The Nutraceutical Value of Carnitine and Its Use in Dietary Supplements
Abstract
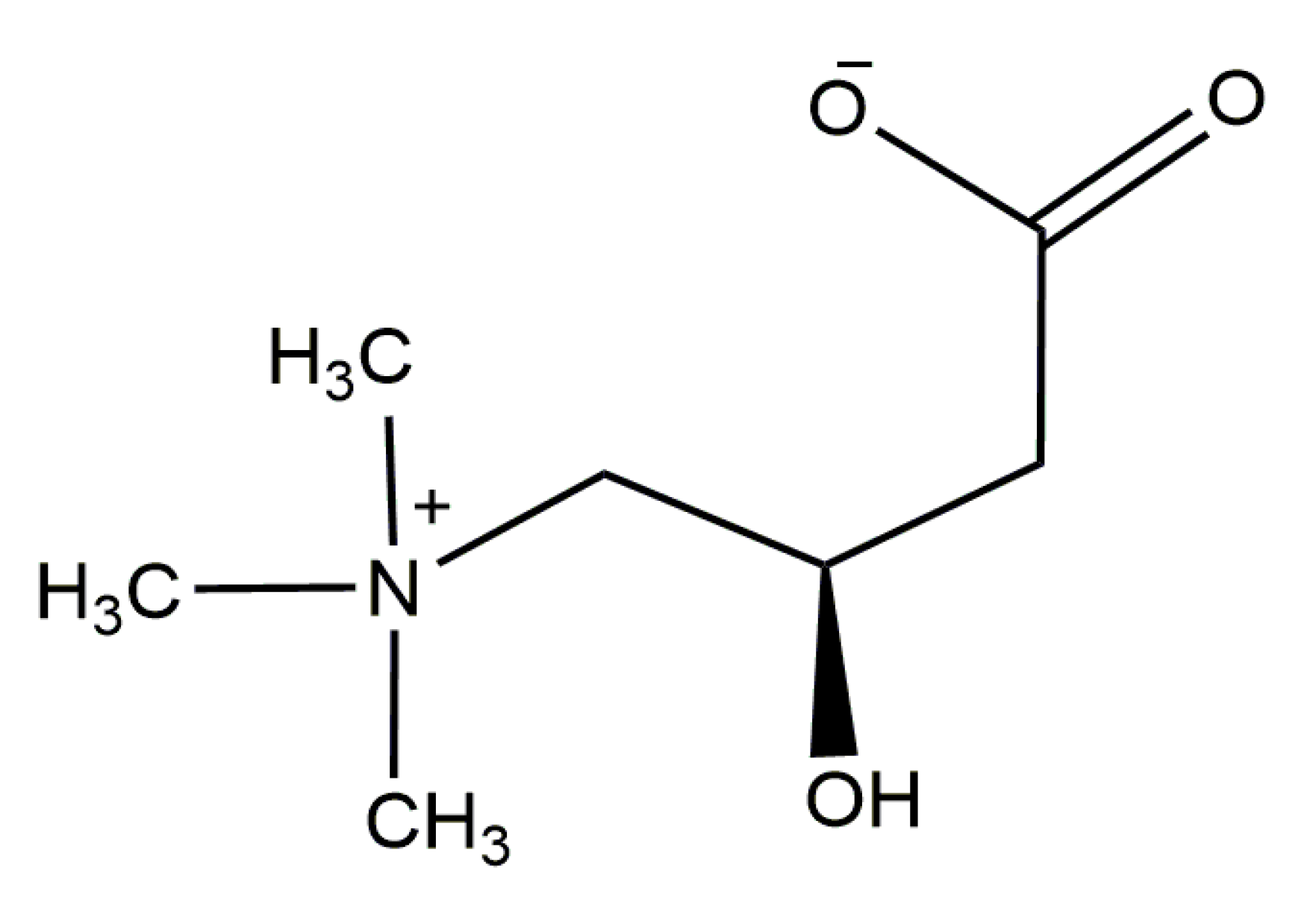
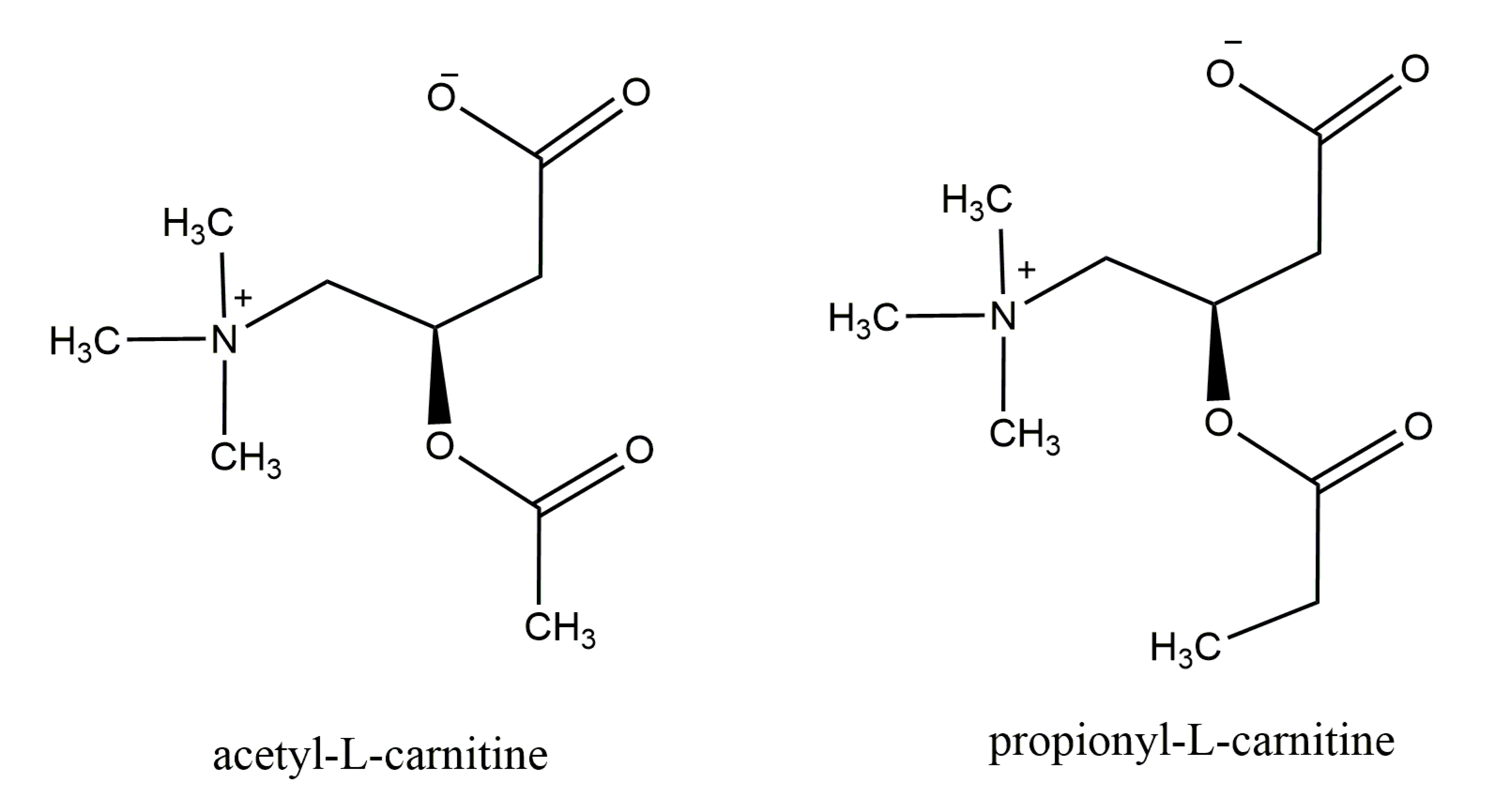
1. Carnitine: An Overview of Its Main Features
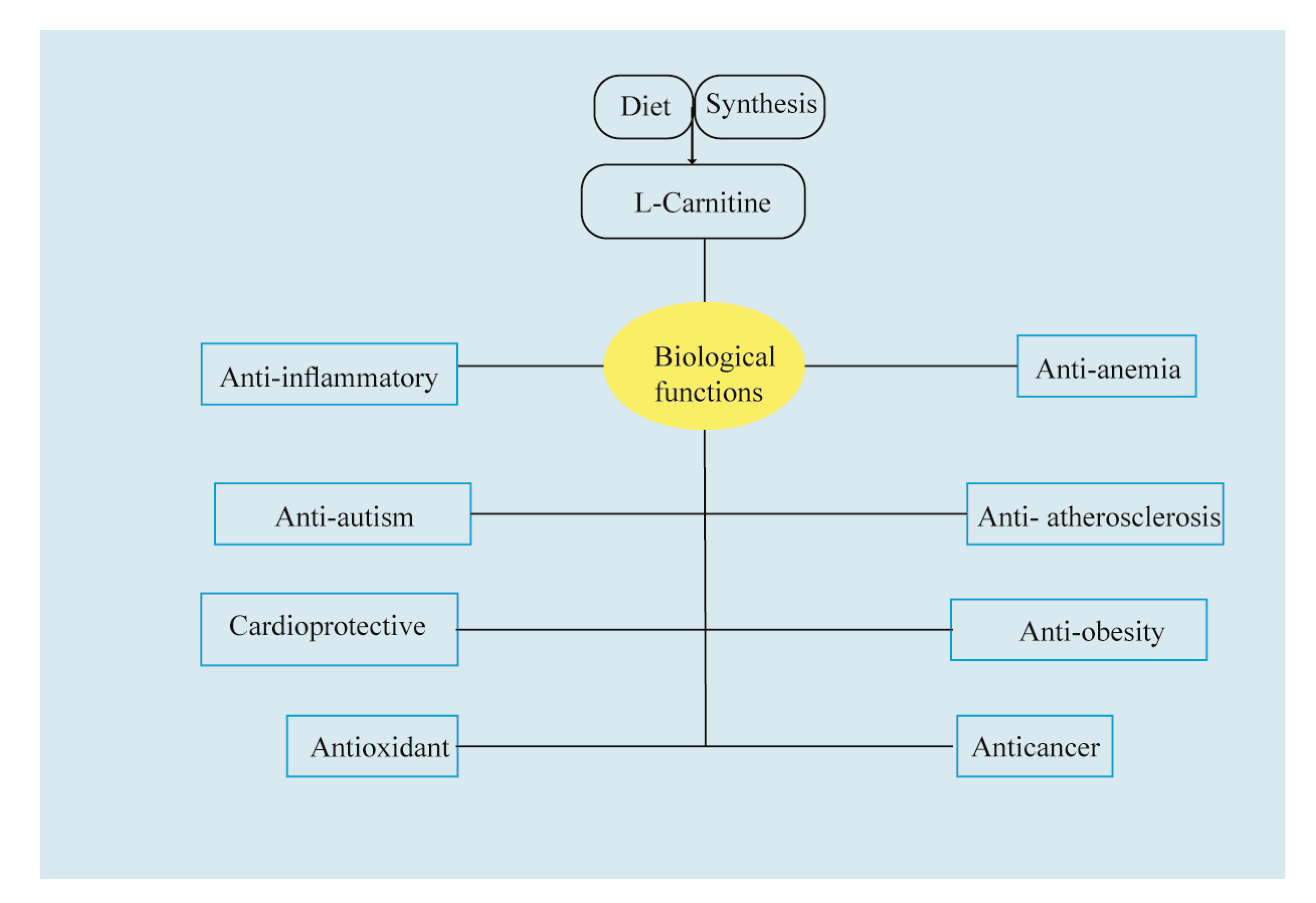
2. An Updated Shot of Beneficial Properties: In Vitro and In Vivo Studies
2.1. In Vitro Activity
2.2. In-Animal Studies
2.3. In Human Studies
3. Carnitine-Based Dietary Supplements
3.1. Monitoring l-Carnitine in Dietary Supplements
3.2. A Shot of Dietary Supplement Label Databases
4. Conclusions and Future Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Alesci, S. Carnitine: The science behind a conditionally essential nutrient. Ann. N. Y. Acad. Sci. 2004, 1033, 1–197. [Google Scholar]
- Wolf, G. The discovery of a vitamin role for carnitine: The first 50 years. J. Nutr. 2006, 136, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Black, S.M. Carnitine homeostasis, mitochondrial function and cardiovascular disease. Drug Discov. Today 2009, 6, e31–e39. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Sirtori, C.; Peracino, A.; Gheorghiade, M.; Borum, P.; Remuzzi, G.; Ardehali, H. Translating the basic knowledge of mitochondrial functions to metabolic therapy: Role of l-carnitine. Transl. Res. 2013, 161, 73–84. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Scaglia, F. Disorders of carnitine biosynthesis and transport. Mol. Genet. Metab. 2015, 116, 107–112. [Google Scholar] [CrossRef]
- Longo, N.; Amat di San Filippo, C.; Pasquali, M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. 2006, 142, 77–85. [Google Scholar] [CrossRef]
- Wang, L.Y.; Chen, N.I.; Chen, P.W.; Chiang, S.C.; Hwu, W.L.; Lee, N.C.; Chien, Y.H. Newborn screening for citrin deficiency and carnitine uptake defect using second-tier molecular tests. BMC Med. Genet. 2013, 14, 24. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chien, Y.H.; Chen, P.W.; Leung-Sang Tang, N.; Chiu, P.C.; Hwu, W.L.; Lee, N.C. Carnitine uptake defect (primary carnitine deficiency): Risk in genotype-phenotype correlation. Hum. Mutat. 2013, 34, 655. [Google Scholar] [CrossRef]
- Lee, N.C.; Tang, N.L.S.; Chien, Y.H.; Chen, C.A.; Lin, S.J.; Chiu, P.C.; Huang, A.C.; Hwu, W.L. Diagnoses of newborns and mothers with carnitine uptake defects through newborn screening. Mol. Genet. Metabol. 2010, 100, 46–50. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochem. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Steiber, A.; Kerner, J.; Hoppel, C.L. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 2004, 25, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines. Clin. Pharmacokin. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Pormsila, W.; Krähenbühl, S.; Hauser, P.C. Determination of carnitine in food and food supplements by capillary electrophoresis with contactless conductivity detection. Electrophoresis 2010, 31, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Odle, J.; Adams, S.H.; Vockley, J. Carnitine. Adv. Nutr. 2014, 5, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metabol. 2010, 7, 30. [Google Scholar] [CrossRef]
- Ribas, G.S.; Vargas, C.R.; Wajner, M. l-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 2014, 533, 469–476. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, W.; Chen, G.; Zhu, W.; Ding, W.; Ge, Z.; Tan, Y.; Ma, T.; Cui, G. l-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exper. Med. 2017, 26, 333–338. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Lavie, C.J.; Fares, H.; Menezes, A.R.; O’Keefe, J.H. l-carnitine in the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Proc. Mayo Clin. Proc. 2013, 88, 544–551. [Google Scholar] [CrossRef]
- Shang, R.; Sun, Z.; Li, H. Effective dosing of l-carnitine in the secondary prevention of cardiovascular disease: A systematic review and meta-analysis. BMC Cardiovasc Disord. 2014, 14, 88. [Google Scholar] [CrossRef]
- Melone, M.A.B.; Valentino, A.; Margarucci, S.; Galderisi, U.; Giordano, A.; Peluso, G. The carnitine system and cancer metabolic plasticity. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A. Risk assessment for carnitine. Regul. Toxicol. Pharmacol. 2006, 46, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ribas, G.S.; Manfredini, V.; de Marco, M.G.; Vieira, R.B.; Wayhs, C.Y.; Vanzin, C.S.; Biancini, G.B.; Wajner, M.; Vargas, C.R. Prevention by l-carnitine of DNA damage induced by propionic and L-methylmalonic acids in human peripheral leukocytes in vitro. Mutat. Res. Genet Toxicol. Environ. Mutagen. 2010, 702, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.; Sharma, R.; Bayachou, M.; Sabanegh, E.; Agarwal, A. Human sperm DNA oxidation, motility and viability in the presence of l-carnitine during in vitro incubation and centrifugation. Andrologia 2012, 44, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Zamani, E.; Shokrzadeh, M.; Modanloo, M.; Shaki, F. In Vitro Study Towards Role of Acrylamide-Induced Genotoxicity in Human Lymphocytes and the Protective Effect of l-carnitine. Braz Arch Bio Technol. 2018, 61. [Google Scholar] [CrossRef]
- Hua, X.; Deng, R.; Li, J.; Chi, W.; Su, Z.; Lin, J.; Pflugfelder, S.C.; Li, D.-Q. Protective effects of l-carnitine against oxidative injury by hyperosmolarity in human corneal epithelial cells. Investig. Ophthal. Visual Sci. 2015, 56, 5503–5511. [Google Scholar] [CrossRef]
- Farahzadi, R.; Fathi, E.; Mesbah-Namin, S.A.; Zarghami, N. Anti-aging protective effect of l-carnitine as clinical agent in regenerative medicine through increasing telomerase activity and change in the hTERT promoter CpG island methylation status of adipose tissue-derived mesenchymal stem cells. Tissue Cell. 2018, 54, 105–113. [Google Scholar] [CrossRef]
- Nakamura, Y.; Iida, H.; Nakatake, R.; Sakaguchi, T.; Kaibori, M.; Okumura, T.; Hamada, Y.; Doi, T. l-carnitine has a liver-protective effect through inhibition of inducible nitric oxide synthase induction in primary cultured rat hepatocytes. Funct. Foods Health Dis. 2018, 8, 212–227. [Google Scholar] [CrossRef]
- Baci, D.; Bruno, A.; Bassani, B.; Tramacere, M.; Mortara, L.; Albini, A.; Noonan, D.M. Acetyl-l-carnitine is an anti-angiogenic agent targeting the VEGFR2 and CXCR4 pathways. Cancer Lett. 2018, 429, 100–116. [Google Scholar] [CrossRef]
- Fernandes, S.; Salta, S.; Bravo, J.; Silva, A.; Summavielle, T. Acetyl-l-carnitine prevents methamphetamine-induced structural damage on endothelial cells via ILK-related MMP-9 activity. Mol. Neurobiol. 2016, 53, 408–422. [Google Scholar] [CrossRef]
- Singh, M.; Miura, P.; Renden, R. Age-related defects in short-term plasticity are reversed by acetyl-l-carnitine at the mouse calyx of Held. Neurobiol. Aging. 2018, 67, 108–119. [Google Scholar] [CrossRef]
- Nicassio, L.; Fracasso, F.; Sirago, G.; Musicco, C.; Picca, A.; Marzetti, E.; Calvani, R.; Cantatore, P.; Gadaleta, M.N.; Pesce, V. Dietary supplementation with acetyl-l-carnitine counteracts age-related alterations of mitochondrial biogenesis, dynamics and antioxidant defenses in brain of old rats. Exp. Gerontol. 2017, 98, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M.; Noland, R.C.; Kovalik, J.P.; Seiler, S.E.; Davies, M.N.; DeBalsi, K.L.; Ilkayeva, O.R.; Stevens, R.D.; Kheterpal, I.; Zhang, J. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabol. 2012, 15, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarem, H.M.; Fadda, L.H.; Hassan, A.A. Potential intervention of α-Lipoic acid and carnitine on insulin sensitivity and anti-inflammatory cytokines levels in fructose-fed rats, a model of metabolic syndrome. J. Diet. Suppl. 2017, 14, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi-Namileh, V.; Sepand, M.R.; Omidi, A.; Aghsami, M.; Seyednejad, S.A.; Kasirzadeh, S.; Sabzevari, O. Acetyl-l-carnitine attenuates arsenic-induced liver injury by abrogation of mitochondrial dysfunction, inflammation, and apoptosis in rats. Environ. Toxicol. Pharmacol. 2018, 58, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Edres, H.A.; Taha, N.M.; Mandour, A.E.W.A.; Lebda, M.A. Impact of l-carnitine on Bisphenol A-Induced Kidney Damage in Rats. Alex. J. Vet. Sci. 2018, 56, 11–17. [Google Scholar] [CrossRef]
- Al-Eisa, R.A.; Al-Salmi, F.A.; Hamza, R.Z.; El-Shenawy, N.S. Role of l-carnitine in protection against the cardiac oxidative stress induced by aspartame in Wistar albino rats. PLoS ONE 2018, 13, e0204913. [Google Scholar] [CrossRef] [PubMed]
- Couturier, A.; Ringseis, R.; Mooren, F.C.; Krüger, K.; Most, E.; Eder, K. Carnitine supplementation to obese Zucker rats prevents obesity-induced type I to type II muscle fiber transition and favors an oxidative phenotype of skeletal muscle. Nutr. Metab. 2013, 10, 48. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Ibrahim, Z.S.; Alkafafy, M.; El-Shazly, S.A. l-carnitine protects against testicular dysfunction caused by gamma irradiation in mice. Acta histochemica 2014, 116, 1046–1055. [Google Scholar] [CrossRef]
- Kim, J.H.; Pan, J.H.; Lee, E.S.; Kim, Y.J. l-carnitine enhances exercise endurance capacity by promoting muscle oxidative metabolism in mice. Biochem. Biophys. Res. Commun. 2015, 464, 568–573. [Google Scholar] [CrossRef]
- Meky, N.H.; Haggag, A.M.; Kamal, A.M.; Ahmed, Z.A. The Protective Effect of l-carnitine against Gamma Irradiation-Induced Cardiotoxicity in Male Albino Rats. Egypt. Acad. J. Bio. Sci. Phys. Mol. Bio. 2017, 9, 9–20. [Google Scholar] [CrossRef]
- Sepand, M.R.; Razavi-Azarkhiavi, K.; Omidi, A.; Zirak, M.R.; Sabzevari, S.; Kazemi, A.R.; Sabzevari, O. Effect of acetyl-l-carnitine on antioxidant status, lipid peroxidation, and oxidative damage of arsenic in rat. Biol. Trace Elem. Res. 2016, 171, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, J.; Chang, H.; Lim, K. l-carnitine supplement reduces skeletal muscle atrophy induced by prolonged hindlimb suspension in rats. Appl. Physiol. Nutr. Metab. 2016, 41, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.L.; Drazul-Schrader, D.; Sulpizio, A.C.; Koster, P.D.; Williamson, Y.; Adelman, S.J.; Owen, K.; Sanli, T.; Bellamine, A. l-carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP. Atherosclerosis 2016, 244, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Canbolat, E.P.; Sagsoz, N.; Noyan, V.; Yucel, A.; Kısa, U. Effects of l-carnitine on oxidative stress parameters in oophorectomized rats. Alex. Med. J. 2017, 53, 55–60. [Google Scholar] [CrossRef]
- Zamani, E.; Shokrzadeh, M.; Ziar, A.; Abedian-Kenari, S.; Shaki, F. Acrylamide attenuated immune tissues’ function via induction of apoptosis and oxidative stress: Protection by l-carnitine. Hum. Exp. Toxicol. 2018, 37, 859–869. [Google Scholar] [CrossRef]
- Zakzok, F.B.; Hegazy, H.M.; Yosef, T.A.; Gomaa, G.M. Mitigating impact of l-carnitine against dimethoate induction of hepatic and testicular genotoxicity in rats: The role of oxidative stress. Toxin Rev. 2018. [Google Scholar] [CrossRef]
- Salama, S.A.; Arab, H.H.; Omar, H.A.; Gad, H.S.; Abd-Allah, G.M.; Maghrabi, I.A. l-carnitine mitigates UVA-induced skin tissue injury in rats through downregulation of oxidative stress, p38/c-Fos signaling, and the proinflammatory cytokines. Chem.-Biol. Interact. 2018, 285, 40–47. [Google Scholar] [CrossRef]
- Cruz, W.M.S.; Guimaraes, S.; Maciel, G.C.; Huguenin, A.B.A.; Carvalho, M.E.D.; Costa, B.O.; Silva, G.A.; Colafranceschi, A.S.; Scalco, F.B.; Ribeiro, M. l-carnitine supplementation in the recovery of plasma l-carnitine in patients with heart failure submitted to coronary artery bypass grafting. Anais da Acad. Bras. de Ciências 2018, 90, 3099–3104. [Google Scholar] [CrossRef]
- Yaghubi, E.; Daneshpazhooh, M.; Djalali, M.; Mohammadi, H.; Sepandar, F.; Fakhri, Z.; Ghaedi, E.; Keshavarz, S.A.; Balighi, K.; Mahmoudi, H. Effects of l-carnitine supplementation on cardiovascular and bone turnover markers in patients with pemphigus vulgaris under corticosteroids treatment: A randomized, double-blind, controlled trial. Dermat. Therap. 2019, 32, e13049. [Google Scholar] [CrossRef]
- Talari, H.R.; Azad, Z.J.; Hamidian, Y.; Samimi, M.; Gilasi, H.R.; Afshar, F.E.; Ostadmohammadi, V.; Asemi, Z. Effects of carnitine administration on carotid intima-media thickness and inflammatory factors in patients with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Int. J. Prev. Med. 2019, 10, 89. [Google Scholar]
- Ravikumar, S.; Prabhu, S.; Vani, R. Effects of l-carnitine on the erythrocytes of stored human blood. Transfus. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Talasaz, A.H.; Alidoosti, M. Preventive effect of l-carnitine and its derivatives on endothelial dysfunction and platelet aggregation. Clin. Nutr. ESPEN 2016, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, G.; Faverzani, J.; Jacques, C.E.D.; Marchetti, D.P.; Sitta, A.; de Moura Coelho, D.; Kayser, A.; Kok, F.; Athayde, L.; Manfredini, V. Oxidative damage in glutaric aciduria type I patients and the protective effects of l-carnitine treatment. J. Cell. Biochem. 2018, 119, 10021–10032. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Longo, S.; Gnoni, G.V.; Giudetti, A.M. Carnitine in Human Muscle Bioenergetics: Can Carnitine Supplementation Improve Physical Exercise? Molecules 2020, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Hashimoto, S.; Matsumoto, H.; Yamochi, T.; Yamanaka, M.; Nakaoka, Y.; Fukuda, A.; Inoue, M.; Ikeda, T.; Morimoto, Y. Oral administration of l-carnitine improves the clinical outcome of fertility in patients with IVF treatment. Gynecol. Endocr. 2018, 34, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Verrina, E.; Caruso, U.; Calevo, M.G.; Emma, F.; Sorino, P.; De Palo, T.; Lavoratti, G.; Dertenois, L.T.; Cassanello, M.; Cerone, R. Effect of carnitine supplementation on lipid profile and anemia in children on chronic dialysis. Ped. Neph. 2007, 22, 727–733. [Google Scholar] [CrossRef]
- Geier, D.A.; Kern, J.K.; Davis, G.; King, P.G.; Adams, J.B.; Young, J.L.; Geier, M.R. A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Med. Sci. Monit. 2011, 17, PI15. [Google Scholar] [CrossRef]
- Fahmy, S.F.; El-hamamsy, M.H.; Zaki, O.K.; Badary, O.A. l-carnitine supplementation improves the behavioral symptoms in autistic children. Res. Autism Spect. Disord. 2013, 7, 159–166. [Google Scholar] [CrossRef]
- Ziats, M.N.; Comeaux, M.S.; Yang, Y.; Scaglia, F.; Elsea, S.H.; Sun, Q.; Beaudet, A.L.; Schaaf, C.P. Improvement of regressive autism symptoms in a child with TMLHE deficiency following carnitine supplementation. Am. J. Med. Genet. A. 2015, 167, 2162–2167. [Google Scholar] [CrossRef]
- Roy, M.J.; Dionne, S.; Marx, G.; Qureshi, I.; Sarma, D.; Levy, E.; Seidman, E.G. In vitro studies on the inhibition of colon cancer by butyrate and carnitine. Nutrition 2009, 25, 1193–1201. [Google Scholar] [CrossRef]
- Baci, D.; Bruno, A.; Cascini, C.; Gallazzi, M.; Mortara, L.; Sessa, F.; Pelosi, G.; Albini, A.; Noonan, D.M. Acetyl-l-carnitine downregulates invasion (CXCR4/CXCL12, MMP-9) and angiogenesis (VEGF, CXCL8) pathways in prostate cancer cells: Rationale for prevention and interception strategies. J. Exp. Clin. Cancer Res. 2019, 38, 464. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, N.; Guo, H.; Liao, S.; Li, X.; Yang, C.; Liu, S.; Song, W.; Liu, C.; Guan, L. l-carnitine is an endogenous HDAC inhibitor selectively inhibiting cancer cell growth in vivo and in vitro. PLoS ONE 2012, 7, e49062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, H.; Zhang, Z.; Wang, T.; Niu, J.; Cui, D.; Xu, S. Neuroprotective effects of pre-treatment with l-carnitine and acetyl-l-carnitine on ischemic injury in vivo and in vitro. Int. J. Mol. Sci. 2012, 13, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Deon, M.; Landgraf, S.S.; Lamberty, J.F.; Moura, D.J.; Saffi, J.; Wajner, M.; Vargas, C.R. Protective effect of l-carnitine on Phenylalanine-induced DNA damage. Metab. Brain Dis. 2015, 30, 925–933. [Google Scholar] [CrossRef]
- Kocsis, K.; Frank, R.; Szabó, J.; Knapp, L.; Kis, Z.; Farkas, T.; Vécsei, L.; Toldi, J. Acetyl-l-carnitine restores synaptic transmission and enhances the inducibility of stable LTP after oxygen–glucose deprivation. Neuroscience 2016, 332, 203–211. [Google Scholar] [CrossRef]
- Bavari, M.; Tabandeh, M.R.; Najafzadeh Varzi, H.; Bahramzadeh, S. Neuroprotective, antiapoptotic and antioxidant effects of l-carnitine against caffeine-induced neurotoxicity in SH-SY5Y neuroblastoma cell line. Drug Chem. Toxicol. 2016, 39, 157–166. [Google Scholar] [CrossRef]
- de Moraes, M.S.; Guerreiro, G.; Sitta, A.; de Moura Coelho, D.; Manfredini, V.; Wajner, M.; Vargas, C.R. Oxidative damage in mitochondrial fatty acids oxidation disorders patients and the in vitro effect of l-carnitine on DNA damage induced by the accumulated metabolites. Arch. Biochem. Biophys. 2020, 679, 108206. [Google Scholar] [CrossRef]
- Montesano, A.; Senesi, P.; Vacante, F.; Mollica, G.; Benedini, S.; Mariotti, M.; Luzi, L.; Terruzzi, I. l-carnitine counteracts in vitro fructose-induced hepatic steatosis through targeting oxidative stress markers. J. Endocrinol. Investig. 2019, 43, 493–503. [Google Scholar] [CrossRef]
- Terruzzi, I.; Montesano, A.; Senesi, P.; Villa, I.; Ferraretto, A.; Bottani, M.; Vacante, F.; Spinello, A.; Bolamperti, S.; Luzi, L. l-carnitine reduces oxidative stress and promotes cells differentiation and bone matrix proteins expression in human osteoblast-like cells. BioMed. Res. Int. 2019, 2019, 5678548. [Google Scholar] [CrossRef]
- Calandrella, N.; De Seta, C.; Scarsella, G.; Risuleo, G. Carnitine reduces the lipoperoxidative damage of the membrane and apoptosis after induction of cell stress in experimental glaucoma. Cell Death Dis. 2010, 1, e62. [Google Scholar] [CrossRef]
- Farahzadi, R.; Mesbah-Namin, S.A.; Zarghami, N.; Fathi, E. l-carnitine effectively induces hTERT gene expression of human adipose tissue-derived mesenchymal stem cells obtained from the aged subjects. Int. J. Stem Cells. 2016, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.G.B.; de Moura Coelho, D.; Sitta, Â.; Jacques, C.E.D.; Hauschild, T.; Manfredini, V.; Bakkali, A.; Struys, E.A.; Jakobs, C.; Wajner, M. Experimental evidence of oxidative stress in patients with l-2-hydroxyglutaric aciduria and that l-carnitine attenuates in vitro DNA damage caused by d-2-hydroxyglutaric and l-2-hydroxyglutaric acids. Toxicol. Vitro 2017, 42, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Le Borgne, F.; Ravaut, G.; Bernard, A.; Demarquoy, J. l-carnitine protects C2C12 cells against mitochondrial superoxide overproduction and cell death. World J. Biol. Chem. 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, M.H.M.; Yenzeel, J.H.; Fakhrildin, M.-B.M. Effect of l-carnitine and COQ10 addition to SMART Pro-medium on human sperm concentration, sperm morphology and chromatin structure during in vitro sperm activation. J. Pharm. Bio. Sci. 2017, 12, 51–55. [Google Scholar]
- Abdel-Emam, R.A.; Ahmed, E.A.; Ali, M.F. The protective role of l-carnitine against 1st- and 2nd-generation antihistamine-induced liver injury in mice. Compar. Clin. Path. 2020, 29, 213–221. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Abd-Eldayem, A.M.; Aboulhagag, N.A. The possible protective effects of vitamin D and l-carnitine against used atorvastatin-induced myopathy and hepatotoxicity. Compar. Clin. Path. 2019, 28, 1751–1759. [Google Scholar] [CrossRef]
- Salic, K.; Gart, E.; Seidel, F.; Verschuren, L.; Caspers, M.; van Duyvenvoorde, W.; Wong, K.E.; Keijer, J.; Bobeldijk-Pastorova, I.; Wielinga, P.Y. Combined Treatment with l-carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. Int. J. Mol. Sci. 2019, 20, 4359. [Google Scholar] [CrossRef]
- Ali, S.A.; Faddah, L.; Abdel-Baky, A.; Bayoumi, A. Protective effect of l-carnitine and coenzyme Q10 on CCl4-induced liver injury in rats. Sci. Pharm. 2010, 78, 881–896. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Skrzydlewska, E.; Figaszewski, Z. Effect of l-carnitine on liver cell membranes in ethanol-intoxicated rats. Chem.-Bio. Interact. 2010, 188, 44–51. [Google Scholar] [CrossRef]
- Xiang, Y.; Piao, S.; Zou, H.; Jin, J.; Fang, M.; Lei, D.; Gao, B.; Yang, C.; Li, C. l-carnitine protects against cyclosporine-induced pancreatic and renal injury in rats. Transpl. Proc. 2013, 45, 3127–3134. [Google Scholar] [CrossRef]
- Ishikawa, H.; Takaki, A.; Tsuzaki, R.; Yasunaka, T.; Koike, K.; Shimomura, Y.; Seki, H.; Matsushita, H.; Miyake, Y.; Ikeda, F. l-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS ONE 2014, 9, e100627. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.; Zheng, J.; Zhang, X.; Shao, J.; Zhao, L.; Hao, J. Anti-Inflammatory and Antioxidant Effects of Acetyl-l-carnitine on Atherosclerotic Rats. Med. Sci. Monit. 2020, 26, e920250. [Google Scholar] [CrossRef] [PubMed]
- Blanca, A.J.; Ruiz-Armenta, M.V.; Zambrano, S.; Miguel-Carrasco, J.L.; Arias, J.L.; Arevalo, M.; Mate, A.; Aramburu, O.; Vazquez, C.M. Inflammatory and fibrotic processes are involved in the cardiotoxic effect of sunitinib: Protective role of l-carnitine. Toxicol. Lett. 2016, 241, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Abd El Fattah, M.A.; Ahmed, K.A.; Moawad, H. Protective effects of Olmesartan and l-carnitine on doxorubicin-induced Cardiotoxicity in rats. Can. J. Physiol. Pharmacol. 2019, 999, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Lapi, D.; Sabatino, L.; Altobelli, G.G.; Mondola, P.; Cimini, V.; Colantuoni, A. Effects of propionyl-l-carnitine on ischemia–reperfusion injury in hamster cheek pouch microcirculation. Front. Physiol. 2010, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Ohtani, T.; Sakata, Y.; Mano, T.; Takeda, Y.; Tamaki, S.; Tsukamoto, Y.; Kamimura, D.; Aizawa, Y.; Miwa, T. l-carnitine prevents the development of ventricular fibrosis and heart failure with preserved ejection fraction in hypertensive heart disease. J. Hypertens. 2012, 30, 1834–1844. [Google Scholar] [CrossRef]
- Zambrano, S.; Blanca, A.J.; Ruiz-Armenta, M.V.; Miguel-Carrasco, J.L.; Arévalo, M.; Vázquez, M.J.; Mate, A.; Vázquez, C.M. l-carnitine protects against arterial hypertension-related cardiac fibrosis through modulation of PPAR-γ expression. Biochem. Pharmacol. 2013, 85, 937–944. [Google Scholar] [CrossRef]
- Alharthi, W.A.; Hamza, R.Z.; Elmahdi, M.M.; Abuelzahab, H.S.H.; Saleh, H. Selenium and l-carnitine Ameliorate Reproductive Toxicity Induced by Cadmium in Male Mice. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Kelek, S.E.; Afşar, E.; Akçay, G.; Danışman, B.; Aslan, M. Effect of chronic l-carnitine supplementation on carnitine levels, oxidative stress and apoptotic markers in peripheral organs of adult Wistar rats. Food Chem. Toxicol. 2019, 134, 110851. [Google Scholar] [CrossRef]
- Dokmeci, D.; Inan, M.; Basaran, U.N.; Yalcin, O.; Aydogdu, N.; Turan, F.N.; Uz, Y.H. Protective effect of l-carnitine on testicular ischaemia–reperfusion injury in rats. Cell Biochem. Funct. Cellul. 2007, 25, 611–618. [Google Scholar] [CrossRef]
- Boyacioglu, M.; Turgut, H.; Akgullu, C.; Eryilmaz, U.; Kum, C.; Onbasili, O.A. The efficient of l-carnitine on oxidative stress responses of experimental contrast-induced nephropathy in rat. J. Vet. Med. Sci. 2014, 76, 13–0202. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elrazek, A.M.; Ahmed-Farid, O.A.H. Protective effect of l-carnitine and L-arginine against busulfan-induced oligospermia in adult rat. Andrologia 2018, 50. [Google Scholar] [CrossRef] [PubMed]
- Masoumi-Ardakani, Y.; Aminizadeh, S.; Fallah, H.; Shahouzehi, B. l-carnitine different doses affect serum and pancreas tissue Antioxidative defense and histopathology in STZ-induced diabetic rats. Biologia 2020. [Google Scholar] [CrossRef]
- Hooshmand, S.; Balakrishnan, A.; Clark, R.M.; Owen, K.Q.; Koo, S.I.; Arjmandi, B.H. Dietary l-carnitine supplementation improves bone mineral density by suppressing bone turnover in aged ovariectomized rats. Phytomedicine 2008, 15, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Jiang, Q.; Song, L.; Liu, Y.; Li, M.; Lin, Q.; Li, Y.; Su, K.; Ma, Z.; Wang, Y.; et al. l-carnitine Is Involved in Hyperbaric Oxygen-Mediated Therapeutic Effects in High Fat Diet-Induced Lipid Metabolism Dysfunction. Molecules 2020, 25, 176. [Google Scholar] [CrossRef] [PubMed]
- Chidiebere, U.; Ambali, S.F.; Ayo, J.O.; Eseivo, K.A. Acetyl-l-carnitine attenuates haemotoxicity induced by subacute chlorpyrifos exposure in Wistar rats. Der. Pharm. Lettre. 2011, 3, 292–303. [Google Scholar]
- Yarizadh, H.; Shab-Bidar, S.; Zamani, B.; Vanani, A.N.; Baharlooi, H.; Djafarian, K. The Effect of l-carnitine Supplementation on Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Am. Coll. Nutr. 2020. [Google Scholar] [CrossRef]
- Chae, H.; Ryu, M.-H.; Ma, J.; Beck, M.; Kang, Y.K. Impact of l-carnitine on imatinib-related muscle cramps in patients with gastrointestinal stromal tumor. Investig. New Drugs. 2020, 38, 493–499. [Google Scholar] [CrossRef]
- Malek Mahdavi, A.; Mahdavi, R.; Kolahi, S. Effects of l-carnitine Supplementation on Serum Inflammatory Factors and Matrix Metalloproteinase Enzymes in Females with Knee Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Am. Coll. Nutr. 2016, 35, 597–603. [Google Scholar] [CrossRef]
- Kazemian, K.; Ala, S.; Mojtahedzadeh, M.; Abedini, M.; Alipour, A.; Abediankenari, S.; Rafati, M.; Abaskhanidavanloo, A.; Mohajerani, F. Evaluation of neuroprotective effects of l-carnitine and Fat emulsion in the CVA patients: A prospective, randomized, double blind, clinical trial. Iran. J. Pharm. Res. 2020, 19, 111–119. [Google Scholar] [CrossRef]
- Di Stefano, G.; Di Lionardo, A.; Galosi, E.; Truini, A.; Cruccu, G. Acetyl- l-carnitine in painful peripheral neuropathy: A systematic review. J. Pain Res. 2019, 12, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Abolfathi, M.; Mohd-Yusof, B.N.; Hanipah, Z.N.; Redzwan, S.M.; Yusof, L.M.; Khosroshahi, M.Z. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 102273. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, P.; Chalmers, J.; Ban, L.; Grindlay, D.; Aithal, G.P. l-carnitine supplementation in non-alcoholic fatty liver disease: A systematic review and meta-analysis. WJMA 2020, 8, 4–14. [Google Scholar] [CrossRef]
- Askarpour, M.; Djafarian, K.; Ghaedi, E.; Sadeghi, O.; Sheikhi, A.; Shab-Bidar, S. Effect of l-carnitine Supplementation on Liver Enzymes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Med. Res. 2020, 51, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kido, J.; Inoue, H.; Shimotsu, H.; Yoshida, Y.; Suzuki, Y.; Nakamura, K.; Endo, F.; Matsumoto, S. Effect of l-carnitine on Amino Acid Metabolism in Elderly Patients Undergoing Regular Hemodialysis. Blood Purif. 2020. [Google Scholar] [CrossRef] [PubMed]
- Safdar, O.; Jambi, G.; Asaad, O.; Hassan, L.; Al Dahlawi, L.; Halawani, A.F.; Jamjoom, M.; Shaikhoon, B.; Azhar, A.; Zaher, Z.F. l-carnitine effect on bone disease in hemodialysis pediatric patients in KAU Hospital, Jeddah Saudi Arabia: An experimental non-randomized study. Int. J. Med.Dev. Ctries. 2020, 4, 612–619. [Google Scholar] [CrossRef]
- Koşan, C.; Sever, L.; Arısoy, N.; Çalışkan, S.; Kasapçopur, Ö. Carnitine supplementation improves apolipoprotein B levels in pediatric peritoneal dialysis patients. Pediatr. Nephrol. 2003, 18, 1184–1188. [Google Scholar] [CrossRef]
- Sheikhi, A.; Djafarian, K.; Askarpour, M.; Shab-Bidar, S. The effects of supplementation with l-carnitine on apolipoproteins: A systematic review and meta-analysis of randomized trials. Eur. J. Pharmacol. 2019, 858, 172493. [Google Scholar] [CrossRef]
- Shimizu, S.; Takashima, H.; Tei, R.; Furukawa, T.; Okamura, M.; Kitai, M.; Nagura, C.; Maruyama, T.; Higuchi, T.; Abe, M. Prevalence of Carnitine Deficiency and Decreased Carnitine Levels in Patients on Peritoneal Dialysis. Nutrition 2019, 11, 2645. [Google Scholar] [CrossRef]
- Bordoni, L.; Sawicka, A.K.; Szarmach, A.; Winklewski, P.J.; Olek, R.A.; Gabbianelli, R. A Pilot Study on the Effects of l-carnitine and Trimethylamine-N-Oxide on Platelet Mitochondrial DNA Methylation and CVD Biomarkers in Aged Women. Int. J. Mol. Sci. 2020, 21, 1047. [Google Scholar] [CrossRef]
- Bavbek, N.; Akay, H.; Uz, B.; Uz, E.; Turgut, F.; Kanbay, M.; Senes, M.; Akcay, A.; Duranay, M. The effects of l-carnitine therapy on respiratory function tests in chronic hemodialysis patients. Ren. Fail. 2010, 32, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Sitta, A.; Vanzin, C.S.; Biancini, G.B.; Manfredini, V.; De Oliveira, A.; Wayhs, C.; Ribas, G.; Giugliani, L.; Schwartz, I.; Bohrer, D. Evidence that l-carnitine and selenium supplementation reduces oxidative stress in phenylketonuric patients. Cellul. Mol. Neurobiol. 2011, 31, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Amirani, E.; Mahdavinia, M.; Mafi, A.; Asemi, Z. Carnitine and chromium co-supplementation affects mental health, hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. J. Psychosom. Obst. Gyn. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, T.C.; Guerreiro, G.; Mescka, C.P.; Coelho, D.M.; Steffens, L.; Moura, D.J.; Manfredini, V.; Vargas, C.R. DNA damage induced by alloisoleucine and other metabolites in maple syrup urine disease and protective effect of l-carnitine. Toxicol. Vitro 2019, 57, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, R.A.; Dvorkin, E.; Homel, P.; Malamud, S.; Culliney, B.; Lapin, J.; Portenoy, R.K.; Esteban-Cruciani, N. Safety, tolerability and symptom outcomes associated with l-carnitine supplementation in patients with cancer, fatigue, and carnitine deficiency: A phase I/II study. J. Pain Symptom Manag. 2006, 32, 551–559. [Google Scholar] [CrossRef]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simon, P.; Weber, E.; Schütte, K.; Stieler, J.; Koula-Jenik, H.; Holzhauer, P. l-carnitine-supplementation in advanced pancreatic cancer (CARPAN)-a randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef]
- Shirali, S.; Daneghian, S.; Hosseini, S.A.; Ashtary-Larky, D.; Daneghian, M.; Mirlohi, M.-S. Effect of caffeine co-ingested with carnitine on weight, body-fat percent, serum leptin and lipid profile changes in male teen soccer players: A randomized clinical trial. Int. J. Pediat. 2016, 4, 3685–3698. [Google Scholar]
- Samimi, M.; Jamilian, M.; Ebrahimi, F.A.; Rahimi, M.; Tajbakhsh, B.; Asemi, Z. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2016, 84, 851–857. [Google Scholar] [CrossRef]
- Ibrahim, N.A.M.; Mansour, Y.S.E.; Sulieman, A.A.; Hussein, H.S. A Local Study on the Effects of L-carnitine Supplement on Serum Lipid Profiles in Libyan Type 2 Diabetic Patients. IJARW 2020, 1, 28–32. [Google Scholar]
- Moradi, M.; Moradi, A.; Alemi, M.; Ahmadnia, H.; Abdi, H.; Ahmadi, A.; Bazargan-Hejazi, S. Safety and efficacy of clomiphene citrate and l-carnitine in idiopathic male infertility: A comparative study. Urol. J. 2010, 7, 188–193. [Google Scholar]
- Malaguarnera, M.; Vacante, M.; Giordano, M.; Motta, M.; Bertino, G.; Pennisi, M.; Neri, S.; Malaguarnera, M.; Volti, G.L.; Galvano, F. l-carnitine supplementation improves hematological pattern in patients affected by HCV treated with Peg interferon-α 2b plus ribavirin. World J. Gastroenterol. WJG 2011, 17, 4414–4420. [Google Scholar] [CrossRef] [PubMed]
- Mescka, C.P.; Guerreiro, G.; Hammerschmidt, T.; Faverzani, J.; de Moura Coelho, D.; Mandredini, V.; Wayhs, C.A.Y.; Wajner, M.; Dutra-Filho, C.S.; Vargas, C.R. l-carnitine supplementation decreases DNA damage in treated MSUD patients. Mutat. Res. 2015, 775, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Giammarioli, S.; Boniglia, C.; Carratu, B.; Ciarrocchi, M.; Chiarotti, F.; Mosca, M.; Sanzini, E. Use of food supplements and determinants of usage in a sample Italian adult population. Public Health Nutr. 2013, 16, 1768–1781. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals: Beyond the diet before the drugs. Curr. Bioact. Compounds 2014, 10, 1–12. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. To nutraceuticals and back: Rethinking a concept. Foods 2017, 6, 74. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. A decade of nutraceutical patents: Where are we now in 2018? Expert Opin. Ther. Pat. 2018, 28, 875–882. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370. [Google Scholar] [CrossRef]
- Durazzo, A. Extractable and Non-extractable polyphenols: An overview. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Pérez-Jiménez, J., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 1–37. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M. A current shot and re-thinking of antioxidant research strategy. Braz. J. Anal. Chem. 2018, 5, 9–11. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals-shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert. Rev. Clin. Pharm. 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M. Extractable and non-extractable antioxidants. Molecules 2019, 24, 1933. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Virji, A. Potential benefits of l-carnitine as dietary supplement. Am. Fam. Phys. 2017, 96, 11. [Google Scholar]
- de Andrés, F.; Castañeda, G.; Ríos, Á. Achiral liquid chromatography with circular dichroism detection for the determination of carnitine enantiomers in dietary supplements and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2010, 51, 478–483. [Google Scholar] [CrossRef]
- Sánchez-Hernández, L.; Castro-Puyana, M.; García-Ruiz, C.; Crego, A.L.; Marina, M.L. Determination of L- and D-carnitine in dietary food supplements using capillary electrophoresis–tandem mass spectrometry. Food Chem. 2010, 120, 921–928. [Google Scholar] [CrossRef]
- Isaguirre, A.C.; Acosta, G.; Cerutti, S.; Fernandez, L.P. New flow injection method for quality control of dietary supplements containing l-carnitine using extraction mediated by sodium taurodeoxycholate coacervate coupled to molecular fluorescence. Microchem. J. 2016, 129, 268–273. [Google Scholar] [CrossRef]
- Voitiuk, O.; Yegorova, A.; Scrypynets, Y.V.; Kashutskyy, S.; Kluchnik, O.; Umetskaya, I. HPLC-determination of active components in dietary supplement « l-carnitine smart». Farmatsevtychnyi zhurnal 2019, 86–96. [Google Scholar] [CrossRef]
- Ellingson, D.J.; Shippar, J.J.; Gilmore, J.M. Determination of free and total choline and carnitine in infant formula and adult/pediatric nutritional formula by liquid chromatography/tandem mass spectrometry (LC/MS/MS): Single-laboratory validation, first action 2015.10. J. AOAC Int. 2016, 99, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W. An acid hydrolysis method for quantification of plasma free and total carnitine by flow injection tandem mass spectrometry. Clin. Biochem. 2010, 43, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.T.; Saldanha, L.G.; Bailen, R.A.; Bailey, R.L.; Costello, R.B.; Betz, J.M.; Chang, F.F.; Goshorn, J.; Andrews, K.W.; Pehrsson, P.R. A free new dietary supplement label database for registered dietitian nutritionists. J. Acad. Nutr. Dietet. 2014, 114, 1517. [Google Scholar] [CrossRef] [PubMed]
- DSLD-Dietary Supplement Label Database. Available online: https://dsld.nlm.nih.gov/dsld/ (accessed on 2 April 2020).
- Brown, A.C. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food. Chem. Toxicol. 2017, 107, 449–471. [Google Scholar] [CrossRef]
- Potischman, N.; Salazar, S.; Susser, J.; Saldanha, L.; Dwyer, J.; Kuzak, A.; Betz, J.; Bailen, R. Testing usability of the Dietary Supplement Label Database (DSLD): A resource for consumers, professionals, and researchers. J. Nutr. Edu. Behav. 2017, 49, S99. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Bailen, R.A.; Saldanha, L.G.; Gahche, J.J.; Costello, R.B.; Betz, J.M.; Davis, C.D.; Bailey, R.L.; Potischman, N.; Ershow, A.G. The dietary supplement label database: Recent developments and applications. J. Nutr. 2018, 148, 1428S–1435S. [Google Scholar] [CrossRef]
- Durazzo, A.; Camilli, E.; D’Addezio, L.; Piccinelli, R.; Mantur-Vierendeel, A.; Marletta, L.; Finglas, P.; Turrini, A.; Sette, S. Development of Dietary Supplement Label Database in Italy: Focus of FoodEx2 Coding. Nutrition 2020, 12, 89. [Google Scholar] [CrossRef]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P. From plant compounds to botanicals and back: A current snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef]
- European Food Safety Authority. Classification and description system FoodEx2 (revision 2). EFSA J. 2015, 12, EN-804. [Google Scholar]
- Goin-Kochel, R.P.; Scaglia, F.; Schaaf, C.P.; Berry, L.N.; Dang, D.; Nowel, K.P.; Laakman, A.L.; Dowell, L.R.; Minard, C.G.; Loh, A. Side effects and behavioral outcomes following high-dose carnitine supplementation among young males with autism spectrum disorder: A pilot study. Glob. Pediatr. Health 2019, 6, 2333794X19830696. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Cauli, O. Effects of l-carnitine in Patients with Autism Spectrum Disorders: Review of Clinical Studies. Molecules 2019, 24, 4262. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Rahimlou, M.; Shishehbor, F.; Mansoori, A. The effect of l-carnitine supplementation on lipid profile and glycaemic control in adults with cardiovascular risk factors: A systematic review and meta-analysis of randomized controlled clinical trials. Clin. Nutr. 2020, 39, 110–122. [Google Scholar] [CrossRef]
| Condition | Activity | Effect | References |
|---|---|---|---|
| In vitro | Anticancer effects | Reduced the levels of methylmalonicacidemia and Propionic acidemia in peripheral leukocytes. | [22] |
| In vitro | Antioxidant effects | l-carnitine could elevate in vitro human sperm motility. | [23] |
| In vitro | Antioxidant effects | Inhibited acrylamide-induced genotoxicity in human lymphocytes through the improvement of oxidative stress. | [24] |
| In vitro | Antioxidant effects | Inhibited ROS production and reduced antioxidant activity. | [25] |
| In vitro | Anti-aging effect | Decreased epigenetic modification of hTERT gene promoter and the numbers of senescent cells, and increased activity of telomerase. | [26] |
| In vitro | Hepatoprotective effect | Inhibited the inflammatory mediator iNOS through the suppression of NF-kB activity in IL-1β-stimulated hepatocytes. | [27] |
| In vitro | Anti-angiogenic effect | Suppressed the activation of ICAM-1 and NF-kB and down-regulated the activation of FAK, CXCR4, CXCL12, VEGFR2 and VEGF pathways. | [28] |
| In vitro | Neuroprotective effect | Inhibited methamphetamine-induced activation of MMP-9 | [29] |
| Condition | Activity | Effect | References |
|---|---|---|---|
| In-animal model | Antioxidant effects | Symptom improvement observed by inducing potential function of the CNS and short-term plasticity. | [30] |
| In-animal model | Antioxidant effects | Impeded age-related mitochondrial dysfunction by reducing oxidative stress, age-related alterations of mitochondrial dynamics and biogenesis, and activation of PGC-1α/β coactivators. | [31] |
| In-animal model | Anti-diabetic effects | An improvement of glucose metabolism in mice with insulin resistant | [32] |
| In-animal model | Anti-diabetic effects | Reduction in the serum levels of adiponectin. | [33] |
| In-animal model | Anti-inflammatory and anti-oxidant effects | Managed histological and inflammation damage, apoptosis, mitochondrial dysfunction and arsenic-induced hepatotoxicity. | [34] |
| In-animal model | Antioxidant effect | Upregulation of nrf2 expression and elevation of GSH and TAC levels. | [35] |
| In-animal model | Cardioprotective effect | Controlled the cardiac toxicity induced by 75- and 150-mg/Kg BW aspartme. | [36] |
| In-animal model | Anti-obesity effect | Reduction in elevated plasma lipids in obese Zucker rats. | [37] |
| In-animal model | Immunostimulatory and radioprotective role | Reduced sperm abnormalities, modified severe tubular degeneration and increased serum testosterone levels. | [38] |
| In-animal model | Enhanced exercise endurance | Reduced body fat, increased maximum running time, and elevated mitochondrial biogenesis, oxidative metabolism and fatty acid adsorption. | [39] |
| In-animal model | Cardioprotective effect | Inhibited 6-Gy γ-radiation-induced toxicity. | [40] |
| In-animal model | Antioxidant effect | Prevented NaAsO2-induced oxidative damage in rat. | [41] |
| In-animal model | Treatment of muscle atrophy | Prevented muscle atrophy by inhibiting the ubiquitin proteasome pathway. | [42] |
| In-animal model | Anti-atherosclerosis effect | Prevented the production of trimethylamine N-oxide. | [43] |
| In-animal model | Antioxidant effect | Decreased the oxidative stress at least in the heart of oophorectomized rats. | [44] |
| In-animal model | Antioxidant effect | Decreased acrylamide-toxicity in spleen and thymus tissues in mice. | [45] |
| In-animal model | Antioxidant effect | l-carnitine (200 mg/kg BW) for 11 weeks prevented dimethoate toxicity in rats. | [46] |
| In-animal model | Antioxidant effect | Reduction in PCC (protein oxidation marker), TBARS (lipid peroxidation marker), caspase-3, DNA fragmentation, cyclobutane pyrimidine dimers, 8-oxo-2′-deoxyguanosine (8-oxo-dG) as well as proinflammatory cytokines IL-1β, IL-6, and TNF-α downregulation, upregulation of PCNA (DNA repair proliferating cell nuclear antigen) protein, removed c-Fos and oxidative stress-sensitive signaling protein p38. | [47] |
| Condition | Activity | Administration | Effect | References |
|---|---|---|---|---|
| Clinical trial | Cardioprotective effect | Daily oral l-carnitine (50 mg/kg) in patients with ischemic heart failure for 10 days | Enhancement of cardiac efficiency, restoration of cardiac energy metabolism, and elimination of toxic mitochondrial products. | [48] |
| Clinical trial | Cardioprotective effect | l-carnitine supplementation at the concentration of 2 g/day for 8 weeks in patients with Pemphigus vulgaris | Reduced serum levels of cystatin C, BMP4 and OPN as well as increased serum levels of carnitine. | [49] |
| Clinical trial | Anti-inflammatory effects | Administration of carnitine (250 mg/day) in females with polycystic ovary syndrome for 12 weeks | Decreased carotid intima-media thickness (CIMT) and plasma nitric oxide. | [50] |
| Clinical trial | Antioxidant effect | l-carnitine supplementation at the concentrations of 10 mM and 30 mM for 55 days | Elevated sulfhydryls and ascorbic acid uptake, preserved glutathione level, enhanced sulfhydryls and ascorbic acid levels, preserved lipid peroxidation, haemolysis and haemoglobin, and modulated antioxidants. | [51] |
| Clinical trial | Antioxidant effects | Administration of l-carnitine (100 mg/kg day) in patients with glutaric acidemia type I for 2 month | Prevented oxidative damage and increased the removal of toxic metabolites in patients with type I glutaric aciduria. | [53] |
| Clinical trial | Embryonic development effect | Administration of l-carnitine (1000 mg/day) for 82 days | An improvement of oocyte developmental competence in patients with in-vitro fertilization-embryo transfer. | [55] |
| Clinical trial | Anti-anemia effect | The administration of l-carnitine (20 mg/kg/day) for three months in dialysis children | A restoration and normalized circulation of plasma free carnitine (FC) levels | [56] |
| Clinical trial | Anti-autism effect | Administration of l-carnitine (50 mg/kg/day bodyweight) for three months | An improvement of autism symptoms based on autism treatment evaluation checklist (ATEC) scores, modified clinical global impression (CGI), and childhood autism rating scale (CARS) | [57] |
| Clinical trial | Anti-autism effect | Administration of l-carnitine (100 mg/kg/day body weight) in children | An enhancement of total and free carnitine levels, a reduction of autism severity and an improvement of autistic behavior | [58] |
| Clinical trial | Anti-autism effect | Administration of l-carnitine (200 mg/kg/day) in male subjects aged 5 years for 4.5 months | A gradual improvement of autism symptoms | [59] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durazzo, A.; Lucarini, M.; Nazhand, A.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. The Nutraceutical Value of Carnitine and Its Use in Dietary Supplements. Molecules 2020, 25, 2127. https://doi.org/10.3390/molecules25092127
Durazzo A, Lucarini M, Nazhand A, Souto SB, Silva AM, Severino P, Souto EB, Santini A. The Nutraceutical Value of Carnitine and Its Use in Dietary Supplements. Molecules. 2020; 25(9):2127. https://doi.org/10.3390/molecules25092127
Chicago/Turabian StyleDurazzo, Alessandra, Massimo Lucarini, Amirhossein Nazhand, Selma B. Souto, Amélia M. Silva, Patrícia Severino, Eliana B. Souto, and Antonello Santini. 2020. "The Nutraceutical Value of Carnitine and Its Use in Dietary Supplements" Molecules 25, no. 9: 2127. https://doi.org/10.3390/molecules25092127
APA StyleDurazzo, A., Lucarini, M., Nazhand, A., Souto, S. B., Silva, A. M., Severino, P., Souto, E. B., & Santini, A. (2020). The Nutraceutical Value of Carnitine and Its Use in Dietary Supplements. Molecules, 25(9), 2127. https://doi.org/10.3390/molecules25092127











