Genetically Modified Mesenchymal Stem Cells: The Next Generation of Stem Cell-Based Therapy for TBI
Abstract
:1. Introduction
2. Search Strategy and Selection Criteria
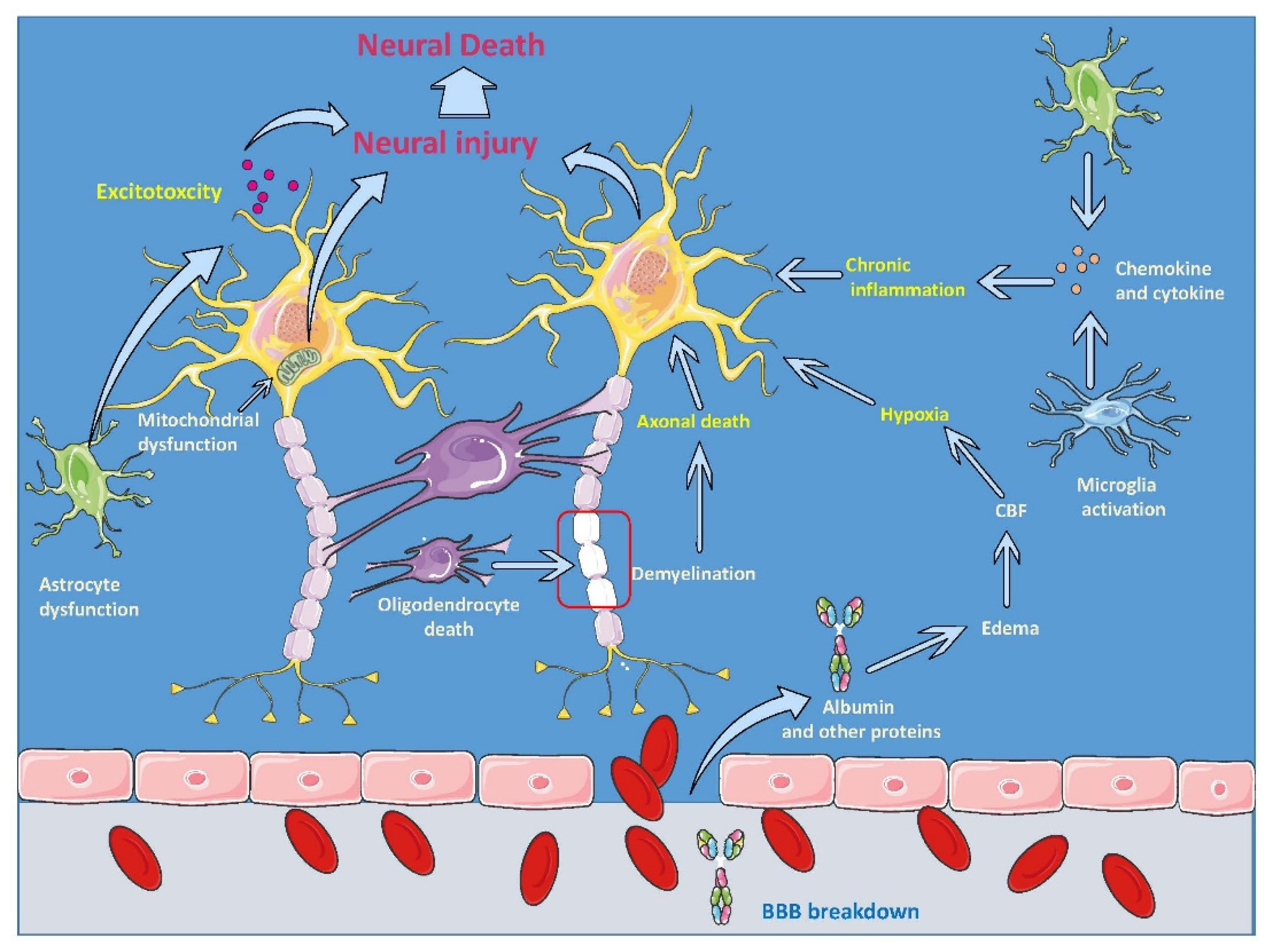
3. Neuroinflammatory Cascade of TBI
4. MSC-Based Therapy for TBI
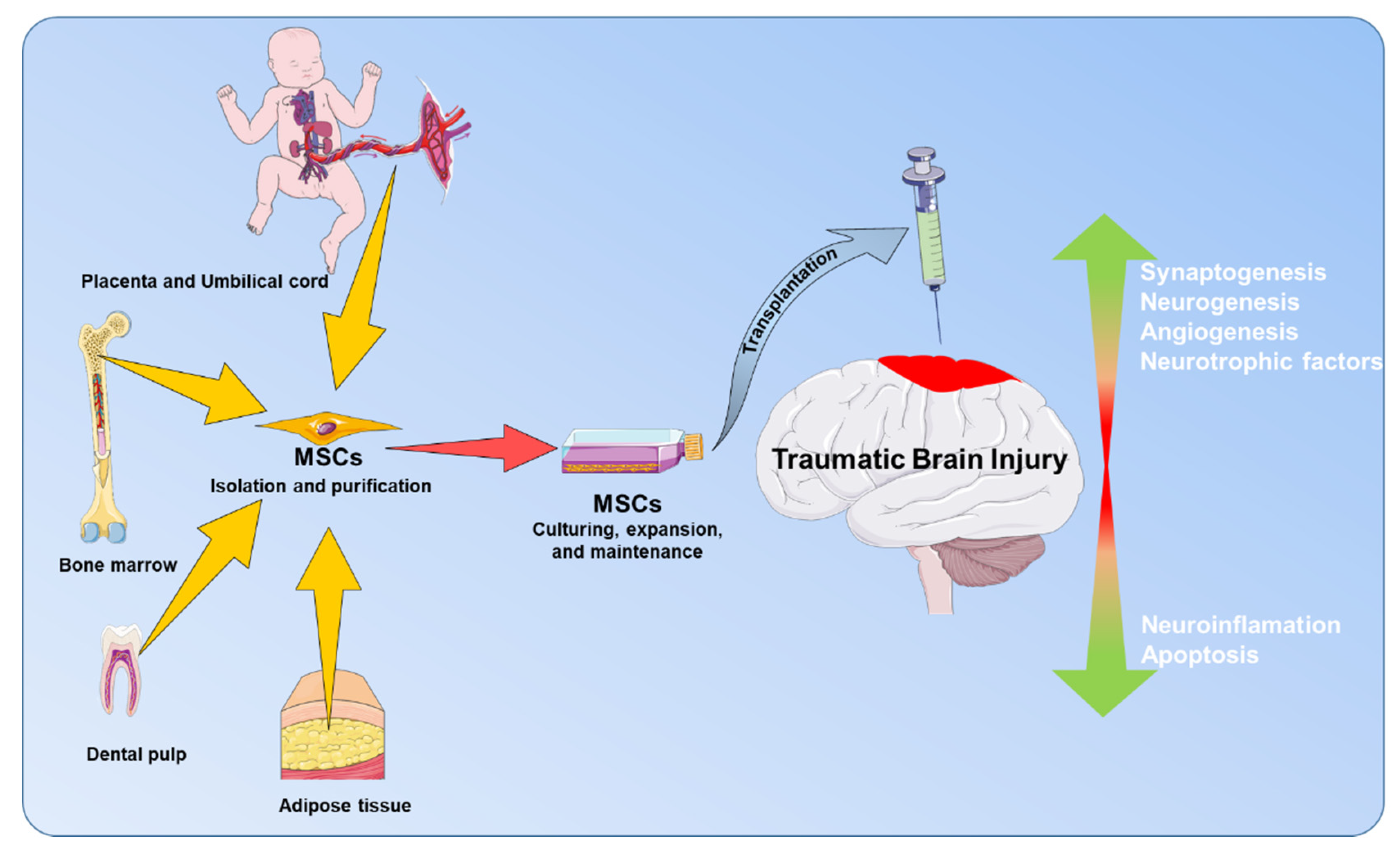
4.1. MSCs’ Biology
4.2. MSCs’ Application for TBI Treatment
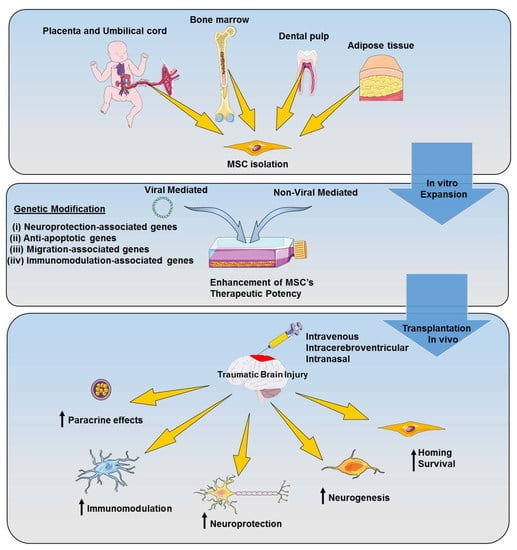
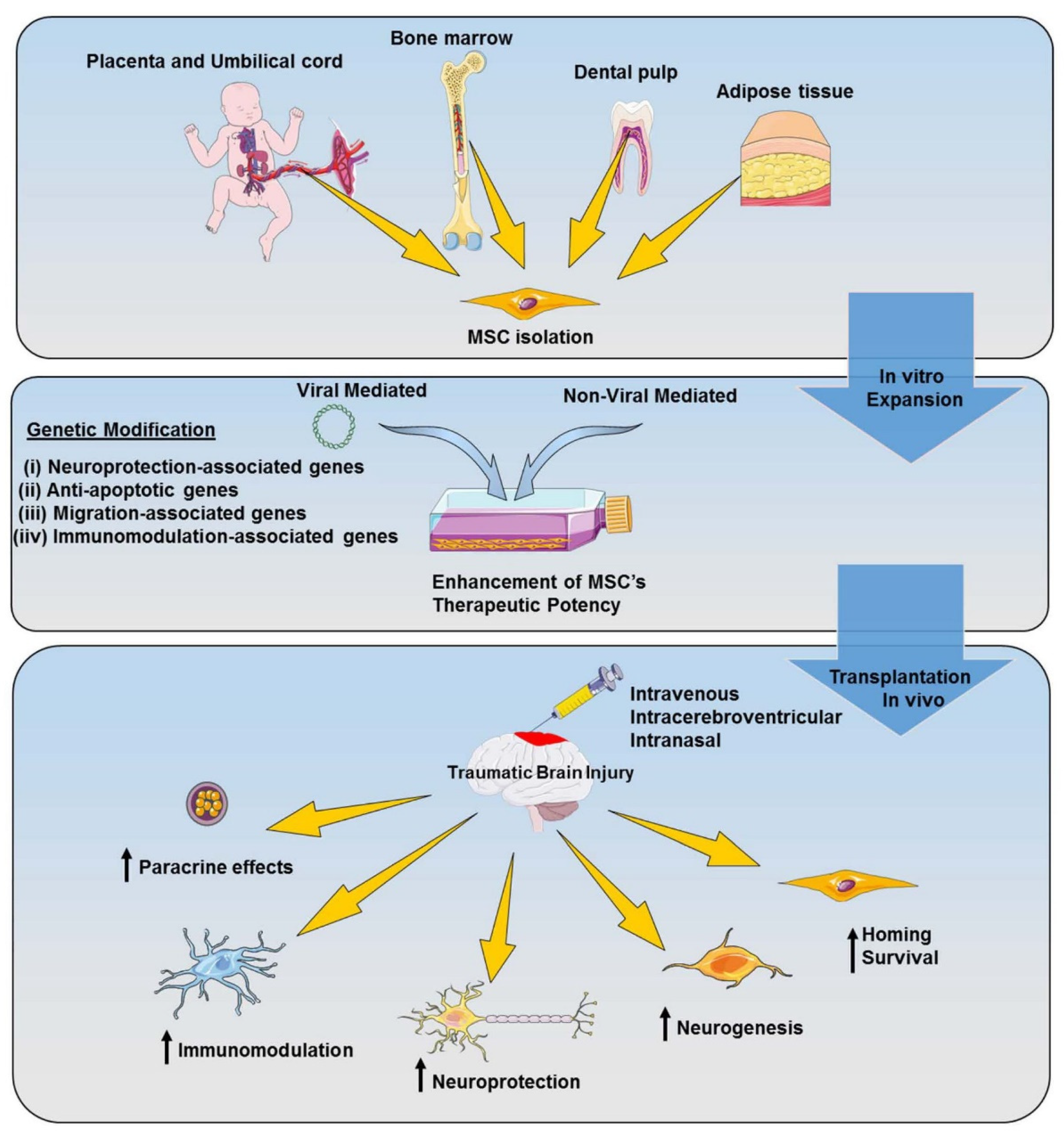
5. Genetically Modified MSC-Based Therapy for TBI
5.1. Viral Vector-Mediated Genetic Modification
5.2. Non-Viral Vector-Mediated Genetic Modification
5.3. Application of Genetically Modified MSCs for TBI Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murray, C.J.; Lopez, A.D. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997, 349, 1269–1276. [Google Scholar] [CrossRef]
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Thurman, D.J.; Alverson, C.; Dunn, K.A.; Guerrero, J.; Sniezek, J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999, 14, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Coronado, V.G.; Xu, L.; Basavaraju, S.V.; McGuire, L.C.; Wald, M.M.; Faul, M.D.; Guzman, B.R.; Hemphill, J.D.; Centers for Disease Control and Prevention. Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill. Summ. 2011, 60, 1–32. [Google Scholar] [PubMed]
- Somayaji, M.R.; Przekwas, A.J.; Gupta, R.K. Combination Therapy for Multi-Target Manipulation of Secondary Brain Injury Mechanisms. Curr. Neuropharmacol. 2018, 16, 484–504. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Lee, N.K.; Na, D.L.; Chang, J.W.; Chang, J.W. Optimal mesenchymal stem cell delivery routes to enhance neurogenesis for the treatment of Alzheimer’s disease. Histol. Histopathol. 2018, 33, 533–541. [Google Scholar] [PubMed]
- Osaka, M.; Honmou, O.; Murakami, T.; Nonaka, T.; Houkin, K.; Hamada, H.; Kocsis, J.D. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010, 1343, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yang, X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transpl. 2019, 28, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Muniswami, D.M.; Kanthakumar, P.; Kanakasabapathy, I.; Tharion, G. Motor Recovery after Transplantation of Bone Marrow Mesenchymal Stem Cells in Rat Models of Spinal Cord Injury. Ann. Neurosci. 2019, 25, 126–140. [Google Scholar] [CrossRef]
- Qi, L.; Xue, X.; Sun, J.; Wu, Q.; Wang, H.; Guo, Y.; Sun, B. The Promising Effects of Transplanted Umbilical Cord Mesenchymal Stem Cells on the Treatment in Traumatic Brain Injury. J. Craniofac. Surg. 2018, 29, 1689–1692. [Google Scholar] [CrossRef]
- Anbari, F.; Khalili, M.A.; Bahrami, A.R.; Khoradmehr, A.; Sadeghian, F.; Fesahat, F.; Nabi, A. Intravenous transplantation of bone marrow mesenchymal stem cells promotes neural regeneration after traumatic brain injury. Neural Regen. Res. 2014, 9, 919–923. [Google Scholar] [CrossRef]
- van Velthoven, C.T.; Sheldon, R.A.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.L.; Maas, M.; Heijnen, C.J.; Ferriero, D.M. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 2013, 44, 1426–1432. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Urnukhsaikhan, E.; Yoon, H.H.; Seo, Y.K.; Park, J.K. Effect of human mesenchymal stem cell transplantation on cerebral ischemic volume-controlled photothrombotic mouse model. Biotechnol. J. 2016, 11, 1397–1404. [Google Scholar] [CrossRef]
- Wang, F.; Tang, H.; Zhu, J.; Zhang, J.H. Transplanting Mesenchymal Stem Cells for Treatment of Ischemic Stroke. Cell Transpl. 2018, 27, 1825–1834. [Google Scholar] [CrossRef]
- Pires, A.O.; Teixeira, F.G.; Mendes-Pinheiro, B.; Serra, S.C.; Sousa, N.; Salgado, A.J. Old and new challenges in Parkinson’s disease therapeutics. Prog. Neurobiol. 2017, 156, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, A.; Yasuhara, T.; Kameda, M.; Morimoto, J.; Takeuchi, H.; Wang, F.; Sasaki, T.; Sasada, S.; Shinko, A.; Wakamori, T.; et al. Intra-Arterial Transplantation of Allogeneic Mesenchymal Stem Cells Mounts Neuroprotective Effects in a Transient Ischemic Stroke Model in Rats: Analyses of Therapeutic Time Window and Its Mechanisms. PLoS ONE 2015, 10, e0127302. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganau, L.; Prisco, L.; Ligarotti, G.K.I.; Ambu, R.; Ganau, M. Understanding the Pathological Basis of Neurological Diseases Through Diagnostic Platforms Based on Innovations in Biomedical Engineering: New Concepts and Theranostics Perspectives. Medecines (Basel) 2018, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- Bains, M.; Hall, E.D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta 2012, 1822, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, M.; Mohapatra, S.; Mohapatra, S.S. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J. Neuroinflammation 2012, 9, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.C.; Liao, Y.E.; Yang, L.Y.; Wang, J.Y.; Tweedie, D.; Karnati, H.K.; Greig, N.H.; Wang, J.Y. Neuroinflammation in animal models of traumatic brain injury. J. Neurosci. Methods 2016, 272, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witcher, K.G.; Bray, C.E.; Dziabis, J.E.; McKim, D.B.; Benner, B.N.; Rowe, R.K.; Kokiko-Cochran, O.N.; Popovich, P.G.; Lifshitz, J.; Eiferman, D.S.; et al. Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation. Glia 2018, 66, 2719–2736. [Google Scholar] [CrossRef]
- Greve, M.W.; Zink, B.J. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. 2009, 76, 97–104. [Google Scholar] [CrossRef]
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 2011, 30, 33–48. [Google Scholar] [CrossRef]
- Morganti-Kossmann, M.C.; Rancan, M.; Stahel, P.F.; Kossmann, T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr. Opin. Crit. Care 2002, 8, 101–105. [Google Scholar] [CrossRef]
- Schmidt, O.I.; Heyde, C.E.; Ertel, W.; Stahel, P.F. Closed head injury—An inflammatory disease? Brain Res. Brain Res. Rev. 2005, 48, 388–399. [Google Scholar] [CrossRef]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef]
- Barreto, G.E.; Gonzalez, J.; Torres, Y.; Morales, L. Astrocytic-neuronal crosstalk: Implications for neuroprotection from brain injury. Neurosci. Res. 2011, 71, 107–113. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayir, H.; Clark, R.S.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, R.C.; Byers, A.L.; Barnes, D.E.; Li, Y.; Boscardin, J.; Yaffe, K. Mild TBI and risk of Parkinson disease: A Chronic Effects of Neurotrauma Consortium Study. Neurology 2018, 90, e1771–e1779. [Google Scholar] [CrossRef]
- Acosta, S.A.; Diamond, D.M.; Wolfe, S.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Hernandez, D.G.; Sanberg, P.R.; Kaneko, Y.; Borlongan, C.V. Influence of post-traumatic stress disorder on neuroinflammation and cell proliferation in a rat model of traumatic brain injury. PLoS ONE 2013, 8, e81585. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Grimmig, B.; Diamond, D.M.; Sanberg, P.R.; Bickford, P.C.; Kaneko, Y.; Borlongan, C.V. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS ONE 2013, 8, e53376. [Google Scholar] [CrossRef]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Sanberg, P.R.; Sanchez-Ramos, J.; Song, S.; Kaneko, Y.; Borlongan, C.V. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS ONE 2014, 9, e90953. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147, S232–S240. [Google Scholar] [CrossRef] [Green Version]
- Lozano, D.; Gonzales-Portillo, G.S.; Acosta, S.; de la Pena, I.; Tajiri, N.; Kaneko, Y.; Borlongan, C.V. Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 2015, 11, 97. [Google Scholar]
- Yang, Y.; Ye, Y.; Su, X.; He, J.; Bai, W.; He, X. MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front. Cell. Neurosci. 2017, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Quan, F.S.; Chen, J.; Zhong, Y.; Ren, W.Z. Comparative effect of immature neuronal or glial cell transplantation on motor functional recovery following experimental traumatic brain injury in rats. Exp. Ther. Med. 2016, 12, 1671–1680. [Google Scholar] [CrossRef] [Green Version]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, T.; Morganti-Kossmann, M.C. The role of markers of inflammation in traumatic brain injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellewell, S.; Semple, B.D.; Morganti-Kossmann, M.C. Therapies negating neuroinflammation after brain trauma. Brain Res. 2016, 1640, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Bergold, P.J. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp. Neurol. 2016, 275 Pt 3, 367–380. [Google Scholar] [CrossRef]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro-and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W. Neuronal ‘On’and ‘Off’signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef]
- Aloisi, F. Immune function of microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Tomaiuolo, F.; Bivona, U.; Lerch, J.P.; Di Paola, M.; Carlesimo, G.A.; Ciurli, P.; Matteis, M.; Cecchetti, L.; Forcina, A.; Silvestro, D.; et al. Memory and anatomical change in severe non missile traumatic brain injury: ∼1 vs. ∼8years follow-up. Brain Res. Bull. 2012, 87, 373–382. [Google Scholar] [CrossRef]
- Wilde, E.A.; Chu, Z.; Bigler, E.D.; Hunter, J.V.; Fearing, M.A.; Hanten, G.; Newsome, M.R.; Scheibel, R.S.; Li, X.; Levin, H.S. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma 2006, 23, 1412–1426. [Google Scholar] [CrossRef]
- Bigler, E. Traumatic brain injury, neuroimaging, and neurodegeneration. Front. Hum. Neurosci. 2013, 7, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocchetti, N.; Zanier, E.R. Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care 2016, 20, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, H.; Kotter, M.R.; Franklin, R.J. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain 2009, 132, 288–295. [Google Scholar] [CrossRef]
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachon-Pena, G.; Yu, G.; Tucker, A.; Wu, X.; Vendrell, J.; Bunnell, B.A.; Gimble, J.M. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J. Cell Physiol. 2011, 226, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Talwadekar, M.D.; Kale, V.P.; Limaye, L.S. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts-a paired sample study. Sci. Rep. 2015, 5, 15784. [Google Scholar] [CrossRef] [Green Version]
- Gala, K.; Burdzinska, A.; Idziak, M.; Makula, J.; Paczek, L. Characterization of bone-marrow-derived rat mesenchymal stem cells depending on donor age. Cell Biol. Int. 2011, 35, 1055–1062. [Google Scholar] [CrossRef]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen. Med. 2010, 5, 933–946. [Google Scholar] [CrossRef] [Green Version]
- Azizi, S.A.; Stokes, D.; Augelli, B.J.; DiGirolamo, C.; Prockop, D.J. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—Similarities to astrocyte grafts. Proc. Natl. Acad. Sci. USA 1998, 95, 3908–3913. [Google Scholar] [CrossRef] [Green Version]
- Galindo, L.T.; Filippo, T.R.; Semedo, P.; Ariza, C.B.; Moreira, C.M.; Camara, N.O.; Porcionatto, M.A. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol. Res. Int. 2011, 2011, 564089. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Lee, J.-H.; Kim, S.-H. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: Secretion of neurotrophic factors and inhibition of apoptosis. J. Neurotrauma 2010, 27, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Opydo-Chanek, M. Bone marrow stromal cells in traumatic brain injury (TBI) therapy: True perspective or false hope? Acta Neurobiol. Exp. 2007, 67, 187. [Google Scholar]
- Gonzalez, M.E.; Martin, E.E.; Anwar, T.; Arellano-Garcia, C.; Medhora, N.; Lama, A.; Chen, Y.-C.; Tanager, K.S.; Yoon, E.; Kidwell, K.M. Mesenchymal stem cell-induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell Rep. 2017, 18, 1215–1228. [Google Scholar] [CrossRef]
- Huang, J.; Basu, S.; Zhao, X.; Chien, S.; Fang, M.; Oehler, V.; Appelbaum, F.; Becker, P. Mesenchymal stromal cells derived from acute myeloid leukemia bone marrow exhibit aberrant cytogenetics and cytokine elaboration. Blood Cancer J. 2015, 5, e302. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, F.; Zheng, Y.; Wan, Y.; Song, J. Loss of interactions between p53 and survivin gene in mesenchymal stem cells after spontaneous transformation in vitro. Int. J. Biochem. Cell Biol. 2016, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Katakowski, M.; Li, Y.; Lu, D.; Wang, L.; Zhang, L.; Chen, J.; Xu, Y.; Gautam, S.; Mahmood, A.; et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: Growth factor production. J. Neurosci. Res. 2002, 69, 687–691. [Google Scholar] [CrossRef]
- Meyerrose, T.; Olson, S.; Pontow, S.; Kalomoiris, S.; Jung, Y.; Annett, G.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv. Drug Deliv. Rev. 2010, 62, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.; Lu, D.; Chopp, M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 2004, 21, 33–39. [Google Scholar] [CrossRef]
- Mahmood, A.; Lu, D.; Lu, M.; Chopp, M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 2003, 53, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Lu, D.; Qu, C.; Goussev, A.; Chopp, M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J. Neurosurg. 2006, 104, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Dekmak, A.; Mantash, S.; Shaito, A.; Toutonji, A.; Ramadan, N.; Ghazale, H.; Kassem, N.; Darwish, H.; Zibara, K. Stem cells and combination therapy for the treatment of traumatic brain injury. Behav. Brain Res. 2018, 340, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chio, C.C.; Cheong, C.U.; Chao, C.M.; Cheng, B.C.; Lin, M.T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. (Lond.) 2013, 124, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Guan, L.X.; Zhang, K.; Zhang, Q.; Dai, L.J. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy 2008, 10, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.A.; Shah, S.K.; Harting, M.T.; Cox, C.S., Jr. Progenitor cell therapies for traumatic brain injury: Barriers and opportunities in translation. Dis. Model Mech. 2009, 2, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Chuang, T.J.; Lin, K.C.; Chio, C.C.; Wang, C.C.; Chang, C.P.; Kuo, J.R. Effects of secretome obtained from normoxia-preconditioned human mesenchymal stem cells in traumatic brain injury rats. J. Trauma Acute Care Surg. 2012, 73, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Liu, Y.; Yan, K.; Chen, L.; Chen, X.R.; Li, P.; Chen, F.F.; Jiang, X.D. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J. Neuroinflammation 2013, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Lin, W.; Su, Y.-C.; Cheng-Yo Hsuan, Y.; Chen, Y.-C.; Chang, C.-P.; Chou, W.; Lin, K.-C. Modulation of parietal cytokine and chemokine gene profiles by mesenchymal stem cell as a basis for neurotrauma recovery. J. Formos. Med. Assoc. 2019, 118, 1661–1673. [Google Scholar] [CrossRef]
- Barker, G.R.; Warburton, E.C. When is the hippocampus involved in recognition memory? J. Neurosci. 2011, 31, 10721–10731. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Deng-Bryant, Y.; Cho, W.; Carrico, K.M.; Hall, E.D.; Chen, J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 2008, 86, 2258–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grady, M.S.; Charleston, J.S.; Maris, D.; Witgen, B.M.; Lifshitz, J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J. Neurotrauma 2003, 20, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.C.; Wu, C.C.; Chuang, D.M.; Chiang, Y.H.; Chen, K.Y. Transplantation of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 Facilitates Cognitive Recovery and Enhances Neurogenesis in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Hu, W.; Wang, X.; Gao, X.; He, C.; Chen, J. Traumatic Brain Injury Causes Aberrant Migration of Adult-Born Neurons in the Hippocampus. Sci. Rep. 2016, 6, 21793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golarai, G.; Greenwood, A.C.; Feeney, D.M.; Connor, J.A. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci. 2001, 21, 8523–8537. [Google Scholar] [CrossRef]
- Kharatishvili, I.; Nissinen, J.P.; McIntosh, T.K.; Pitkänen, A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 2006, 140, 685–697. [Google Scholar] [CrossRef]
- Babb, T.L.; Kupfer, W.R.; Pretorius, J.K.; Crandall, P.H.; Levesque, M.F. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience 1991, 42, 351–363. [Google Scholar] [CrossRef]
- Atkins, C.M.; Truettner, J.S.; Lotocki, G.; Sanchez-Molano, J.; Kang, Y.; Alonso, O.F.; Sick, T.J.; Dietrich, W.D.; Bramlett, H.M. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur. J. Neurosci. 2010, 32, 1912–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef]
- Swartz, B.E.; Houser, C.R.; Tomiyasu, U.; Walsh, G.O.; DeSalles, A.; Rich, J.R.; Delgado-Escueta, A. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia 2006, 47, 1373–1382. [Google Scholar] [CrossRef]
- Chen, J.; Hu, J.; Liu, H.; Xiong, Y.; Zou, Y.; Huang, W.; Shao, M.; Wu, J.; Yu, L.; Wang, X.; et al. FGF21 Protects the Blood-Brain Barrier by Upregulating PPARgamma via FGFR1/beta-klotho after Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.R.; Stoutenger, B.R.; Robinson, A.P.; Spees, J.L.; Prockop, D.J. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc. Natl. Acad. Sci. USA 2005, 102, 18171–18176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Mahmood, A.; Wang, L.; Li, Y.; Lu, M.; Chopp, M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport 2001, 12, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Lu, D.; Wang, L.; Li, Y.; Lu, M.; Chopp, M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery 2001, 49, 1196–1204. [Google Scholar] [PubMed]
- Jackson, J.S.; Golding, J.P.; Chapon, C.; Jones, W.A.; Bhakoo, K.K. Homing of stem cells to sites of inflammatory brain injury after intracerebral and intravenous administration: A longitudinal imaging study. Stem Cell Res. Ther. 2010, 1, 17. [Google Scholar] [CrossRef] [Green Version]
- Chapel, A.; Bertho, J.M.; Bensidhoum, M.; Fouillard, L.; Young, R.G.; Frick, J.; Demarquay, C.; Cuvelier, F.; Mathieu, E.; Trompier, F.; et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J. Gene Med. 2003, 5, 1028–1038. [Google Scholar] [CrossRef]
- Devine, S.M.; Cobbs, C.; Jennings, M.; Bartholomew, A.; Hoffman, R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 2003, 101, 2999–3001. [Google Scholar] [CrossRef]
- Kraitchman, D.L.; Tatsumi, M.; Gilson, W.D.; Ishimori, T.; Kedziorek, D.; Walczak, P.; Segars, W.P.; Chen, H.H.; Fritzges, D.; Izbudak, I.; et al. Dynamic Imaging of Allogeneic Mesenchymal Stem Cells Trafficking to Myocardial Infarction. Circulation 2005, 112, 1451–1461. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.-Z.; Liu, B.-R.; Xu, J.; Gao, F.-L.; Hu, Z.-J.; Wang, X.-H.; Pei, F.-H.; Hong, Y.; Hu, H.-Y.; Han, M.-Z. Ex vivo-expanded bone marrow stem cells home to the liver and ameliorate functional recovery in a mouse model of acute hepatic injury. Hepatobiliary Pancreatic Dis. Int. 2012, 11, 66–73. [Google Scholar] [CrossRef]
- Steingen, C.; Brenig, F.; Baumgartner, L.; Schmidt, J.; Schmidt, A.; Bloch, W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell Cardiol. 2008, 44, 1072–1084. [Google Scholar] [CrossRef]
- Chamberlain, G.; Wright, K.; Rot, A.; Ashton, B.; Middleton, J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: Comparison with human. PLoS ONE 2008, 3, e2934. [Google Scholar] [CrossRef] [PubMed]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Aomparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Smith, H.; Rainger, G.E.; Middleton, J. Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: Effects of chemokines and shear. PLoS ONE 2011, 6, e25663. [Google Scholar] [CrossRef] [PubMed]
- De Becker, A.; Van Hummelen, P.; Bakkus, M.; Vande Broek, I.; De Wever, J.; De Waele, M.; Van Riet, I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 2007, 92, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Menge, T.; Zhao, Y.; Zhao, J.; Wataha, K.; Gerber, M.; Zhang, J.; Letourneau, P.; Redell, J.; Shen, L.; Wang, J.; et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci. Transl. Med. 2012, 4, 161ra150. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Naldini, L.; Blomer, U.; Gage, F.H.; Trono, D.; Verma, I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA 1996, 93, 11382–11388. [Google Scholar] [CrossRef] [Green Version]
- Schroder, A.R.; Shinn, P.; Chen, H.; Berry, C.; Ecker, J.R.; Bushman, F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002, 110, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Nienhuis, A.W.; Dunbar, C.E.; Sorrentino, B.P. Genotoxicity of retroviral integration in hematopoietic cells. Molecules 2006, 13, 1031–1049. [Google Scholar] [CrossRef]
- Dewey, R.; Morrissey, G.; Cowsill, C.; Stone, D.; Bolognani, F.; Dodd, N.; Southgate, T.; Klatzmann, D.; Lassmann, H.; Castro, M. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: Implications for clinical trials. Nat. Med. 1999, 5, 1256–1263. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Zhao, J.; Zhang, Z.; Yang, R.; Xie, J.; Liu, X.; Qi, S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE 2014, 9, e96161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Liu, J.; Zhao, J.; Xiao, L.; An, S.; Gou, Y.; Quan, H.; Cheng, Q.; Zhang, Y.; He, W. Effects of hypoxia on the immunomodulatory properties of human Gingiva–derived mesenchymal stem cells. J. Dent. Res. 2015, 94, 69–77. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, C.M.; McCarthy, H.O. Cancer gene therapy–key biological concepts in the design of multifunctional non-viral delivery systems. In Gene Therapy-Tools and Potential Applications; IntechOpen: London, UK, 2013. [Google Scholar]
- Stenler, S.; Blomberg, P.; Smith, C.I. Safety and efficacy of DNA vaccines: Plasmids vs. minicircles. Hum. Vaccin. Immunother. 2014, 10, 1306–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, R.; Spohn, G.; Baer, P.C. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus. Med. Hemother. 2016, 43, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, M.; Nito, C.; Sowa, K.; Suda, S.; Nishiyama, Y.; Nakamura-Takahashi, A.; Nitahara-Kasahara, Y.; Imagawa, K.; Hirato, T.; Ueda, M.; et al. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol. Methods Clin. Dev. 2017, 6, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Manning, E.; Pham, S.; Li, S.; Vazquez-Padron, R.I.; Mathew, J.; Ruiz, P.; Salgar, S.K. Interleukin-10 delivery via mesenchymal stem cells: A novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum. Gene 2010, 21, 713–727. [Google Scholar] [CrossRef]
- Choi, J.J.; Yoo, S.A.; Park, S.J.; Kang, Y.J.; Kim, W.U.; Oh, I.H.; Cho, C.S. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin. Exp. Immunol. 2008, 153, 269–276. [Google Scholar] [CrossRef]
- Liao, W.; Pham, V.; Liu, L.; Riazifar, M.; Pone, E.J.; Zhang, S.X.; Ma, F.; Lu, M.; Walsh, C.M.; Zhao, W. Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmune encephalomyelitis. Biomaterials 2016, 77, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Min, C.K.; Kim, B.G.; Park, G.; Cho, B.; Oh, I.H. IL-10-transduced bone marrow mesenchymal stem cells can attenuate the severity of acute graft-versus-host disease after experimental allogeneic stem cell transplantation. Bone Marrow Transpl. 2007, 39, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Peruzzaro, S.T.; Andrews, M.M.M.; Al-Gharaibeh, A.; Pupiec, O.; Resk, M.; Story, D.; Maiti, P.; Rossignol, J.; Dunbar, G.L. Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury. J. Neuroinflammation 2019, 16, 2. [Google Scholar] [CrossRef]
- Shahror, R.A.; Ali, A.A.A.; Wu, C.C.; Chiang, Y.H.; Chen, K.Y. Enhanced Homing of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 to Injury Site in a Mouse Model of Traumatic Brain Injury. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Cao, S.; Liu, J. Role of angiopoietin-2 in the cardioprotective effect of fibroblast growth factor 21 on ischemia/reperfusion-induced injury in H9c2 cardiomyocytes. Exp. Med. 2017, 14, 771–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhu, Y.; Sun, C.; Wang, T.; Shen, Y.; Cai, W.; Sun, J.; Chi, L.; Wang, H.; Song, N.; et al. Feedback Activation of Basic Fibroblast Growth Factor Signaling via the Wnt/beta-Catenin Pathway in Skin Fibroblasts. Front. Pharm. 2017, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Ding, J.; Jin, R.; Jung, J.; Li, S.; Yang, J.; Wang, A.; Li, Z. Expression and purification of FGF21 in Pichia pastoris and its effect on fibroblast-cell migration. Mol. Med. Rep. 2016, 13, 3619–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Pan, S.; Sun, Z.; Dan, Q.; Liu, J. Brain-derived neurotrophic factor-modified umbilical cord mesenchymal stem cell transplantation improves neurological deficits in rats with traumatic brain injury. Int. J. Neurosci. 2014, 124, 524–531. [Google Scholar] [CrossRef]
- Heile, A.M.; Wallrapp, C.; Klinge, P.M.; Samii, A.; Kassem, M.; Silverberg, G.; Brinker, T. Cerebral transplantation of encapsulated mesenchymal stem cells improves cellular pathology after experimental traumatic brain injury. Neurosci. Lett. 2009, 463, 176–181. [Google Scholar] [CrossRef]
- Zou, L.; Huang, L.; Hayes, R.; Black, C.; Qiu, Y.; Perez-Polo, J.; Le, W.; Clifton, G.; Yang, K. Liposome-mediated NGF gene transfection following neuronal injury: Potential therapeutic applications. Gene Ther. 1999, 6, 994–1005. [Google Scholar] [CrossRef] [Green Version]
- Philips, M.F.; Mattiasson, G.; Wieloch, T.; Björklund, A.; Johansson, B.B.; Tomasevic, G.; Martínez-Serrano, A.; Lenzlinger, P.M.; Sinson, G.; Grady, M.S. Neuroprotective and behavioral efficacy of nerve growth factor—Transfected hippocampal progenitor cell transplants after experimental traumatic brain injury. J. Neurosurg. 2001, 94, 765–774. [Google Scholar] [CrossRef]
- Kim, C.D.; Shin, H.K.; Lee, H.S.; Lee, J.H.; Lee, T.H.; Hong, K.W. Gene transfer of Cu/Zn SOD to cerebral vessels prevents FPI-induced CBF autoregulatory dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1836–H1842. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Shan, Y.; Hu, Y.; Fang, Z.; Tong, Y.; Chen, M.; Wei, X.; Fu, X.; Xu, X. FGF2 Attenuates Neural Cell Death via Suppressing Autophagy after Rat Mild Traumatic Brain Injury. Stem Cells Int. 2017, 2017, 2923182. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, S.; Teramoto, T.; Whalen, M.J.; Irizarry, M.C.; Takagi, Y.; Qiu, J.; Harada, J.; Waeber, C.; Breakefield, X.O.; Moskowitz, M.A. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J. Clin. Investig. 2003, 112, 1202–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalin, I.; Alyautdin, R.; Wong, T.W.; Gnanou, J.; Kocherga, G.; Kreuter, J. Brain-derived neurotrophic factor delivered to the brain using poly (lactide-co-glycolide) nanoparticles improves neurological and cognitive outcome in mice with traumatic brain injury. Drug Deliv. 2016, 23, 3520–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.J.; Love, C.; Spector, M.; Cool, S.M.; Nurcombe, V.; Lo, E.H. Endogenous regeneration: Engineering growth factors for stroke. Neurochem. Int. 2017, 107, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Redell, J.B.; Moore, A.N.; Ward III, N.H.; Hergenroeder, G.W.; Dash, P.K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma 2010, 27, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Redell, J.B.; Liu, Y.; Dash, P.K. Traumatic brain injury alters expression of hippocampal microRNAs: Potential regulators of multiple pathophysiological processes. J. Neurosci. Res. 2009, 87, 1435–1448. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, H.; Dai, G.; Wang, X.; Hua, R.; Liu, X.; Wang, P.; Chen, G.; Yue, W.; An, Y. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013, 1532, 76–84. [Google Scholar] [CrossRef]
- Palfi, S.; Gurruchaga, J.M.; Lepetit, H.; Howard, K.; Ralph, G.S.; Mason, S.; Gouello, G.; Domenech, P.; Buttery, P.C.; Hantraye, P. Long-term follow-up of a phase I/II study of ProSavin, a lentiviral vector gene therapy for Parkinson’s disease. Hum. Gene Ther. Clin. Dev. 2018, 29, 148–155. [Google Scholar] [CrossRef]
- Hwu, W.-L.; Muramatsu, S.-I.; Tseng, S.-H.; Tzen, K.-Y.; Lee, N.-C.; Chien, Y.-H.; Snyder, R.O.; Byrne, B.J.; Tai, C.-H.; Wu, R.-M. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci. Transl. Med. 2012, 4, 134ra161. [Google Scholar] [CrossRef] [Green Version]
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S. AAV2-GAD gene therapy for advanced Parkinson’s disease: A double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef]
- Mittermeyer, G.; Christine, C.W.; Rosenbluth, K.H.; Baker, S.L.; Starr, P.; Larson, P.; Kaplan, P.L.; Forsayeth, J.; Aminoff, M.J.; Bankiewicz, K.S. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum. Gene Ther. 2012, 23, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Stoessl, A.J. Gene therapy for Parkinson’s disease: A step closer? Lancet 2014, 383, 1107–1109. [Google Scholar] [CrossRef]
- Niethammer, M.; Tang, C.C.; Vo, A.; Nguyen, N.; Spetsieris, P.; Dhawan, V.; Ma, Y.; Small, M.; Feigin, A.; During, M.J. Gene therapy reduces Parkinson’s disease symptoms by reorganizing functional brain connectivity. Sci. Transl. Med. 2018, 10, eaau0713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahror, R.A.; Wu, C.-C.; Chiang, Y.-H.; Chen, K.-Y. Genetically Modified Mesenchymal Stem Cells: The Next Generation of Stem Cell-Based Therapy for TBI. Int. J. Mol. Sci. 2020, 21, 4051. https://doi.org/10.3390/ijms21114051
Shahror RA, Wu C-C, Chiang Y-H, Chen K-Y. Genetically Modified Mesenchymal Stem Cells: The Next Generation of Stem Cell-Based Therapy for TBI. International Journal of Molecular Sciences. 2020; 21(11):4051. https://doi.org/10.3390/ijms21114051
Chicago/Turabian StyleShahror, Rami Ahmad, Chung-Che Wu, Yung-Hsiao Chiang, and Kai-Yun Chen. 2020. "Genetically Modified Mesenchymal Stem Cells: The Next Generation of Stem Cell-Based Therapy for TBI" International Journal of Molecular Sciences 21, no. 11: 4051. https://doi.org/10.3390/ijms21114051