Bifidobacterium longum subsp. longum OLP-01 Supplementation during Endurance Running Training Improves Exercise Performance in Middle- and Long-Distance Runners: A Double-Blind Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic
2.2. Participants
2.3. Experimental Design
2.4. The 12-min Cooper Running/Walking Test
2.5. Body Composition
2.6. Blood Routine and Serum Biochemical Analysis
2.7. Bacterial DNA Extraction and 16S rRNA Sequencing
2.8. Statistical Analysis
3. Results
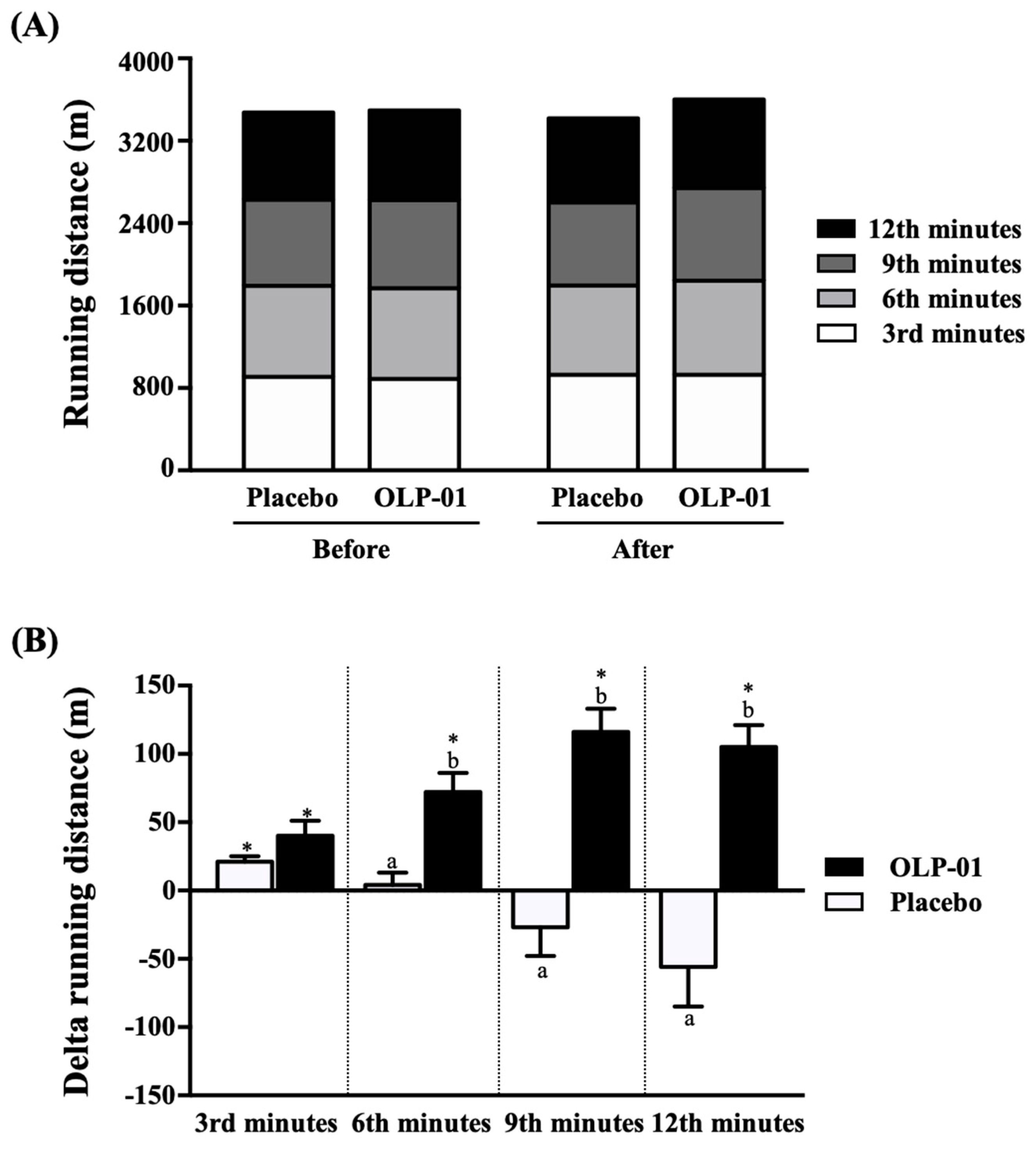
3.1. Effect of OLP-01 Supplementation on Distance in 12-min Cooper Running/Walking Test
3.2. Effect of OLP-01 Supplementation on Body Composition
3.3. Effect of OLP-01 Supplementation on Blood Biochemistry
3.4. Effect of OLP-01 Supplementation on Complete Blood Count Profiles
3.5. Effect of OLP-01 Supplementation on Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thiel, C.; Foster, C.; Banzer, W.; De Koning, J. Pacing in Olympic track races: Competitive tactics versus best performance strategy. J. Sports Sci. 2012, 30, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Sandford, G.N.; Pearson, S.; Allen, S.V.; Malcata, R.M.; Kilding, A.E.; Ross, A.; Laursen, P.B. Tactical behaviors in men’s 800-m Olympic and world-championship medalists: A changing of the guard. Int. J. Sports Physiol. Perform. 2018, 13, 246–249. [Google Scholar] [CrossRef]
- Knechtle, B.; Nikolaidis, P.T. Sex- and age-related differences in half-marathon performance and competitiveness in the world’s largest half-marathon—The GöteborgsVarvet. Res. Sports Med. 2018, 26, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.; Hettinga, F.J. Champions are racers, not pacers: An analysis of qualification patterns of Olympic and IAAF World Championship middle distance runners. J. Sports Sci. 2018, 36, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Kenneally, M.; Casado, A.; Santos-Concejero, J. The effect of periodization and training intensity distribution on middle- and long-distance running performance: A systematic review. Int. J. Sports Physiol. Perform. 2018, 13, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.I. Metabolic factors limiting performance in marathon runners. PLoS Comput. Biol. 2010, 6, e1000960. [Google Scholar] [CrossRef]
- Damasceno, M.V.; Pasqua, L.A.; Lima-Silva, A.E.; Bertuzzi, R. Energy system contribution in a maximal incremental test: Correlations with pacing and overall performance in a 10-km running trial. Braz. J. Med. Biol. Res. 2015, 48, 1048–1054. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Bovim, I.M.; Whitfield, J. Contemporary nutrition interventions to optimize performance in middle-distance runners. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 106–116. [Google Scholar] [CrossRef]
- Lorenzo Calvo, J.; Alorda-Capo, F.; Pareja-Galeano, H.; Jiménez, S.L. Influence of nitrate supplementation on endurance cyclic sports performance: A systematic review. Nutrients 2020, 12, 1796. [Google Scholar] [CrossRef]
- Rivera-Brown, A.M.; Frontera, W.R. Principles of exercise physiology: Responses to acute exercise and long-term adaptations to training. Pm R 2012, 4, 797–804. [Google Scholar] [CrossRef]
- Artioli, G.G.; Bertuzzi, R.C.; Roschel, H.; Mendes, S.H.; Lancha, A.H., Jr.; Franchini, E. Determining the contribution of the energy systems during exercise. J. Vis. Exp. 2012, 61, e3413. [Google Scholar] [CrossRef] [PubMed]
- Tanji, F.; Tsuji, T.; Shimazu, W.; Nabekura, Y. Relationship between 800-m running performance and aerobic and anaerobic energy metabolism capacities in well-trained middle-distance runners. Int. J. Sport Health Sci. 2018, 201724. [Google Scholar] [CrossRef]
- Lambert, J.E.; Myslicki, J.P.; Bomhof, M.R.; Belke, D.D.; Shearer, J.; Reimer, R.A. Exercise training modifies gut microbiota in normal and diabetic mice. Appl. Physiol. Nutr. Metab. 2015, 40, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Kazgan, N.; Metukuri, M.R.; Purushotham, A.; Lu, J.; Rao, A.; Lee, S.; Pratt-Hyatt, M.; Lickteig, A.; Csanaky, I.L.; Zhao, Y.; et al. Intestine-specific deletion of SIRT1 in mice impairs DCoH2-HNF-1α-FXR signaling and alters systemic bile acid homeostasis. Gastroenterology 2014, 146, 1006–1016. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 1–21. [Google Scholar] [CrossRef]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.B.; West, N.P.; Cox, A.J.; Cripps, A.W. Probiotics supplementation for athletes—Clinical and physiological effects. Eur. J. Sport Sci. 2015, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.S.; Student, A.S.R.M.; West, N.P.; Lancha, A.H., Jr. Probiotics and sports: A new magic bullet? Nutrition 2019, 60, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Sung, H.C.; Huang, C.C. Lactobacillus salivarius Subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef]
- Huang, W.C.; Lee, M.C.; Lee, C.C.; Ng, K.S.; Hsu, Y.J.; Tsai, T.Y.; Young, S.L.; Lin, J.S.; Huang, C.C. Effect of Lactobacillus plantarum TWK10 on exercise physiological adaptation, performance, and body composition in healthy humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Chuang, H.L.; Hsieh, P.S.; Ho, H.H.; Chen, W.L.; Chiu, Y.S.; Huang, C.C. In Vivo ergogenic properties of the Bifidobacterium longum OLP-01 isolated from a weightlifting gold medalist. Nutrients 2019, 11, 2003. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Huang, C.C.; Liu, H.C.; Lee, M.C. Exercise training combined with Bifidobacterium longum OLP-01 supplementation improves exercise physiological adaption and performance. Nutrients 2020, 12, 1145. [Google Scholar] [CrossRef]
- Cooper, K.H. A means of assessing maximal oxygen intake: Correlation between field and treadmill testing. JAMA 1968, 203, 201–204. [Google Scholar] [CrossRef]
- Bandyopadhyay, A. Validity of Cooper’s 12-minute run test for estimation of maximum oxygen uptake in male university students. Biol. Sport 2015, 32, 59–63. [Google Scholar] [CrossRef]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Salehzadeh, K. The effects of probiotic yogurt drink on lipid profile, CRP and record changes in aerobic athletes. Int. J. Life Sci. 2015, 9, 32–37. [Google Scholar] [CrossRef]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of probiotic (Bifidobacterium longum 35624) supplementation on exercise performance, immune modulation, and cognitive outlook in division I female swimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef]
- Samedi, L.; Charles, A.L. Viability of 4 probiotic bacteria microencapsulated with arrowroot starch in the simulated Gastrointestinal Tract (GIT) and yoghurt. Foods 2019, 8, 175. [Google Scholar] [CrossRef]
- Lacerda, A.C.; Alecrim, P.; Damasceno, W.C.; Gripp, F.; Pinto, K.M.; Silami-Garcia, E. Carbohydrate ingestion during exercise does not delay the onset of fatigue during submaximal cycle exercise. J. Strength Cond. Res. 2009, 23, 1276–1281. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood lactate measurements and analysis during exercise: A guide for clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569. [Google Scholar] [CrossRef]
- Arakawa, K.; Hosono, A.; Shibata, K.; Ghadimi, R.; Fuku, M.; Goto, C.; Imaeda, N.; Tokudome, Y.; Hoshino, H.; Marumoto, M.; et al. Changes in blood biochemical markers before, during, and after a 2-day ultramarathon. Open Access J. Sports Med. 2016, 7, 43–50. [Google Scholar] [CrossRef][Green Version]
- Van Rensburg, J.P.; Kielblock, A.J.; van der Linde, A. Physiologic and biochemical changes during a triathlon competition. Int. J. Sports Med. 1986, 7, 30–35. [Google Scholar] [CrossRef]
- Nakano, S. Illustration: The Merits and Demerits of Physical Exercise on Human Body, 2nd ed.; Ishiyaku: Tokyo, Japan, 1997. [Google Scholar]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Sovran, B.; Hugenholtz, F.; Elderman, M.; Van-Beek, A.A.; Graversen, K.; Huijskes, M.; Boekschoten, M.V.; Savelkoul, H.F.J.; De Vos, P.; Dekker, J.; et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Di Martino, M.; Catalano, C.; Lenzi, A.; Donini, L.M. The decline in muscle strength and muscle quality in relation to metabolic derangements in adult women with obesity. Clin. Nutr. 2019, 38, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M.J.; Kerimi, A.; Mouly, V.; Tumova, S.; Williamson, G. Gut microbiome catabolites as novel modulators of muscle cell glucose metabolism. FASEB J. 2019, 33, 1887–1898. [Google Scholar] [CrossRef]
- Passos, B.N.; Lima, M.C.; Sierra, A.P.R.; Oliveira, R.A.; Maciel, J.F.S.; Manoel, R.; Rogante, J.I.; Pesquero, J.B.; Cury-Boaventura, M.F. Association of daily dietary intake and inflammation induced by marathon race. Mediat. Inflamm. 2019, 2019, 1537274. [Google Scholar] [CrossRef]
- Bernecker, C.; Scherr, J.; Schinner, S.; Braun, S.; Scherbaum, W.A.; Halle, M. Evidence for an exercise induced increase of TNF-α and IL-6 in marathon runners. Scand. J. Med. Sci. Sports 2013, 23, 207–214. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Yu, J.Y.; Choi, W.J.; Lee, H.S.; Lee, J.W. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: An observational study. Medicine 2019, 98, e14740. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International society of sports nutrition position stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]


| Characteristic | Placebo | OLP-01 |
|---|---|---|
| Age (y) | 21.2 ± 0.4 | 21.6 ± 0.7 |
| Height (cm) | 168.7 ± 1.5 | 169.5 ± 2.3 |
| Weight (kg) | 57.1 ± 1.9 | 56.4 ± 1.1 |
| BMI (kg/m2) | 20.0 ± 0.4 | 19.7 ± 0.3 |
| Characteristics | Before Intervention | After Intervention | Change (After-Before) | |||
|---|---|---|---|---|---|---|
| Placebo | OLP-01 | Placebo | OLP-01 | Placebo | OLP-01 | |
| BW (kg) | 57.1 ± 1.9 | 56.4 ± 1.1 | 56.7 ± 2.0 | 56.3 ± 1.2 | −0.5 ± 0.4 | −0.1 ± 0.3 |
| BMI (kg/cm2) | 20.0 ± 0.4 | 19.7 ± 0.3 | 19.9 ± 0.5 | 19.6 ± 0.3 | −0.1 ± 0.2 | 0.0 ± 0.1 |
| LBM (kg) | 27.8 ± 1.3 | 27.9 ± 1.2 | 27.6 ± 1.3 | 27.8 ± 1.2 | −0.1 ± 0.2 * | −0.1 ± 0.2 |
| FBM (%) | 13.1 ± 1.4 | 12.1 ± 2.1 | 13.3 ± 1.3 | 12.3 ± 2.0 | 0.2 ± 0.4 | 0.2 ± 0.2 |
| Characteristics | Before Intervention | After Intervention | ||
|---|---|---|---|---|
| Placebo | OLP-01 | Placebo | OLP-01 | |
| Lactate (mmol/L) | 2.41 ± 0.10 | 2.41 ± 0.12 | 1.86 ± 0.12 | 2.07 ± 0.07 |
| NH3 (μmol/L) | 117 ± 6 | 119 ± 4 | 88 ± 13 | 88 ± 12 |
| CK (U/L) | 187 ± 25 | 183 ± 21 | 191 ± 21 | 179 ± 16 |
| Glucose (mg/dL) | 89 ± 2 | 85 ± 2 | 91 ± 2 | 89 ± 2 |
| AST (U/L) | 26 ± 2 | 23 ± 2 | 24 ± 3 | 24 ± 3 |
| ALT (U/L) | 22 ± 3 | 21 ± 1 | 19 ± 2 | 18 ± 1 |
| ALB (mg/dL) | 4.9 ± 0.1 | 4.9 ± 0.1 | 4.9 ± 0.1 | 4.9 ± 0.1 |
| TC (mg/dL) | 168 ± 8 | 185 ± 1 | 163 ± 7 | 169 ± 1 |
| TG (mg/dL) | 77 ± 7 | 77 ± 5 | 71 ± 6 | 69 ± 4 |
| HDL (mg/dL) | 67.4 ± 2.6 | 67.2 ± 3.0 | 63.8 ± 2.2 | 70.3 ± 3.1 |
| LDL (mg/dL) | 86.5 ± 5.1 | 93.6 ± 3.6 | 88.6 ± 4.9 | 81.7 ± 3.0 |
| BUN (mg/dL) | 16.5 ± 0.9 | 16.3 ± 0.8 | 16.2 ± 0.7 | 15.6 ± 0.8 |
| CREA (mg/dL) | 1.09 ± 0.04 | 1.10 ± 0.03 | 1.09 ± 0.04 | 1.09 ± 0.02 |
| UA (mg/dL) | 5.7 ± 0.4 | 5.1 ± 0.4 | 6.3 ± 0.6 | 6.0 ± 0.4 |
| TP (mg/dL) | 6.9 ± 0.1 | 6.9 ± 0.2 | 7.0 ± 0.2 | 7.0 ± 0.1 |
| Characteristics | Before Intervention | After Intervention | ||
|---|---|---|---|---|
| Placebo | OLP-01 | Placebo | OLP-01 | |
| WBC (cells/mcL) | 7089 ± 471 | 7071 ± 320 | 7083 ± 487 | 7106 ± 456 |
| Neutrophils (%) | 51.7 ± 2.4 | 53.0 ± 2.8 | 51.9 ± 2.1 | 55.0 ± 2.4 |
| Lymphocytes (%) | 39.2 ± 2.2 | 36.7 ± 2.6 | 39.1 ± 2.2 | 35.3 ± 2.0 |
| Monocytes (%) | 5.5 ± 0.3 | 5.8 ± 0.4 | 5.8 ± 0.2 | 6.0 ± 0.3 |
| Eosinophil (%) | 2.9 ± 0.5 | 3.8 ± 1.0 | 2.4 ± 0.6 | 3.1 ± 0.5 |
| Basophil (%) | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| Platelet (103/mcL) | 252 ± 13 | 273 ± 15 | 271 ± 13 | 267 ± 11 |
| NLR | 1.39 ± 0.15 | 1.60 ± 0.25 | 1.40 ± 0.14 | 1.65 ± 0.17 |
| PLR | 96 ± 8 | 112 ± 10 | 102 ± 7 | 115 ± 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-L.; Hsu, Y.-J.; Ho, H.-H.; Chang, Y.-C.; Kuo, Y.-W.; Yeh, Y.-T.; Tsai, S.-Y.; Chen, C.-W.; Chen, J.-F.; Huang, C.-C.; et al. Bifidobacterium longum subsp. longum OLP-01 Supplementation during Endurance Running Training Improves Exercise Performance in Middle- and Long-Distance Runners: A Double-Blind Controlled Trial. Nutrients 2020, 12, 1972. https://doi.org/10.3390/nu12071972
Lin C-L, Hsu Y-J, Ho H-H, Chang Y-C, Kuo Y-W, Yeh Y-T, Tsai S-Y, Chen C-W, Chen J-F, Huang C-C, et al. Bifidobacterium longum subsp. longum OLP-01 Supplementation during Endurance Running Training Improves Exercise Performance in Middle- and Long-Distance Runners: A Double-Blind Controlled Trial. Nutrients. 2020; 12(7):1972. https://doi.org/10.3390/nu12071972
Chicago/Turabian StyleLin, Che-Li, Yi-Ju Hsu, Hsieh-Hsun Ho, Yung-Cheng Chang, Yi-Wei Kuo, Yao-Tsung Yeh, Shin-Yu Tsai, Ching-Wei Chen, Jui-Fen Chen, Chi-Chang Huang, and et al. 2020. "Bifidobacterium longum subsp. longum OLP-01 Supplementation during Endurance Running Training Improves Exercise Performance in Middle- and Long-Distance Runners: A Double-Blind Controlled Trial" Nutrients 12, no. 7: 1972. https://doi.org/10.3390/nu12071972
APA StyleLin, C.-L., Hsu, Y.-J., Ho, H.-H., Chang, Y.-C., Kuo, Y.-W., Yeh, Y.-T., Tsai, S.-Y., Chen, C.-W., Chen, J.-F., Huang, C.-C., & Lee, M.-C. (2020). Bifidobacterium longum subsp. longum OLP-01 Supplementation during Endurance Running Training Improves Exercise Performance in Middle- and Long-Distance Runners: A Double-Blind Controlled Trial. Nutrients, 12(7), 1972. https://doi.org/10.3390/nu12071972







