Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues
Abstract
:1. Introduction
2. Corrosion
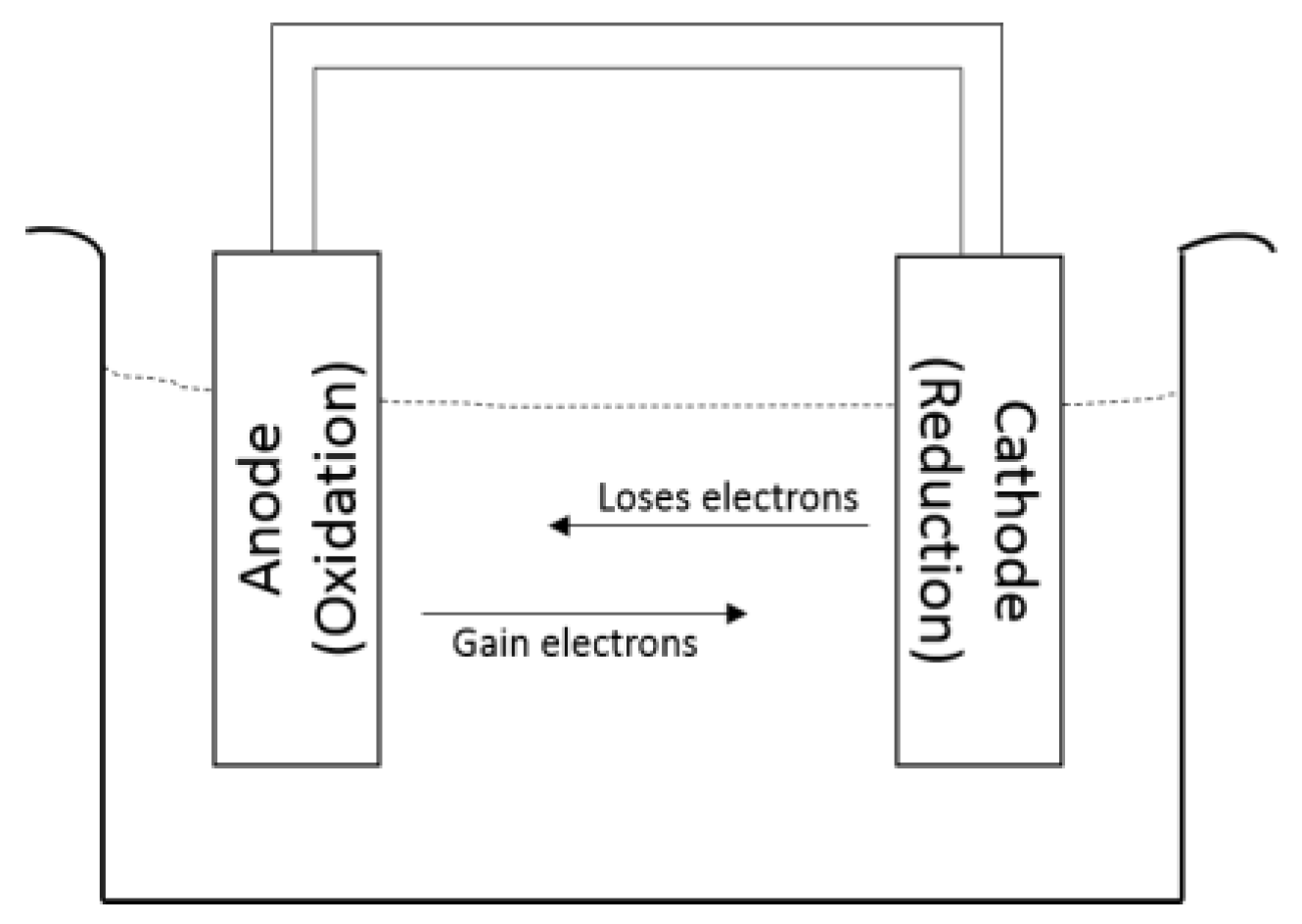
2.1. Mechanism of Corrosion
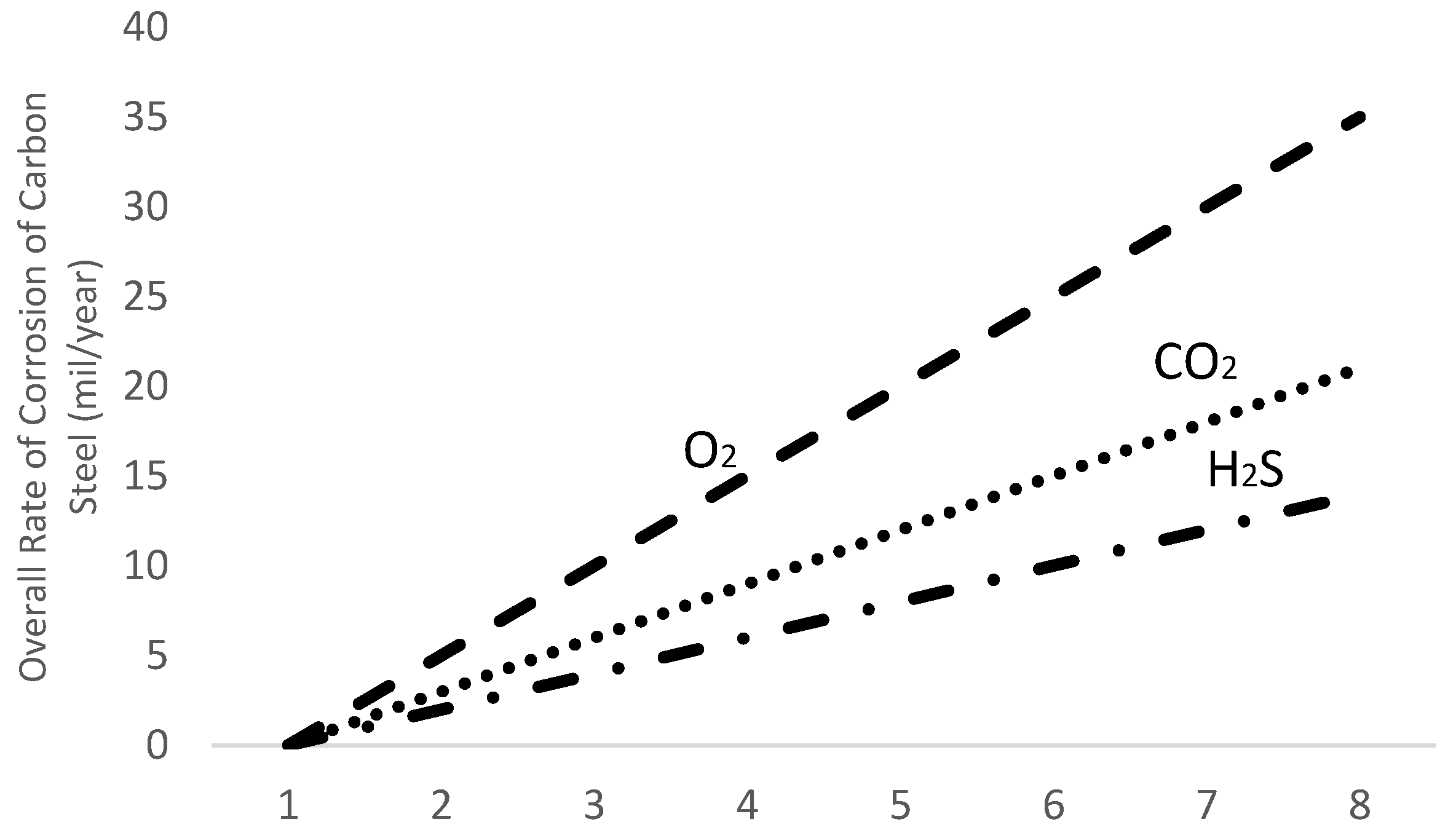
2.2. Sources of Corrosion
- a.
- Hydrogen Sulfide
- b.
- Chloride
- c.
- Carbon dioxide
- d.
- Oxygen
- a.
- Bacteria
- b.
- Water Cut
- c.
- Strong Acids
- d.
- Brines
3. Commercial Corrosion Inhibitors
4. Green-Based Corrosion Inhibitors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simon, M.R. Report of Offshore Technology Conference (OTC) Presentation; NACE International Oil and Gas production: Houston, TX, USA, 2008. [Google Scholar]
- Chen, R.; Li, X.; Du, C.; Cheng, Y. Effect of cathodic protection on corrosion of pipeline steel under disbonded coating. Corros. Sci. 2009, 51, 2242–2245. [Google Scholar] [CrossRef]
- Yabuki, A.; Tanabe, S.; Fathona, I. Comparative studies of two benzaldehyde thiosemicarbazone derivatives as corrosion inhibitors for mild steel in 1.0 M HCl. Surf. Coat. Technol. 2018, 341, 71–77. [Google Scholar] [CrossRef]
- Lyon, S.; Bingham, R.; Mills, D. Corrosion Protection of Carbon Steel by Pongamia glabra Oil- Based Polyetheramide Coatings. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Chengduan, W.; Chuan, L.; Bin, X.; Xiaogang, G.; Dong, F.; Bin, L. Corrosion inhibition of mild steel in HCl medium by S-benzyl-O,O′-bis(2-naphthyl)dithiophosphate with ultra-long lifespan. Results Phys. 2018, 10, 558–567. [Google Scholar]
- Salman, T.; Al-Azawi, K.; Mohammed, I.; Al-Baghdadi, S.; Al-Amiery, A.; Gaaz, T. Experimental and quantum chemical simulations on the corrosion inhibition of mild steel by 3-((5-(3,5-dinitrophenyl)-1,3,4-thiadiazol-2-yl)imino)indolin-2-one. Results Phys. 2018, 10, 291–296. [Google Scholar] [CrossRef]
- Odewunmi, N.; Umoren, S.; Gasem, Z.; Ganiyu, S.; Muhammad, Q. Electrochemical and quantum chemical studies on carbon steel corrosion protection in 1 M H2SO4 using new eco-friendly Schiff base metal complexes. J. Taiwan Inst. Chem. Eng. 2015, 51, 177–185. [Google Scholar] [CrossRef]
- Zeino, A.; Abdulazeez, I.; Khaled, M.; Jawich, M.; Obot, I. Electrochemical Corrosion Performance of Aromatic Functionalized Imidazole Inhibitor Under Hydrodynamic Conditions on API X65 Carbon Steel in 1 M HCl Solution. J. Mol. Liq. 2018, 250, 50–62. [Google Scholar] [CrossRef]
- Umoren, S.; Eduok, U. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- Yadav, D.; Maiti, B.; Quraishi, M. Electrochemical and quantum chemical studies of 3,4-dihydropyrimidin-2(1H)-ones as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2010, 52, 3586–3598. [Google Scholar] [CrossRef]
- Hu, K.; Zhuang, J.; Zheng, C.; Ma, Z.; Yan, L.; Gu, H.; Zeng, X.; Ding, J. Effect of novel cytosine-l-alanine derivative based corrosion inhibitor on steel surface in acidic solution. J. Mol. Liq. 2016, 222, 109–117. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Vakili, H.; Amini, R. The effects of addition of poly (vinyl) alcohol (PVA) as a green corrosion inhibitor to the phosphate conversion coating on the anticorrosion and adhesion properties of the epoxy coating on the steel substrate. Appl. Surf. Sci. 2015, 327, 174–181. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R. Plant-derived nanostructures: Types and applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Varma, R. Journey on greener pathways: From the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chem. 2014, 16, 2027–2041. [Google Scholar] [CrossRef]
- Jeon, H.; Lim, C.; Lee, M.; Kim, S. Chemical assay-guided natural product isolation via solid-supported chemodosimetric fluorescent probe. Chem. Sci. 2015, 6, 2806–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, M.; Tiwari, P.; Srivastava, S.; Prakash, R.; Ji, G. Electrochemical investigation of Irbesartan drug molecules as an inhibitor of mild steel corrosion in 1 M HCl and 0.5 M H2SO4 solutions. J. Mol. Liq. 2017, 236, 184–197. [Google Scholar] [CrossRef]
- Mo, S.; Li, L.; Luo, H.; Li, N. An example of green copper corrosion inhibitors derived from flavor and medicine: Vanillin and isoniazid. J. Mol. Liq. 2017, 242, 822–830. [Google Scholar] [CrossRef]
- Diamanti, M.; Velardi, U.; Brenna, A.; Mele, A.; Pedeferri, M.; Ormellese, M. Compatibility of imidazolium-based ionic liquids for CO2 capture with steel alloys: A corrosion perspective. Electrochim. Acta 2016, 192, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Lozano, I.; Mazario, E.; Olivares-Xometl, C.; Likhanova, N.; Herrasti, P. Corrosion behaviour of API 5LX52 steel in HCl and H2SO4 media in the presence of 1, 3-dibencilimidazolio acetate and 1,3-dibencilimidazoliododecanoate ionic liquids as inhibitors. Mater. Chem. Phys. 2014, 147, 191–197. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Messali, M.; Aljuhani, A.; Aouad, M.; Hammouti, B.; Belghiti, M.; Chauhan, D.; Quraishi, M. Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: Electrochemical and molecular dynamics simulation studies. J. Mol. Liq. 2018, 249, 997–1008. [Google Scholar] [CrossRef]
- Mahmoodian, M.; Qingi, C. Failure assessment and safe life prediction of corroded oil and gas pipelines. J. Pet. Sci. Eng. 2017, 151, 434–438. [Google Scholar] [CrossRef]
- Wang, W.; Natelson, R.; Stikeleather, L.; Roberts, W. CFD simulation of transient stage of continuous countercurrent hydrolysis of canola oil. Comput. Chem. Eng. 2012, 43, 108–119. [Google Scholar] [CrossRef]
- Nesic, S. Key issues related to modelling of internal corrosion of oil and gaspipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Revie, R. Oil, Gas Pipelines. In Integrity and Safety Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Papavinasm, S. Corrosion Control in the Oil and Gas Industry; Elsevier: Houston, TX, USA, 2013. [Google Scholar]
- Regulator, A.E. Report 2013-B: Pipeline Performance in Albarta, 1990–2012; Alberta Energy Regulator: Calgary, AB, Canada, 2013. [Google Scholar]
- Mahmood, A.; Dawood, H. A Comprehensive Review of Corrosion and its Inhibition in the Oil and Gas Industry. In Proceedings of the SPE Kuwait Oil and Gas Show and Conference, Mishref, Kuwait, 11–14 October 2015; Society of Petroleum Engineers. [Google Scholar] [CrossRef]
- Szyprowski, A. Methods of Investigation on Hydrogen Sulfide Corrosion of Steel and Its Inhibitors. Corrosion. Corrosion 2003, 59, 68–81. [Google Scholar] [CrossRef]
- Carter, D.R.; Adams, N.J. Hydrogen Sulfide in the Drilling Industry. In Proceedings of the 4th Deep Drilling and Production Symposium, Amarillo, TX, USA, 1–3 April 1979; Society of Petroleum Engineers of AIME: Dallas, TX, USA, 1979; pp. 123–135. [Google Scholar]
- Carrell, M.A. Reclaiming Produced Water for Steam Generation in the Kern River Field. In Proceedings of the 54th Ann. Conf. Soc. Pet Eng. A.I.M.E., SPE 8411, Las Vegas, NV, USA, 23–26 September 1979. [Google Scholar]
- Fang, H.; Nesic, S.; Brown, B.; Wang, S. General CO2 Corrosion in High Salinity Brines. Corrosion 2006. [Google Scholar]
- Ramachandran, S.; Bartrip, K.; Menendez, C.; Coscio, S. Preventing Erosion and Erosion Corrosion Using Specialty Chemicals. In International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: Houston, TX, USA, 2003. [Google Scholar]
- Yesudass, S.; Olasunkanmi, L.O.; Bahadur, I.; Kabanda, M.M.; Obot, I.B.; Ebenso, E.E. Experimental and theoretical studies on some selected ionic liquids with different cations/anions as corrosion inhibitors for mild steel in acidic medium. J. Taiwan Inst. Chem. Eng. 2016, 64, 252–268. [Google Scholar] [CrossRef]
- Farquhar, G. A review of trends in Microbiologically influenced corrosion. Mater. Perform. 1993, 32, 53. [Google Scholar]
- Umoren, S.; Solomon, M. Recent developments on the use of polymers as corrosion Inhibitors—A review. Open Mater. Sci. J. 2014, 8, 39–54. [Google Scholar] [CrossRef]
- Abreu, C.; Izquierdo, M.; Merino, P.; Nóvoa, X.; Pérez, C. A new approach to the determination of the cathodic protection period in zinc-rich paints. Corrosion 1999, 55, 1173–1181. [Google Scholar] [CrossRef]
- Mansfeld, F.; Tsai, C. Determination of coating deterioration with EIS: I. Basic relationships. Corrosion 1991, 47, 958–963. [Google Scholar] [CrossRef]
- Umoren, S.; Obot, I.; Madhankumar, A.; Gasem, Z. Performance evaluation of pectin as ecofriendly corrosion inhibitor for X60 pipeline steel in acid medium. Carbohydr. Polym. 2015, 124, 280–291. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design graphic and mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.; Singh, A. 5-Substituted 1H-tetrazoles as effective corrosion inhibitors for mild steel in 1M hydrochloric acid. J. Taibah Univ. Sci. 2016, 10, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Azhar, M.; Mernari, M.; Traisnel, M.; Bentiss, F.; Lagrenee, M. Corrosion inhibition of mild steel by the new class of inhibitors [2, 5-bis(n-pyridyl)-1,3,4-thiadiazoles] in acidic media. Corros. Sci. 2011, 43, 2229–2243. [Google Scholar] [CrossRef]
- Mobin, M.; Zehra, S.; Parveen, M. l-Cysteine as corrosion inhibitor for mild steel in 1M HCl and synergistic effect of anionic, cationic and non-ionic surfactants. J. Mol. Liq. 2016, 216, 598–607. [Google Scholar] [CrossRef]
- Da Silva, A.; D’Elia, E.; Gomes, J. Carbon steel corrosion inhibition in hydrochloric acid solution using a reduced Schiff base of ethylenediamine. Corros. Sci. 2010, 52, 788–793. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Z.; Gao, F.; Zhang, S.; Li, H. Synthesis of new benzotriazole derivatives containing carbon chains as the corrosion inhibitors for copper in sodium chloride solution. Ind. Eng. Chem. Res. 2015, 54, 12245–12253. [Google Scholar] [CrossRef]
- Ghazoui, A.; Saddik, R.; Benchat, N.; Guenbour, M.; Hammouti, B.; Al-Deyab, S.S.; Zarrouk, A. Comparative study of pyridine and pyrimidine derivaitves as corrosion inhibitors of C38 steel in molar HCl. Int. J. Electrochem. Sci. 2012, 7, 7080–7097. [Google Scholar]
- Raja, P.; Sethuraman, M. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.; Bahadur, I.; Quraishi, M. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Pillard, D.; Cornell, J.; Dufresne, D.; Hernandez, M. Toxicity of benzotriazole and benzotriazole derivatives to three aquatic species. Water Res. 2001, 35, 557–560. [Google Scholar] [CrossRef]
- Abdallah, M.; Megahed, H.; Radwan, M.; Abdfattah, E. Polyethylene glycol compounds as corrosion inhibitors for aluminum in 0.5 M hydrochloric acid solution. J. Am. Sci. 2012, 8, 49–55. [Google Scholar]
- Awad, M.; Metwally, M.; Soliman, S.; El-Zomrawy, A.; Bedair, M. Experimental and quantum chemical studies of the effect of polyethylene glycol as corrosion inhibitors of aluminum surface. J. Ind. Eng. Chem. 2013, 20, 796–808. [Google Scholar] [CrossRef]
- Umoren, S.; Solomon, M.; Israel, A.; Eduok, U.; Jonah, A. Comparative study of the corrosion inhibition efficacy of polypropylene glycol and poly(methacrylic acid) for mild steel in acid solution. J. Dispers. Sci. Technol. 2015, 36, 1721–1735. [Google Scholar] [CrossRef]
- Al-Otaibi, M.; Al-Mayouf, A.; Khan, M.; Mousa, A.; Al-Mazroa, S.; Alkhathlan, H. Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab J. Chem. 2014, 7, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Gerengi, H.; Jazdzewska, A.; Kurtay, M. A comprehensive evaluation of mimosa extract as a corrosion inhibitor on AA6060 alloy in acid rain solution: Part I. Electrochemical AC methods. J. Adhes. Sci. Technol. 2015, 29, 36–48. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose—Inhibition of cast iron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef]
- Solomon, M.; Umoren, S.; Udousoro, I.; Udoh, A. Inhibitive and adsorption behavior of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Hussin, M.; Rahim, A.; Ibrahim, M.; Brosse, N. The capability of ultrafiltrated alkaline and organosolv oil palm (Elaeis guineensis) fronds lignin as green corrosion inhibitor formild steel in 0.5 M HCl solution. Measurement 2016, 78, 90–103. [Google Scholar] [CrossRef]
- Mohammed, M.; Khan, Z.; Siddiquee, A. Surface modifications of titanium materials for developing corrosion behavior in human body environment: A review. Proc. Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Negm, N.; Kandile, N.; Aiad, I.; Mohammad, M. New eco-friendly cationic surfactants synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1 M HCl. Colloids Surf. A Phys. Eng. Asp. 2011, 391, 224–233. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Etre, A. Inhibition of C-steel corrosion in acidic solution using the aqueous extract of zallouh root. Mater. Chem. Phys. 2008, 108, 278–282. [Google Scholar] [CrossRef]
- Ji, G.; Dwivedi, P.; Sundaram, S.; Prakash, R. Inhibitive effect of Chlorophytum borivilianum root extract on mild steel corrosion in HCl and H2SO4 solutions. Ind. Eng. Chem. Res. 2013, 52, 10673–10681. [Google Scholar] [CrossRef]
- Ji, G.; Dwivedi, P.; Sundaram, S.; Prakash, R. Aqueous extract of Argemone Mexicana roots for effective protection of mild steel in an HCl environment. Res. Chem. Intermed. 2016, 42, 439–459. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Ravichandran, J. Effect of aqueous extract of leaves of Morinda tinctoria on corrosion inhibition of aluminium surface in HCl medium. Trans. Nonferrous Met. Soc. China 2014, 24, 2704–2712. [Google Scholar] [CrossRef]
- Sanatkumar, B.; Nayak, J.; Shetty, A. Influence of 2-(4-chlorophenyl)-2-oxoethyl benzoate on the hydrogen evolution and corrosion inhibition of 18 Ni 250 grade weld aged maraging steel in 1.0 M sulfuric acid medium. Int. J. Hydrog. Energy 2012, 37, 9431–9442. [Google Scholar] [CrossRef]
- Manamela, K.; Murulana, L.; Kabanda, M.; Ebenso, E. Adsorptive and DFT studies of some imidazolium based ionic liquids as corrosion inhibitors for zinc in acidic medium.of some imidazolium based ionic liquids as corrosion inhibitors for zinc in acidic medium. Int. J. Electrochem. Sci. 2014, 9, 3029–3046. [Google Scholar]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.; Khalaj, M. Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv. 2014, 4, 47313–47318. [Google Scholar] [CrossRef]
- Sharghi, H.; Khalifeh, R.; Doroodmand, M. Copper nanoparticles on charcoal for multicomponent catalytic synthesis of 1, 2, 3-triazole derivatives from benzyl halides or alkyl halides, terminal alkynes and sodium Azide in water as a “green” solvent. Adv. Synth. Catal. 2009, 351, 207–218. [Google Scholar] [CrossRef]
- Bose, D.; Fatima, L.; Mereyala, H.B. Green chemistry approaches to the synthesis of 5-alkoxycarbonyl-4-aryl-3, 4-dihydropyrimidin-2 (1 H)-ones by a three component coupling of one-pot condensation reaction: Comparison of ethanol, water, and solvent-free connditions. J. Organomet. Chem. 2003, 68, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Varma, R. Greener and sustainable trends in synthesis of organics and nanomaterials. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef]
- Mohamad, N.; Arham, N.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Adv. Mater. Res. Trans. Tech. Publ. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Seo, J.; Lee, S.; Elam, M.; Johnson, S.; Kang, J.; Arjmandi, B. Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidantefficacy. Food Sci. Nutr. 2014, 2, 174–180. [Google Scholar] [CrossRef]
- Aida, Z.; Razika, A.; Laid, M.; Kamel, B.; Boualem, S. Inhibition of acid corrosion of mild steel by aqueous nettle extracts. Pigment. Resin Technol. 2014, 43, 127–138. [Google Scholar]
- Devarayan, K.; Mayakrishnan, G.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. [Google Scholar]
- Gobara, M.; Zaghloul, B.; Baraka, A.; Elsayed, M.; Zorainy, M.; Kotb, M.; Elnabarawy, H. Green corrosion inhibition of mild steel to aqueous sulfuric acid by the extract of Corchorus ollitorius stems. Mater. Res. Express 2017, 4, 391–401. [Google Scholar] [CrossRef]
- McCafferty, E. Thermodynamic aspects of the crevice corrosion of iron in chromate/chloride solutions. J. Electrochem. Soc. 1979, 126. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.; Calvino, J.; Marcos, M.; Rodriguez-Chacon, M. Lanthanide compounds as environmentally-friendly corrosion inhibitors of aluminium alloys: A review. Corros. Sci. 1998, 40, 1803–1819. [Google Scholar] [CrossRef]
- Michodjehoun-Mestres, L.; Jean-Marc, S.; Fulcrand, H.; Bouchut, C.; Reynes, M.; Jean-Marc, B. Characterisation of highly polymerised prodelphinidins from skin and flesh of four cashew apple (Anacardium occidentale L.) genotypes. Food Chem. 2009, 112, 851. [Google Scholar] [CrossRef]
- Saleh, R.; Ismail, A.; Hosary, A.E.B. Inhibitory mechanism of low-carbon steel corrosion by mimosa tannin in sulphuric acid solutions. Corros. J. 1982, 3, 17. [Google Scholar]
- Dehghani, A.; Bahlakeha, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential of Borage flower aqueous extract as an environmentally sustainable corrosion inhibitor for acid corrosion of mild steel: Electrochemical and theoretical studies. J. Mol. Liq. 2019, 277, 895–911. [Google Scholar] [CrossRef]
- Othman, N.K.; Yahya, S.; Ismail, M.C. Corrosion inhibition of steel in 3.5% NaCl by rice straw extract. J. Ind. Eng. Chem. 2019, 70, 299–310. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechnics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Asadi, N.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1 M HCl solution: A detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Taiwan Inst. Chem. Eng. 2019, 95, 252–272. [Google Scholar] [CrossRef]
- Haldhara, R.; Prasada, D.; Saxenaa, A.; Kumarb, R. Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. Sustain. Chem. Pharm. 2018, 9, 95–105. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. J. Environ. Chem. Eng. 2018, 6, 2290–2301. [Google Scholar] [CrossRef]
- Hassannejad, H.; Nouri, A. Sunflower seed hull extract as a novel green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2018, 254, 377–382. [Google Scholar] [CrossRef]
- Ikeuba, A.; Okafor, P. Green corrosion protection for mild steel in acidic media: Saponins and crude extracts of Gongronema latifolium. Pigment Resin Technol. 2018, 48, 57–64. [Google Scholar] [CrossRef]
- Jmiai, A.; El Ibrahimi, B.; Tara, A.; Chadili, M.; El Issami, S.; Jbara, O.; Khallaayoun, A.; Bazzi, L. Application of Zizyphus Lotuse-pulp of Jujube extract as green and promising corrosion inhibitor for copper in acidic medium. J. Mol. Liq. 2018, 268, 102–113. [Google Scholar] [CrossRef]
- Khadom, A.; Abd, A.; Ahmed, N. Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: Kinetics and mathematical studies. S. Afr. J. Chem. Eng. 2018, 25, 13–21. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Investigation of corrosion inhibition effect and adsorption activities of Cuscuta reflexa extract for mild steel in 0.5 M H2SO4. Bioelectrochemistry 2018, 124, 156–164. [Google Scholar] [CrossRef]
- Ugi, B.U.; Obeten, M.E.; Magu, T.O. Phytochemical constituents of Taraxacum officinale leaves as eco-friendly and nontoxic organic inhibitors for stainless steel corrosion in 0.2 M HCl acid medium. Int. J. Chem. Sci. 2018, 2, 35–43. [Google Scholar]
- Aribo, S.; Olusegun, S.J.; Ibhadiyi, L.J.; Oyetunji, A.; Folorunso, D. Green inhibitors for corrosion protection in acidizing oilfield environment. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 24, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Gerengia, H.; Uygura, I.; Solomona, M.; Yildiza, M.; Goksub, H. Evaluation of the inhibitive effect of Diospyros kaki (Persimmon) leaves extract on St37 steel corrosion in acid medium. Sustain. Chem. Pharm. 2016, 4, 57–66. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M.; Solomon, M.M.; Udoh, A.P. Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab. J. Chem. 2016, 9, S209–S224. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Deng, S.; Fu, H. Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corros. Sci. 2012, 62, 163–175. [Google Scholar] [CrossRef]
- Quraishi, M.; Singh, A.; Singh, K.V.; Yadav, D.; Singh, A. Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater. Chem. Phys. 2010, 122, 114–122. [Google Scholar] [CrossRef]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Liao, L.; Moa, S.; Luo, H.; Li, N. Corrosion protection for mild steel by extract from the waste of lychee fruit in HCl solution: Experimental and theoretical studies. J. Colloid Interface Sci. 2018, 520, 41–49. [Google Scholar] [CrossRef]
- Tiwari, P.; Srivastava, M.; Mishra, G.R.; Prakash, R. Economic use of waste Musa paradisica peels for effective control of mild steel loss in aggressive acid solutions. J. Environ. Chem. Eng. 2018, 6, 4773–4783. [Google Scholar] [CrossRef]
- Liao, L.; Mo, S.; Luo, H.; Li, N. Longan seed and peel as environmentally friendly corrosion inhibitor for mild steel in acid solution: Experimental and theoretical studies. J. Colloid Interface Sci. 2017, 499, 110–119. [Google Scholar] [CrossRef]
- Paul, S.; Koley, I. Corrosion Inhibition of Carbon Steel in Acidic Environment by Papaya Seed as Green Inhibitor. J. Bio Tribo Corros. 2016, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.; Sanketi, B.; Kumar, S. Corrosion inhibition of mild steel by Capsicum annuum fruit paste. Perspect. Sci. 2016, 8, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Lin, Y.; Ebenso, E.; Liu, W.; Pan, J.; Huang, B. Gingko biloba fruit extract as an eco-friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5% NaCl solution. J. Ind. Eng. Chem. 2015, 24, 219–228. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, P.; Gupta, K. The litchi (Litchi chinensis) peels extract as a potential green inhibitor in prevention of corrosion of mild steel in 0.5 M H2SO4 solution. Arab. J. Chem. 2015. [Google Scholar] [CrossRef] [Green Version]
- Odewunmi, N.; Umoren, S.; Gasem, Z. Watermelonwaste products as green corrosion inhibitors for mild steel in HCl solution. J. Environ. Chem. Eng. 2015, 3, 286–296. [Google Scholar] [CrossRef]
- Yaro, A.; Khadom, A.; Wael, R. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandra Eng. J. 2013, 52, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.; Gomes, J.P.; D’Elia, E. Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extracts. Corros. Sci. 2010, 52, 2341–2348. [Google Scholar] [CrossRef]
- Ossai, C. Advances in Asset Management Techniques: An Overview of Corrosion Mechanisms and Mitigation Strategies for Oil and Gas Pipelines. Int. Sch. Res. Not. 2012, 2012, 570143. [Google Scholar] [CrossRef] [Green Version]
| Details | Gap | Reference/Year |
|---|---|---|
| (a) Borage flower (b) Experiments: Weight loss, EIS, surface analysis (c) Parameters: Concentration (200, 400, 600, 800 ppm) and immersion time (0.5, 2.5, 5.0 h) (d) Results: 800 ppm; 2.5 h; 91% IE | Limitation: Constant temperature (25 °C) Remark: Vary the temperature from 25 up to 90 ° | [83]/2019 |
| (a) Rice straw extract (b) Experiments: Weight loss, surface and morphology analysis, and electrochemical test (c) Parameters: Immersion time (7, 14, 21, 28, 35, 42 days) at room temperature (25 °C) (d) Results: Immersion time of 7 to 14 days and 85% IE | Limitation: Constant temperature (25 °C) used throughout 42 days Remark: Vary the temperature from 25 up to 90 °C | [84]/2019 |
| (a) Glycyrrhiza glabra (Pea and bean family) leaves (b) Experiments: EIS, surface characterization (c) Parameters: Concentration of inhibitor (200, 400, 600, 800 ppm) (d) Results: 800 ppm gave 88% IE | Limitation:Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [85]/2018 |
| (a) Lemon balm extracts (b) Experiments: Characterization technique (LBE, EIS, surface analysis) (c) Parameters: Inhibitor concentration (200, 400, 600, 800 ppm) and immersion time (0.5, 2, 4, 6, 12, 24 h) (d) Result: 800 ppm with immersion time of 24 h and 94.6% IE | Limitation: Constant temperature (25 °C) Remark: Vary the temperature from 25 up to 90 °C | [86]/2018 |
| (a) Ficus religiose (leaf, bodhi tree) (b) Experiments: EIS, gravimetric measurements, quantum chemical study, SEM (c) Parameters: Temperature (25, 35, 45 °C), inhibitor concentration (100–500 ppm) (d) Results: 50 ppm gave 88.29% IE at 25 °C | Limitation: Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [87]/2018 |
| (a) Myristica fragrans (nutmeg fruit) (b) Experiments: Weight loss, UV-vis spectroscopy, FT-IR spectroscopy, NMR analysis, quantum chemical studies, SEM (c) Parameter: Inhibitor concentration (100, 200, 300, 400, 500 ppm) D) Results: 500 ppm gave 87.81% IE | Limitation: Constant temperature (25 °C) and immersion time (24 h) Remark: Vary the temperature from 25 up to 90 °C and extend the immersion time from 3 up to 30 days | [88]/2018 |
| (a) Sunflower seed hull (flower) (b) Experiments: FT-IR, UV-vis (c) Parameters: Inhibitor concentration (50, 100, 200, 300, 400 ppm) and temperatures (25, 40, 50, 60 °C) (d) Result: 400 ppm gave 98.46% IE at 60 °C | Limitation: Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [89]/2018 |
| (a) Gongronema latifolium (utazi, herb) (b) Experiments: Gasometric method (c) Parameters: Inhibitor concentration (50, 100, 250, 500, 1000ppm) and temperature (30, 40, 50, 60 °C) (d) Results i. EEGL: 1000 ppm gave 93.7% IE at 30 °C ii. SEGL: 1000 ppm gave 96.5% IE at 50 °C | Limitation: Immersion time was not stated in this article, and only one major experiment was carried out Remark: Immersion time should be tested from 3 up to 30 days | [90]/2018 |
| (a) Zizyphus lotuse (lotus) (b) Active ingredients: Vitamin C (ascorbic acid), linoleic acid, oleanolic acid, flavonoid compound, triterpenoic acid, jujuboside (c) Experiments: Electrochemical methods, potentiodynamic polarization, SEM and EDS analysis (d) Parameters: Concentration of inhibitors (0.05–2 g L−1) and Temperatures (25, 35, 45, 55 °C) (e) Results: i. Concentration effect: 1000 ppm gave 93% IE ii. Temperature effect at 1000 ppm: 25 °C; 93.16% IE | Limitation: Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [91]/2018 |
| (a) Xanthium strumarium (cocklebur) leaf extract (b) Experiments: SEM, FTIR, weight loss (c) Parameters: Inhibitor concentration (200, 400, 600, 800, 1000 ppm) and temperature (30, 40, 50, 60 °C) (d) Results: 1000 ppm gave 94.82% IE at 60 °C | Limitation: Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [92]/2018 |
| (a) Cuscuta reflexa (morning glory family, fruit extract) (b) Experiments: Weight loss, electrochemical measurement, UV-visible spectroscopy, FT-IR spectroscopy, surface analyses, quantum chemical studies (c) Parameter: Inhibitor concentration (100, 200, 300, 400, 500 ppm) (d) Results: 500 ppm gave 95.47% IE | Limitation: Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [93]/2018 |
| (a) Taraxacum officinale (dandelion, flower) (b) Experiments: Weight loss, Thermometric measurements, Electrochemical measurements, Gravimetric (c) Parameters: Type of crude (saponins—SETOL; flavonoids—FETOL; alkaloids—AETOL) and inhibitor concentration (10, 30, 70, 150, 300 ppm) and temperature (25, 40, 60 °C) (d) Results i. Gravimetric AETOL 300 ppm; 99.3% IE ii. Thermometric AETOL 300 ppm; 25 °C; 98.2% IE iii. EIS AETOL; 79.0% IE | Limitation: Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [94]/2018 |
| (a) Tridax procumbens (daisy flower) and Chromolaena odorata (Christmas bush-leaf) (b) Experiments: EIS and adsorption isotherm (c) Parameters: Inhibitor concentration (100, 200, 300, 400 ppm) (d) Results: 100 ppm gave 95.06% IE | Limitation: Constant temperature (40 °C) and immersion time Remark: Vary the temperature from 25 up to 90 °C and the immersion time from 3 up to 30 days | [95]/2018 |
| (a) Diospyros kaki (persimmon) (b) Experiments: EIS and polarization, weight loss, surface analysis (c) Results: 225 ppm gave 83.45% IE at immersion time of 6 h | Limitation: Constant temperature (25 °C) and short immersion time (3–6 h) Remark: Vary the temperature from 25 up to 90 °C and extend the immersion time from 3 up to 30 days | [96]/2016 |
| (a) Sida acuta leaves and stem (wireweed) (b) Experiments: Weight loss, hydrogen evaluation measurement, spectrophotometric analysis (c) Parameters: Temperature (30–60 °C) (d) Results: 500 ppm gave 85% (leaves) and 52% (stem) IE at 30 °C | Limitation: Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [97]/2016 |
| (a) Bamboo leaf extract (b) Experiments: Weight loss measurements, electrochemical measurements and atomic force microscope (c) Parameters: Acid concentration (1 M HCl and 0.5 M H2SO4), temperatures (20, 30, 40, 50 °C), immersion time (6–160 h) (d) Results: i. 1 M HCl 40 °C temperature 91.2% IE ii. 0.5 M H2SO4 50 °C temperature 86.5% IE iii. Immersion time 36 to 160 h 95% IE; 1 M HCl 86% IE; 0.5 M H2SO4 | Limitation: Constant inhibitor concentration was used Remark: Vary the concentration from 50 to 250 ppm | [98]/2012 |
| (a) Murraya koenigii (curry leaves) (b) Experiments: Weight loss method, EIS (c) Parameters: Inhibitor concentration, acid concentration (d) Results i. Concentration: 600 ppm 96.66% and 94.66% IE in HCl and H2SO4 ii. Acid concentration 1 M HCl; 97.54% IE | Limitation: Small temperature range (35–65 °C) and short immersion time (2 to 8 h) used Remark: Vary the temperature from 25 up to 90 °C and extend the immersion time from 3 up to 30 days | [99]/2010 |
| Details | Gap | References/Year |
|---|---|---|
| (a) Rosa canina fruit (b) Experiments: Characterization, quantum chemical and EIS (c) Parameters: Inhibitor concentration (200, 400, 600, 800 ppm) and immersion time (2, 4, 6, 24, 48 h) (d) Result: 600 ppm gave 85.7% IE at immersion time of 6 h | Limitation: Constant temperature (25 °C) Remark: Vary the temperature from 25 up to 90 °C | [100]/2019 |
| (a) Lychee waste (b) Experiments: Weight loss, EIS, FTIR and SEM, and computational studies (c) Parameters: Extraction process (blank, etoh-U, etoh-R, H2O-U), immersion time (1.5, 3.0, 4.5 h) and inhibitor concentration (300, 400, 500, 600, 700 ppm) (d) Results Etoh-U: 97.95% IE 1.5 h: 97.95% IE 600 ppm: 97.95% IE | Limitation: Constant temperature (25 °C) Remark: Vary the temperature from 25 up to 90 °C | [101]/2018 |
| (a) Musa paradisica peels (banana) (b) Experiments: EIS, polarization, surface analysis (c) Parameters: Acid solution (1 M HCl and 0.5 M H2SO4) and inhibitor concentration (200, 300, 400 ppm) (d) Results: 1 M HCl, 400 ppm gave 90% IE | Limitation: Constant temperature (25 °C) and immersion time (24 h) Remark: Vary the temperature from 25 up to 90 °C and extend the immersion time from 3 up to 30 days | [102]/2018 |
| (a) Longan seed and peel (b) Experiment: EIS, Weight loss, FTIR, SEM and computational studies (c) Parameters: Inhibitor concentration (300, 400, 500, 600 ppm) and temperature (25, 35, 45, 55 °C) (d) Results: 600ppm: 92.93% IE 55 °C: 89.29% IE | Limitation: Constant immersion time (24 h) Remark: Vary the immersion time from 3 up to 30 days | [103]/2017 |
| (a) Papaya Seed (b) Experiments: Electrochemical studies, adsorption studies (c) Parameter: Different H2SO4 solutions (0.5 M, 1 M, 3 M) (d) Results: 3 M H2SO4 gave 90% IE | Limitation: Constant temperature (25 °C) and immersion time (24 h) Remark: Vary the temperature from 25 up to 90 °C and extend the immersion time from 3 up to 30 days | [104]/2016 |
| (a) Capsicum annuum fruit paste (b) Experiments: Weight loss, contact angle measurements, analysis of protective film (c) Parameter: Immersion time (24, 96, 168 h) (d) Results: 96.48% IE at immersion time of 24 h | Limitation: Constant temperature (25 °C) and concentration Remark: Vary the temperature from 25 up to 90 °C and concentrations from 50 to 250 ppm | [105]/2016 |
| (a) Gingko biloba fruit (b) Experiments: MS, FTIR, EIS, contact angle measurement and SEM (c) Parameters: Inhibitor concentration (250, 500, 1000 ppm) (d) Results: 1000 ppm gave 97% IE | Limitation: Constant temperature (25 °C) and immersion time (24 h) Remark: Vary the temperature from 25 up to 90 °C and extend the immersion time from 3 up to 30 days | [106]/2015 |
| (a) Litchi fruit (b) Experiments: Weight loss, EIS, surface analysis (c) Parameter: Inhibitor concentration (25, 75, 100, 150, 200, 300 ppm) (d) Results: 300 ppm gave 97.8% IE | Limitation: Constant temperature (25 °C) and immersion time (24 h) Remark: Vary the temperature from 25 up to 90 °C and extend the immersion time from 3 up to 30 days | [107]/2015 |
| (a) Watermelon waste (b) Experiments: EIS, SEM, UV-vis and FTIR (c) Parameters: Watermelon waste (rind, seed, peel) and inhibitor concentration (10, 50, 100, 200 ppm) (d) Results: Rind: 200 ppm, 79.86% IE Seed: 200 ppm, 83.67% IE Peel: 200 ppm, 72.42% IE | Limitation: Constant temperature (25 °C) and immersion time (24 h) Remark: Vary the temperature from 25 up to 90 °C and extend the immersion time from 3 up to 30 days | [108]/2015 |
| (a) Apricot juice (b) Experiments: Adsorption study and inhibition mechanism (c) Parameters: Inhibitor concentration (100, 200, 300, 400 ppm) and temperature (30, 40, 50, 60 °C) (d) Results: 400 ppm gave 75% IE at 30 °C | Limitation: Constant immersion time (24 h) used throughout experiment Remark: Vary the immersion time from 3 up to 30 days | [109]/2013 |
| (a) Fruit peels (b) Experiments: EIS, polarization, weight loss, SEM (c) Parameters: Type of fruit peels (mango, orange, passion, and cashew), inhibitor concentration (100–800 ppm), immersion time (1, 4, 24 h) and temperature (25, 40, 60 °C) (d) Results: i. Mango 600 mg/L: 91% IE Orange 400 mg/L: 95% IE Passion 500 mg/L: 90% IE Cashew 800 mg/L: 80% IE ii. Immersion time 24 h: 96% IE iii. Temperature 25 °C: 92% IE | No limitation or remarks | [110]/2010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamalmani, K.; Husin, H. Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues. Appl. Sci. 2020, 10, 3389. https://doi.org/10.3390/app10103389
Tamalmani K, Husin H. Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues. Applied Sciences. 2020; 10(10):3389. https://doi.org/10.3390/app10103389
Chicago/Turabian StyleTamalmani, Kausalya, and Hazlina Husin. 2020. "Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues" Applied Sciences 10, no. 10: 3389. https://doi.org/10.3390/app10103389
APA StyleTamalmani, K., & Husin, H. (2020). Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues. Applied Sciences, 10(10), 3389. https://doi.org/10.3390/app10103389






