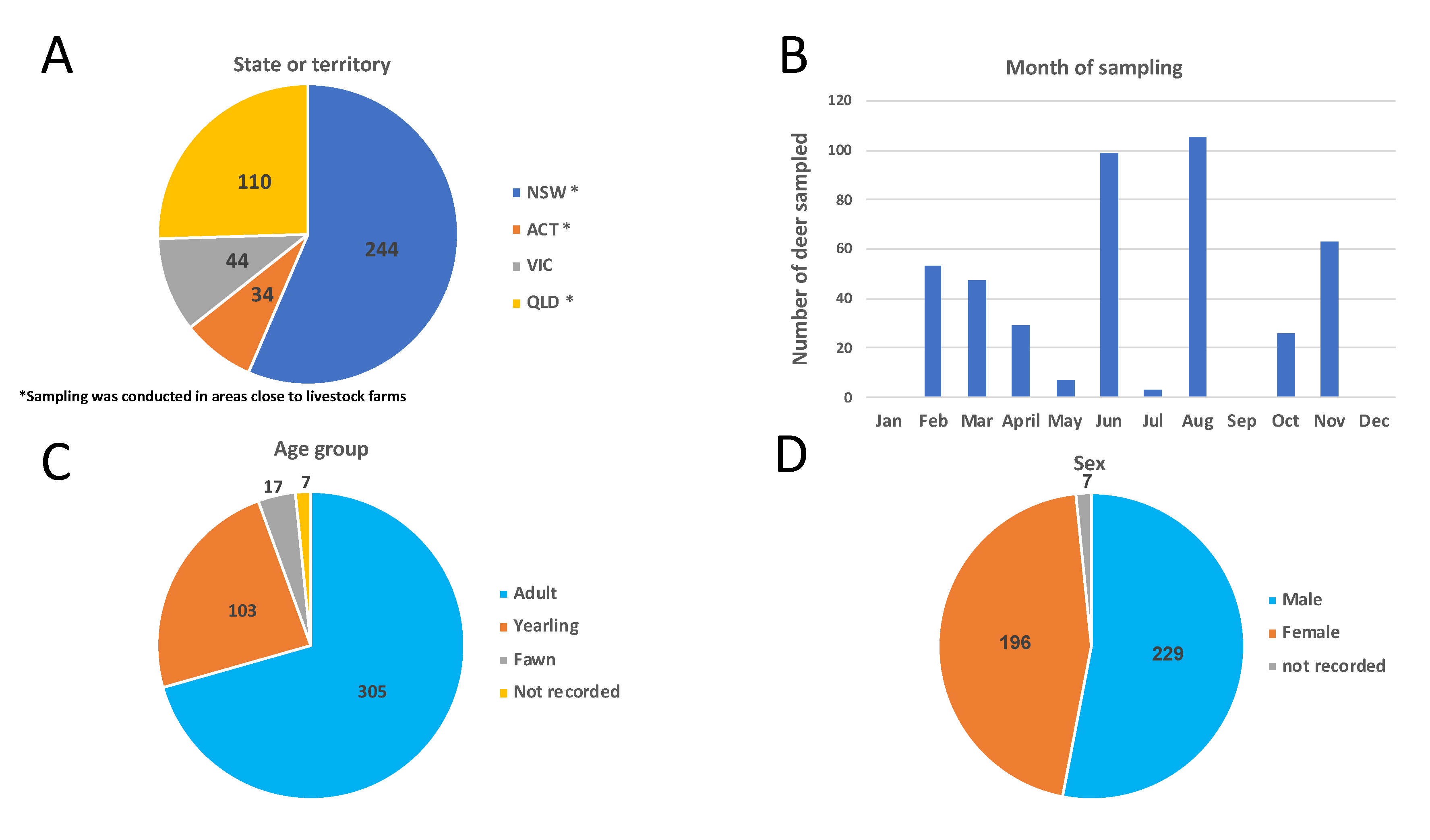
| States or Territory | Animals | Sampling Location | Species | Sex | Age Groups | Month of Sampling | No. Deer Tested by | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | N.r. | Ad | Yrl | Fw | N.r. | Feb | Mar | Apr | May | Jun | Jul | Aug | Oct | Nov | ELISA Ab | ELISA Ag | PCR | ||||
| NSW | 244 | Liverpool Plains * | Fallow | 74 | 52 | 0 | 74 | 47 | 5 | 0 | 0 | 0 | 0 | 0 | 39 | 0 | 87 | 0 | 0 | 126 | 126 | 42 |
| Eden * | 12 | 21 | 5 | 21 | 9 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 15 | 5 | 38 | 0 | 18 | |||
| Wollongong * | Rusa | 69 | 11 | 0 | 68 | 12 | 0 | 0 | 43 | 0 | 14 | 0 | 13 | 0 | 0 | 10 | 0 | 80 | 66 | 7 | ||
| ACT | 34 | Canberra * | Fallow | 14 | 20 | 0 | 26 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 34 | 0 | 0 | 0 | 0 | 34 | 0 | 10 |
| VIC | 44 | Alpine National Park | Sambar | 17 | 14 | 1 | 19 | 6 | 6 | 1 | 10 | 0 | 15 | 7 | 0 | 0 | 0 | 0 | 0 | 32 | 16 | 17 |
| Upper Yarra Flats | 2 | 6 | 1 | 6 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 1 | 0 | 9 | 8 | 4 | |||
| Yellinbo | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | |||
| Fallow | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | |||
| QLD | 110 | North east Queensland * | Chital | 41 | 69 | 0 | 90 | 20 | 0 | 0 | 0 | 47 | 0 | 0 | 5 | 0 | 0 | 0 | 58 | 110 | 105 | 43 |
| Total | 432 | 229 | 196 | 7 | 305 | 103 | 17 | 7 | 53 | 47 | 29 | 7 | 99 | 3 | 105 | 26 | 63 | 432 | 321 | 144 | ||
| Virus | Target Region | Primer Name | Sequence 5′–3′ | Amplicon Length (bp) | PCR Condition | Reference |
|---|---|---|---|---|---|---|
| Pestivirus | 5′UTR | 324 | ATGCCCWTAGTAGGACTAGCA | 288 | 95 °C × 2 min 40 cycles (95 °C × 45 s, 52 °C × 45 s, 72 °C × 45 s) 72 °C × 5 min | [11] |
| 326 | WCAACTCCATGTGCCATGTAC | |||||
| Simbu Serogroup | Segment S | Uni-S-59F | GATGWCCWCAACGGAAT | 215 | 95 °C × 2 min 40 cycles (95 °C × 45 s, 55 °C × 45 s, 72 °C × 45 s) 72 °C × 5 min | [12] |
| Uni-S-254R | TGGGGAAAATGGTTATTAAC | |||||
| BEFV | Glucoprotein G | GF | ATGTTCAAGGTCCTCATAATTACC | 1871 | 95 °C × 2 min 40 cycles (95°C × 45 s, 52 °C × 45 s, 72 °C × 2 min) 72 °C × 5 min | [13] |
| GR | TAATGATCAAAGAACCTATCATCA | |||||
| EHDV | NS3 | NS3F | CAGCGCYWTATWCGATATTG | 533 | 95 °C × 2 min 40 cycles (95 °C × 45 s, 55 °C × 45 s, 72 °C × 60 s) 72 °C × 5 min | [14] |
| NS3R | TCCGGAGATACCTCCATTAC |
| Sample | Species | Sampling Month | Sex | Age | Location | Anti-Pestivirus ELISA | |||
|---|---|---|---|---|---|---|---|---|---|
| Serum | Plasma | ||||||||
| Result | %INH a | Result | %INH a | ||||||
| 1 | Fallow | June | F | Ad | New South Wales | WP | 65 | Neg | - |
| 2 | Fallow | June | F | Ad | New South Wales | SP | 91.9 | SP | 91.4 |
| 3 | Fallow | June | F | Ad | New South Wales | WP | 73.7 | WP | 57.4 |
| 4 | Fallow | June | F | Yrl | New South Wales | WP | 73.6 | WP | 59.0 |
| 5 | Fallow | June | F | Ad | New South Wales | WP | 61.6 | WP | 67.0 |
| 6 | Fallow | August | M | Ad | New South Wales | WP | 71.6 | WP | 67.1 |
| 7 | Fallow | August | M | Ad | New South Wales | SP | 80.1 | WP | 79.4 |
| 8 | Fallow | August | M | Ad | New South Wales | SP | 84.4 | SP | 83.3 |
| 9 | Fallow | August | M | Ad | New South Wales | SP | 87.2 | SP | 80.1 |
| 10 | Fallow | August | M | Ad | New South Wales | SP | 87.2 | WP | 78.9 |
| 11 | Rusa | February | ND | ND | New South Wales | WP | 51.8 | NS | - |
| 12 | Rusa | October | F | Ad | New South Wales | Neg | - | WP | 60.1 |
| 13 | Fallow | June | M | Ad | Australian Capital Territory | NS | - | WP | 56.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huaman, J.L.; Pacioni, C.; Forsyth, D.M.; Pople, A.; Hampton, J.O.; Carvalho, T.G.; Helbig, K.J. Serosurveillance and Molecular Investigation of Wild Deer in Australia Reveals Seroprevalence of Pestivirus Infection. Viruses 2020, 12, 752. https://doi.org/10.3390/v12070752
Huaman JL, Pacioni C, Forsyth DM, Pople A, Hampton JO, Carvalho TG, Helbig KJ. Serosurveillance and Molecular Investigation of Wild Deer in Australia Reveals Seroprevalence of Pestivirus Infection. Viruses. 2020; 12(7):752. https://doi.org/10.3390/v12070752
Chicago/Turabian StyleHuaman, Jose L., Carlo Pacioni, David M. Forsyth, Anthony Pople, Jordan O. Hampton, Teresa G. Carvalho, and Karla J. Helbig. 2020. "Serosurveillance and Molecular Investigation of Wild Deer in Australia Reveals Seroprevalence of Pestivirus Infection" Viruses 12, no. 7: 752. https://doi.org/10.3390/v12070752
APA StyleHuaman, J. L., Pacioni, C., Forsyth, D. M., Pople, A., Hampton, J. O., Carvalho, T. G., & Helbig, K. J. (2020). Serosurveillance and Molecular Investigation of Wild Deer in Australia Reveals Seroprevalence of Pestivirus Infection. Viruses, 12(7), 752. https://doi.org/10.3390/v12070752






