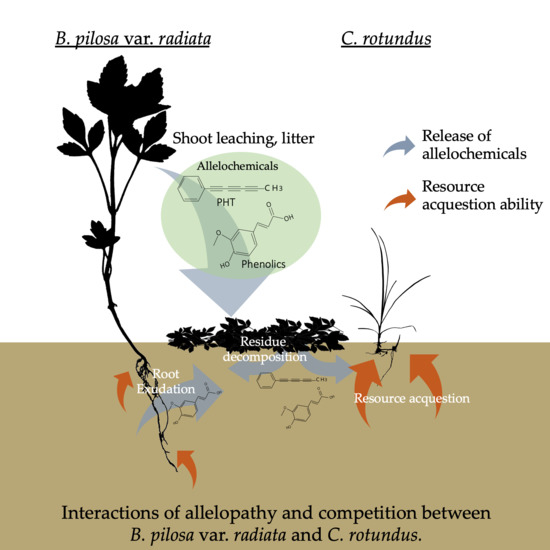
Allelopathic Effects of Bidens pilosa L. var. radiata Sch. Bip. on the Tuber Sprouting and Seedling Growth of Cyperus rotundus L.
Abstract
:1. Introduction
2. Results
2.1. Experiment 1: Density-Dependent Phytotoxicity
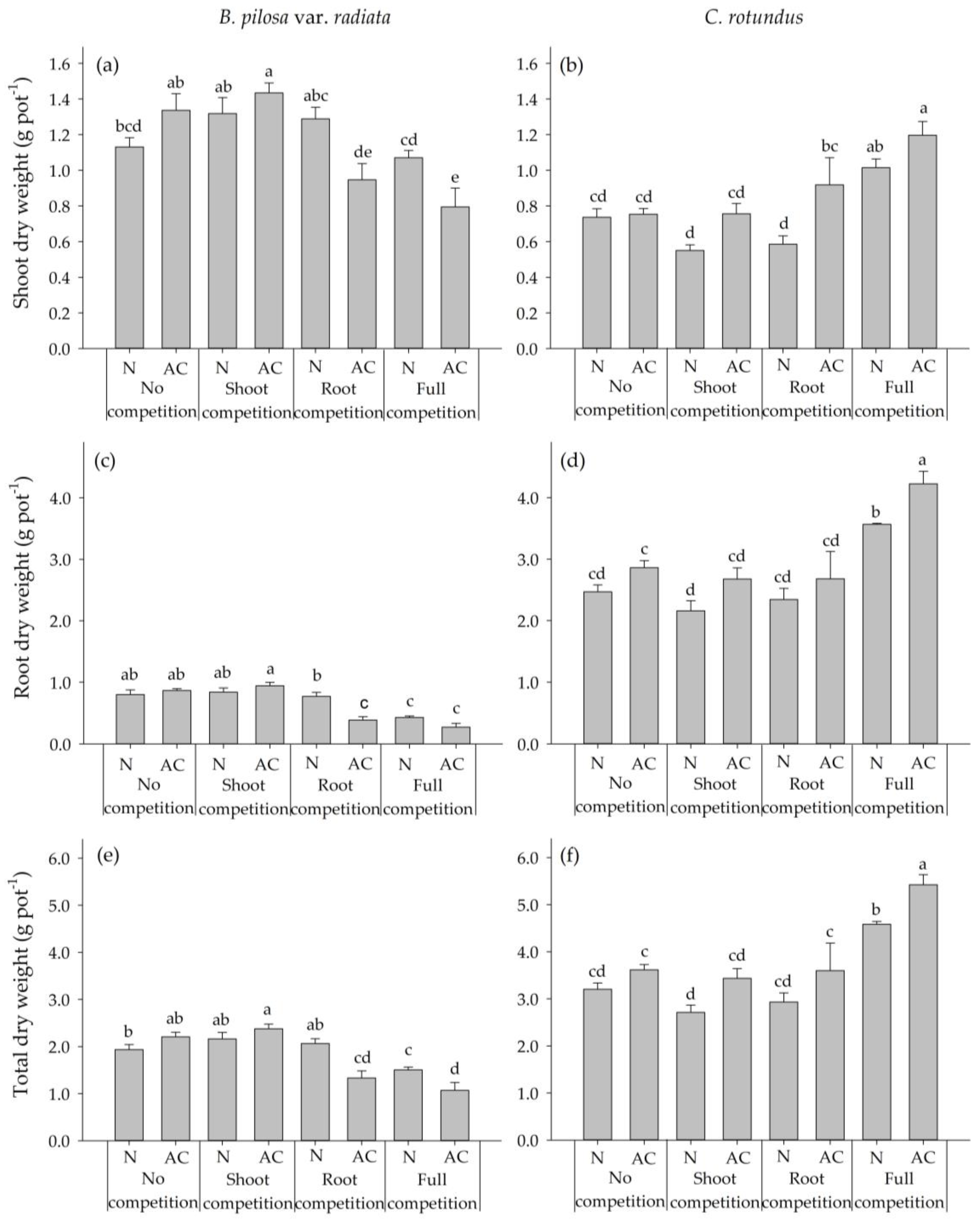
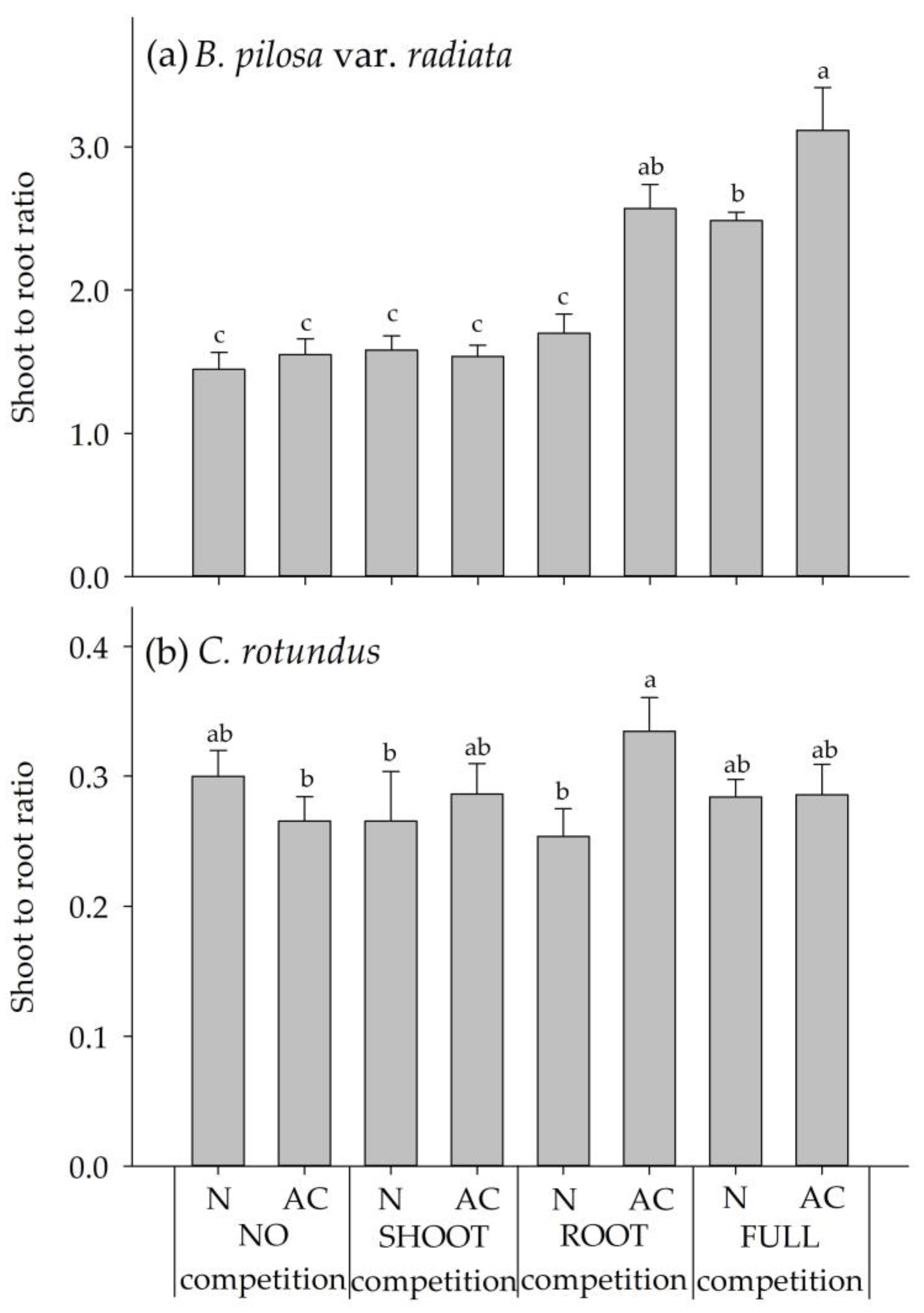
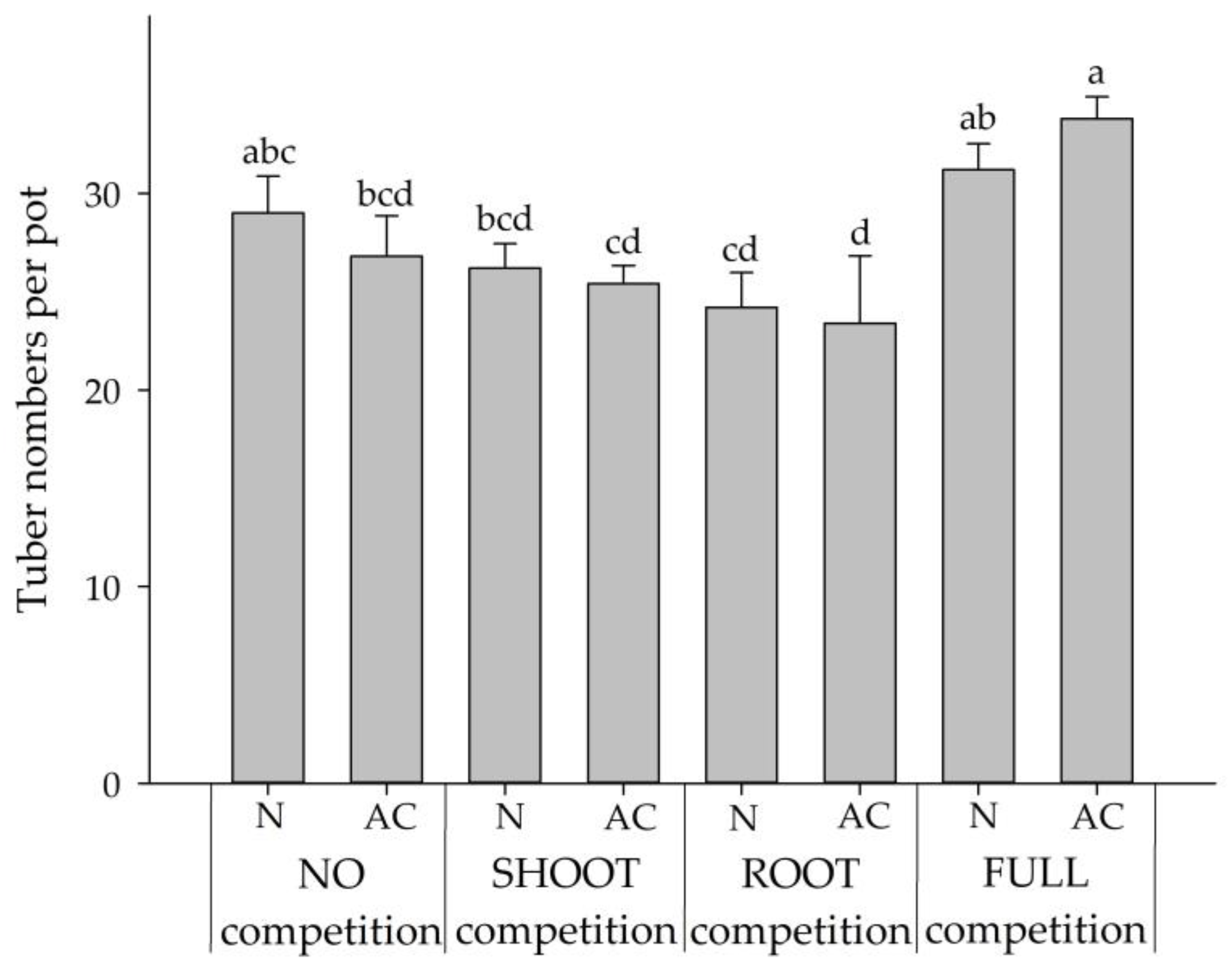
2.2. Experiment 2: Interspecies Competition between BPr and CR
2.3. Experiment 3: The Tuber Sprouting of CR in the Field of Mature BPr Vegetation
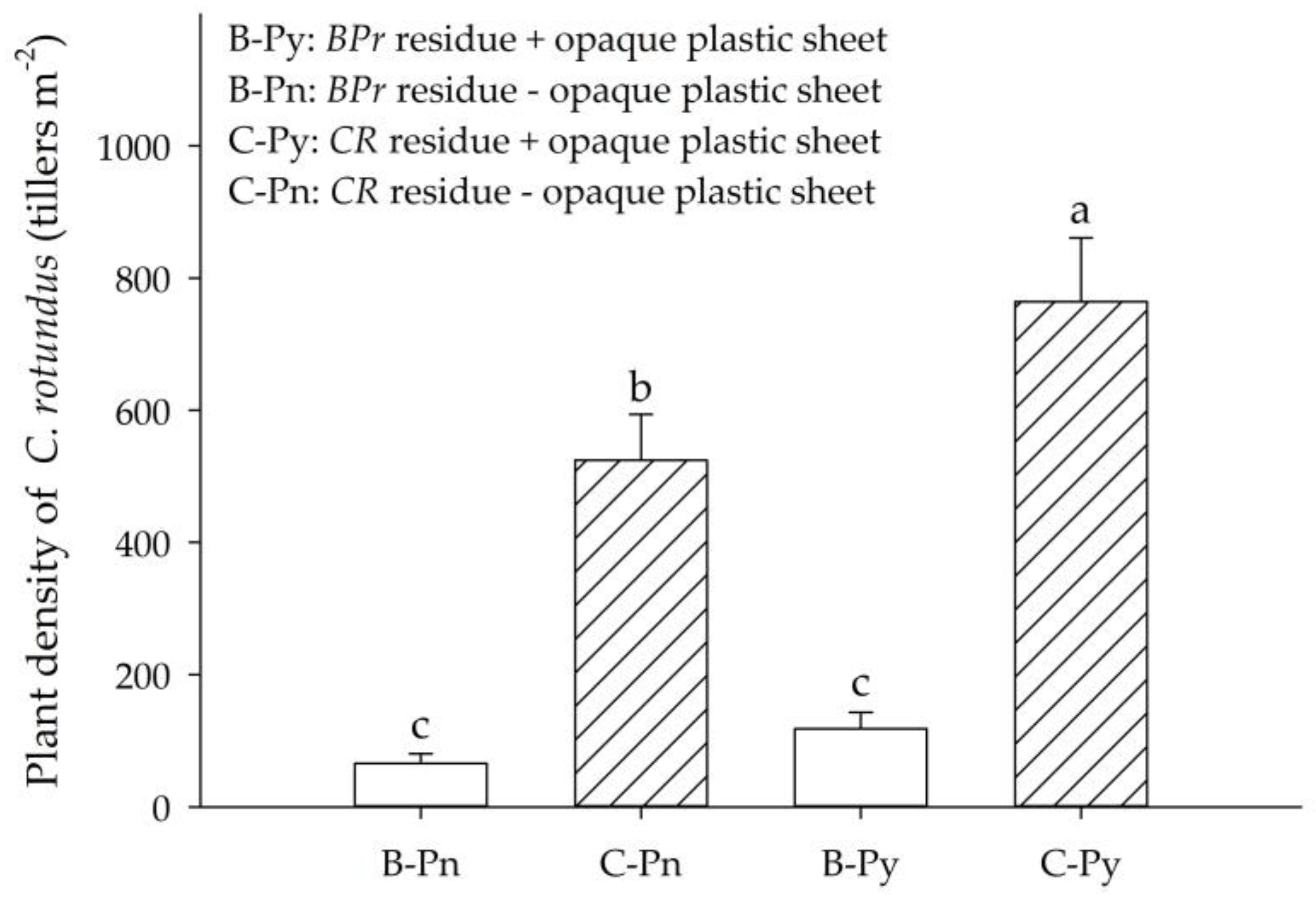
2.4. Experiment 4: The Effects of Vegetation and Residue Mulch of BPr on the Reproduction of CR
3. Discussion
3.1. Distinguishing Allelopathy from Competition
3.2. The Influence of B. pilosa var. Radiata on the Reproduction of CR in the Field
4. Materials and Methods
4.1. Plant Material Preparation
4.2. General Experimental Design
4.3. Experiment 1: Density-Dependent Phytotoxicity
4.4. Experiment 2: Interspecies Competition between B. pilosa var. radiata and C. rotundus
4.5. Experiment 3: The Tuber Sprouting of CR in the Field of Mature BPr Vegetation
4.6. Experiment 4: The Effects of Vegetation and Residue Mulch of BPr on the Reproduction of CR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peerzada, A.M. Biology, agricultural impact, and management of Cyperus rotundus L.: The world’s most tenacious weed. Acta Physiol. Plant. 2017, 39, 270. [Google Scholar] [CrossRef]
- Bendixen, L.E.; Nandihalli, U.B. Worldwide distribution of purple and yellow nutsedge (Cyperus rotundus and C. esculentus). Weed Technol. 1987, 1, 61–65. [Google Scholar] [CrossRef]
- Baloch, A.H.; Rehman, H.; Ibrahim, Z.; Buzdar, M.A.; Ahmad, S. The biology of Balochistani weed: Cyperus rotundus Linnaeus. A review. Pure Appl. Biol. 2015, 4, 171–180. [Google Scholar] [CrossRef]
- Nishimoto, R.K. Purple nutsedge tuber sprouting. Weed Biol. Manag. 2001, 1, 203–208. [Google Scholar] [CrossRef]
- Bangarwa, S.K.; Norsworthy, J.K.; Jha, P.; Malik, M. Purple nutsedge (Cyperus rotundus) management in an organic production system. Weed Sci. 2008, 56, 606–613. [Google Scholar] [CrossRef]
- William, R.D.; Warren, G.F. Competition between purple nutsedge and vegetables. Weed Sci. 1975, 23, 317–323. [Google Scholar] [CrossRef]
- Morales-Payan, J.P.; Santos, B.M.; Stall, W.M.; Bewick, T.A. Effects of purple nutsedge (Cyperus rotundus) on tomato (Lycopersicon esculentum) and bell pepper (Capsicum annuum) vegetative growth and fruit yield. Weed Technol. 1997, 11, 672–676. [Google Scholar] [CrossRef]
- Geretharan, T.; Sangakkara, U.R.; Arulnandhy, V. Competitive performance of purple nutsedge (Cyperus rotundus L.) and onion (Allium cepa L.) as affected by different sources of nitrogen. Trop. Agric. Res. 2012, 23, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Cirujeda, A.; Anzalone, A.; Aibar, J.; Moreno, M.M.; Zaragoza, C. Purple nutsedge (Cyperus rotundus L.) control with paper mulch in processing tomato. Crop. Prot. 2012, 39, 66–71. [Google Scholar] [CrossRef]
- Inderjit; del Moral, R. Is separating resource competition from allelopathy realistic? Bot. Rev. 1997, 63, 221–230. [Google Scholar] [CrossRef]
- Duke, S.O. Proving allelopathy in crop–weed interactions. Weed Sci. 2015, 63, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic interactions and allelochemicals: New possibilities for sustainable weed management. Crit. Rev. Plant Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Viji, N.; Chinnamuthu, C.R. Breaking dormancy and inducing germination of the world worst weed the Cyperus rotundus using nanoparticles. Ann. Plant Soil Res. 2015, 17, 361–363. [Google Scholar]
- Viji, N.; Chinnamuthu, C.R. Nanoparticle effect on degradation of vanillic acid, a germination inhibiting dormancy factor present in Cyperus rotundus. Indian J. Weed Sci. 2019, 51, 98–100. [Google Scholar] [CrossRef]
- Babu, R.C.; Kandasamy, O.S. Allelopathic effect of Eucalyptus globulus Labill. on Cyperus rotundus L. and Cynodon dactylon L. Pers. J. Agron. Crop Sci. 1997, 179, 123–126. [Google Scholar] [CrossRef]
- Cheema, Z.A.; Khaliq, A.; Saeed, S. Weed control in maize (Zea mays L.) through sorghum allelopathy. J. Sustain. Agric. 2004, 23, 73–86. [Google Scholar] [CrossRef]
- Iqbal, J.; Cheema, Z.A.; An, M. Intercropping of field crops in cotton for the management of purple nutsedge (Cyperus rotundus L.). Plant Soil 2007, 300, 163–171. [Google Scholar] [CrossRef]
- Campbell, G.; Lambert, J.D.H.; Arnason, T.; Towers, G.H.N. Allelopathic properties of α-terthienyl and phenylheptatriyne, naturally occurring compounds from species of Asteraceae. J. Chem. Ecol. 1982, 8, 961–972. [Google Scholar] [CrossRef]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Herbicidal and fungicidal activities and identification of potential phytotoxins from Bidens pilosa L. var. radiata Scherff. Weed Biol. Manag. 2007, 7, 77–83. [Google Scholar] [CrossRef]
- Hsu, H.M.; Kao, W.Y. Contrasting effects of aqueous tissue extracts from an invasive plant, Bidens pilosa L. var. radiata, on the performance of its sympatric plant species. Taiwania 2009, 54, 255–260. [Google Scholar]
- Zhang, K.; Shen, Y.; Fang, Y.M.; Liu, Y. Changes in gametophyte physiology of Pteris multifida induced by the leaf leachate treatment of the invasive Bidens pilosa. Environ. Sci. Pollut. Res. 2016, 23, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Khanh, T.D. Chemistry and pharmacology of Bidens pilosa: An overview. J. Pharm. Investig. 2016, 46, 91–132. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Poolkum, S. Utilization of Bidens pilosa var. radiata (Sch. Bip.) Sherff integrated with water irrigation for paddy weed control and rice yield production. Weed Biol. Manag. 2019, 19, 31–38. [Google Scholar] [CrossRef]
- Galon, L.; Concenço, G.; Perin, G.F.; da Silva, A.F.; Forte, C.T.; David, F.A.; Radüz, L.L.; Radunz, A.L.; Andres, A.; Tironi, S.P. Comparison of experimental methods to assess the competitive ability of weed species. Am. J. Plant Sci. 2015, 6, 2185–2196. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Feng, Z.; Liang, X.; Xu, W.; Su, Y.; Song, Y.; Zeng, R. Comparative allelopathic and competitive abilities of 3-native forage legumes and the invasive weed Bidens pilosa L. Allelopath. J. 2012, 29, 297–306. [Google Scholar]
- Weidenhamer, J.D. Distinguishing allelopathy from resource competition: The role of density. In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M.J., Pedrol, N., Gonzalez, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 85–103. [Google Scholar]
- Weidenhamer, J.D.; Hartnett, D.C.; Romeo, J.T. Density-dependent phytotoxicity: Distinguishing resource competition and allelopathic interference in plants. J. Appl. Ecol. 1989, 26, 613–624. [Google Scholar] [CrossRef]
- Blum, D.J.W.; Suffet, I.H.; Duguet, J.P. Estimating the activated carbon adsorption of organic chemicals in water. Crit. Rev. Environ. Sci. Technol. 1993, 23, 121–136. [Google Scholar] [CrossRef]
- Nilsson, M.C. Separation of allelopathy and resource competition by the boreal dwarf shrub Empetrum hermaphroditum Hagerup. Oecologia 1994, 98, 1–7. [Google Scholar] [CrossRef]
- Mahall, B.E.; Callaway, R.M. Root communication mechanisms and intracommunity distributions of two Mojave Desert shrubs. Ecology 1992, 73, 2145–2151. [Google Scholar] [CrossRef]
- Khaliq, A.; Matloob, A.; Irshad, M.S.; Tanveer, A.; Zamir, M.S.I. Organic weed management in maize (Zea mays L.) through integration of allelopathic crop residues. Pak. J. Weed Sci. Res. 2010, 16, 409–420. [Google Scholar]
- Matloob, A.; Khaliq, A.; Farooq, M.; Cheema, Z.A. Quantification of allelopathic potential of different crop residues for the purple nutsedge suppression. Pak. J. Weed Sci. Res. 2010, 16, 1–12. [Google Scholar]
- Fuerst, E.P.; Putnam, A.R. Separating the competitive and allelopathic components of interference. J. Chem. Ecol. 1983, 9, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Lavanya, K.; Singh, H.P.; Kohli, R.K. Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul. 2007, 51, 119–128. [Google Scholar] [CrossRef]
- Weiner, J. Asymmetric competition in plant populations. Trends Ecol. Evol. 1990, 5, 360–364. [Google Scholar] [CrossRef]
- Tuor, F.A.; Froud-Williams, R.J. Influence of nitrogen on competition between purple nutsedge, maize and soybean. Int. J. Pest Manag. 2002, 48, 73–79. [Google Scholar] [CrossRef]
- Horowitz, M. Competitive effects of three perennial weeds, Cynodon dactylon (L.) Pers., Cyperns rotundus L. and Sorghum halepense (L.) Pers., on young citrus. J. Hortic. Sci. 1973, 48, 135–147. [Google Scholar] [CrossRef]
- Kobe, R.K.; Iyer, M.; Walters, M.B. Optimal partitioning theory revisited: Nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology 2010, 91, 166–179. [Google Scholar] [CrossRef]
- Williams, R.D.; Quimby, P.C.; Frick, K.E. Intraspecific competition of purple nutsedge (Cyperus rotundus) under greenhouse conditions. Weed Sci. 1977, 25, 477–481. [Google Scholar] [CrossRef]
- Bergmark, C.L.; Jackson, W.A.; Volk, R.J.; Blum, U. Differential inhibition by ferulic acid of nitrate and ammonium uptake in Zea mays L. Plant Physiol. 1992, 98, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Schenk, H.J. Root competition: Beyond resource depletion. J. Ecol. 2006, 94, 725–739. [Google Scholar] [CrossRef]
- El-Rokiek, K.G.; El-Masry, R.R.; Messiha, N.K.; Ahmed, S.A. The allelopathic effect of mango leaves on the growth and propagative capacity of purple nutsedge (Cyperus rotundus L.). J. Am. Sci. 2010, 6, 151–159. [Google Scholar]
- Einhellig, F.A. Mechanism of action of allelochemicals in allelopathy. In Allelopathy, Organisms, Processes and Applications; Inderjit, Dakshini, K.M.M., Einhellig, F.A., Eds.; ACS Symposium Series; American chemical society: Washington, DC, USA, 1995; Volume 520, pp. 96–116. [Google Scholar]
- Abenavoli, M.R.; Lupini, A.; Oliva, S.; Sorgonà, A. Allelochemical effects on net nitrate uptake and plasma membrane H+-ATPase activity in maize seedlings. Biol. Plant. 2010, 54, 149–153. [Google Scholar] [CrossRef]
- Blum, U. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J. Chem. Ecol. 1998, 24, 685–708. [Google Scholar] [CrossRef]
- Blum, U. Allelopathy: A soil system perspective. In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M.J., Pedrol, N., Gonzalez, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 299–340. [Google Scholar]
- Schmidt, S.; Ley, R.E. Microbial competition and soil structure limit the expression of allelochemicals in nature. In Principles and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, Dakshini, K.M.M., Chester, L.F., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 339–351. [Google Scholar]
- Mahmood, A.R.I.F.; Cheema, Z.A. Influence of sorghum mulch on purple nutsedge (Cyperus rotundus L.). Int. J. Agric. Biol. 2004, 6, 86–88. [Google Scholar]
- Boz, O. Allelopathic effects of wheat and rye straw on some weeds and crops. Asian J. Plant Sci. 2003, 2, 772–778. [Google Scholar]
- Patterson, D.T. Suppression of purple nutsedge (Cyperus rotundus) with polyethylene film mulch. Weed Technol. 1998, 12, 275–280. [Google Scholar] [CrossRef]
- Wang, G.; McGiffen Jr, M.E.; Ogbuchiekwe, E.J. Crop rotation effects on Cyperus rotundus and C. esculentus population dynamics in southern California vegetable production. Weed Res. 2008, 48, 420–428. [Google Scholar] [CrossRef]
- Politycka, B.; Adamska, D. Release of phenolic compounds from apple residues decomposing in soil and the influence of temperature on their degradation. Pol. J. Environ. Stud. 2003, 12, 95–98. [Google Scholar]
- Stevens, K.L. Polyacetylenes as allelochemicals. In The Science of Allelopathy; Putnam, A.R., Tang, C.S., Eds.; John Wiley & Sons: New York, NY, USA, 1986; pp. 219–228. [Google Scholar]
- Xuan, T.D.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Minh, T.N.; Khanh, T.D.; Trung, K.H. Weed allelochemicals and possibility for pest management. Int. Lett. Nat. Sci. 2016, 56, 25–39. [Google Scholar] [CrossRef]
- Ng, C.C.; Wu, S.J.; Wang, C.Y.; Tzeng, W.S.; Shyu, Y.T. Emergence and growth of beggarticks (Bidens pilosa var. radiata) in different plant communities under experimental field conditions. J. Agric. Sci. Technol. A 2011, 1, 950–962. [Google Scholar]
- Hong, N.H.; Xuan, T.D.; Eiji, T.; Khanh, T.D. Paddy weed control by higher plants from Southeast Asia. Crop Prot. 2004, 23, 255–261. [Google Scholar] [CrossRef]
- Krumsri, R.; Suwunnamek, U.; Homhaul, W.; Laosinwattana, C.; Poonpaiboonpipattana, T. Allelopathic effects of Bidens pilosa var. radiata and its preliminary utilization to control weeds in rice. J. Agric. Technol. 2015, 11, 1875–1886. [Google Scholar]
- Scavo, A.; Restuccia, A.; Abbate, C.; Mauromicale, G. Seeming field allelopathic activity of Cynara cardunculus L. reduces the soil weed seed bank. Agron. Sustain. Dev. 2019, 39, 41. [Google Scholar] [CrossRef]
- Hsiao, C.L. Safe production techniques of Bidens pilosa for high content of functional compounds. Stock-Farming Tendays 2017, 1908, 43–44. [Google Scholar]
- Tembo, Y.; Mkindi, A.G.; Mkenda, P.A.; Mpumi, N.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.A.; Belmain, S.R. Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front. Plant Sci. 2018, 9, 1425. [Google Scholar] [CrossRef]
- Snaydon, R.W. An analysis of competition between plants of Trifolium repens L. populations collected from contrasting soils. J. Appl. Ecol. 1971, 8, 687–697. [Google Scholar] [CrossRef]
- Shen, M.L. Experimental Designs; Jeou Chou Book Co., Ltd.: Taipei, Taiwan, 2010. [Google Scholar]
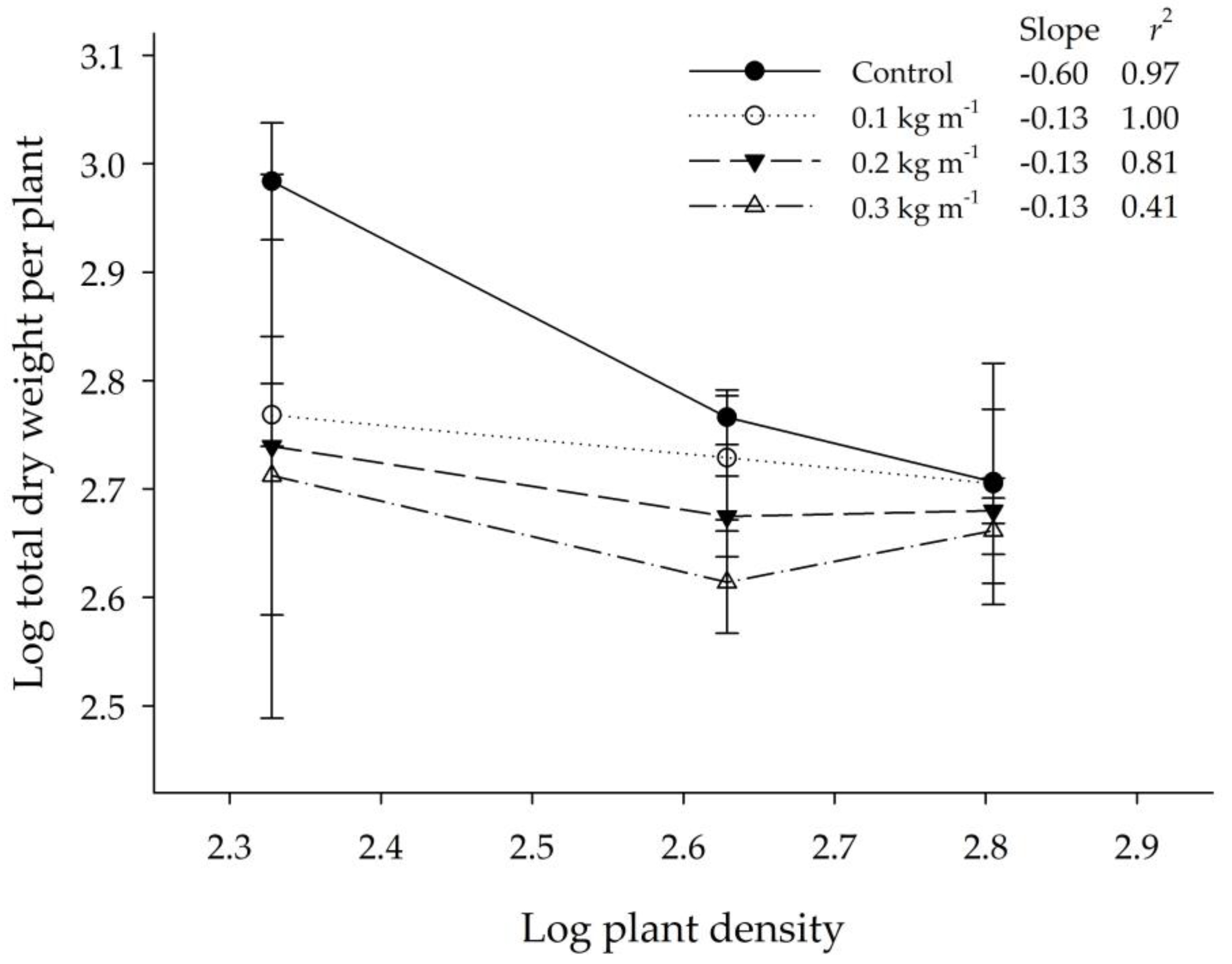


| Application Rate, Residue of BPr (kg m−2) | 3 Plants Pot−1 | 6 Plants Pot−1 | 9 plants Pot−1 |
|---|---|---|---|
| Shoot dry weight per plant (mg) | |||
| 0 | 451.11 ± 9.09 Aa (100) | 253.89 ± 17.01 Ba (100) | 194.44 ± 15.76 Ca (100) |
| 0.1 | 325.00 ± 9.08 Aab (72) | 230.00 ± 14.24 Bab (91) | 193.33 ± 25.00 Ba (99) |
| 0.2 | 305.56 ± 84.71 Aab (68) | 217.78 ± 20.21 Aab (86) | 209.26 ± 3.29 Aa (108) |
| 0.3 | 291.11 ± 53.48 Ab (65) | 192.78 ± 16.84 ABb (76) | 192.78 ± 7.61 Ba (99) |
| Root dry weight per plant (mg) | |||
| 0 | 512.22 ± 59.92 Aa (100) | 330.00 ± 6.94 Ba (100) | 314.44 ± 22.33 Ba (100) |
| 0.1 | 261.67 ± 14.56 Ab (51) | 305.56 ± 28.81 Aab (93) | 313.33 ± 45.03 Aa (100) |
| 0.2 | 243.33 ± 85.05 Ab (48) | 255.00 ± 26.03 Abc (77) | 269.26 ± 9.86 Aa (86) |
| 0.3 | 224.44 ± 42.92 Ab (44) | 218.33 ± 15.28 Ac (66) | 265.83 ± 18.93 Aa (85) |
| Total dry weight per plant (mg) | |||
| 0 | 963.33 ± 67.41 Aa (100) | 583.89 ± 19.35 Ba (100) | 508.89 ± 37.74 Ba (100) |
| 0.1 | 586.67 ± 20.14 Ab (61) | 535.56 ± 39.84 Aab (92) | 506.67 ± 69.15 Aa (100) |
| 0.2 | 548.89 ±168.88 Ab (57) | 472.78 ± 22.96 Abc (81) | 478.52 ± 7.52 Aa (94) |
| 0.3 | 515.56 ± 83.23 Ab (54) | 411.11 ± 24.95 Ac (70) | 458.61 ± 24.97 Aa (90) |
| Application Rate, Residue of BPr (kg m−2) | 3 Plants Pot−1 | 6 Plants Pot−1 | 9 Plants Pot−1 |
|---|---|---|---|
| 0 | 0.90 ± 0.09 Ab | 0.77 ± 0.05 Aa | 0.62 ± 0.01 Bb |
| 0.1 | 1.25 ± 0.06 Aab | 0.76 ± 0.06 Ba | 0.62 ± 0.03 Bb |
| 0.2 | 1.32 ± 0.10 Aa | 0.88 ± 0.14 Ba | 0.78 ± 0.04 Ba |
| 0.3 | 1.36 ± 0.23 Aa | 0.89 ± 0.09 ABa | 0.73 ± 0.04 Ba |
| Application Rate, Residue of BPr (kg m−2) | 3 Plants Pot−1 | 6 Plants Pot−1 | 9 Plants Pot−1 |
|---|---|---|---|
| Tuber numbers per plant | |||
| 0 | 5.33 ± 0.69 Aa (100) | 3.06 ± 0.20 Ba (100) | 2.86 ± 0.16 Ba (100) |
| 0.1 | 3.67 ± 0.14 Ab (69) | 2.94 ± 0.24 Ba (96) | 2.85 ± 0.16 Ba (100) |
| 0.2 | 3.11 ± 0.73 Ab (58) | 2.94 ± 0.20 Aa (96) | 2.37 ± 0.07 Ab (83) |
| 0.3 | 3.78 ± 0.40 Aab (71) | 2.72 ± 0.20 Aba (89) | 2.36 ± 0.08 Bb (83) |
| Tiller numbers per plant | |||
| 0 | 2.78 ± 0.29 Aa (100) | 1.83 ± 0.10 Ba (100) | 1.75 ± 0.05 Bab (100) |
| 0.1 | 2.33 ± 0.14 Aa (84) | 1.83 ± 0.00 Ba (100) | 1.56 ± 0.06 Bb (89) |
| 0.2 | 2.00 ± 0.33 Aa (72) | 2.17 ± 0.25 Aa (118) | 1.93 ± 0.20 Aa (110) |
| 0.3 | 2.44 ± 0.40 Aa (88) | 1.94 ± 0.36 Aa (106) | 1.67 ± 0.08 Aab (95) |
| Treatments | Tuber Sprouting Percentage (%) | Mean Sprouts per Quadrat | Dry Weight per Sprout (mg) |
|---|---|---|---|
| OP | 81.00 ± 0.05 a | 30.75 ± 2.25 a | 9.98 ± 1.76 a |
| VN | 52.00 ± 0.09 ab | 18.00 ± 3.03 ab | 5.26 ± 0.76 ab |
| VS | 1.00 ± 0.01 b | 0.25 ± 0.25 b | 1.50 ± 1.50 b |
| Cover Plants | Tuber Density (tubers dm−2) | Dry Weight per Tuber (mg tuber−1) |
|---|---|---|
| 0 DAS (18 October 2019) | ||
| BPr | 55.51 ± 5.30 a | 98.41 ± 6.68 a |
| CR | 60.44 ± 6.90 a | 101.12 ± 7.81 a |
| 69 DAS (26 December 2019) | ||
| BPr | 92.50 ± 9.59 a | 49.51 ± 4.69 b |
| CR | 104.91 ± 9.38 a | 63.31 ± 4.94 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, M.-T.; Fan, C.; Chang, W.-L. Allelopathic Effects of Bidens pilosa L. var. radiata Sch. Bip. on the Tuber Sprouting and Seedling Growth of Cyperus rotundus L. Plants 2020, 9, 742. https://doi.org/10.3390/plants9060742
Hsueh M-T, Fan C, Chang W-L. Allelopathic Effects of Bidens pilosa L. var. radiata Sch. Bip. on the Tuber Sprouting and Seedling Growth of Cyperus rotundus L. Plants. 2020; 9(6):742. https://doi.org/10.3390/plants9060742
Chicago/Turabian StyleHsueh, Ming-Tung, Chihhao Fan, and Wen-Lian Chang. 2020. "Allelopathic Effects of Bidens pilosa L. var. radiata Sch. Bip. on the Tuber Sprouting and Seedling Growth of Cyperus rotundus L." Plants 9, no. 6: 742. https://doi.org/10.3390/plants9060742
APA StyleHsueh, M.-T., Fan, C., & Chang, W.-L. (2020). Allelopathic Effects of Bidens pilosa L. var. radiata Sch. Bip. on the Tuber Sprouting and Seedling Growth of Cyperus rotundus L. Plants, 9(6), 742. https://doi.org/10.3390/plants9060742