A Guide to Targeting the Endocannabinoid System in Drug Design
Abstract
:1. Introduction
2. Endocannabinoid System
3. Molecular Mechanisms of the Main Proteins of ECS—Implications for Drug Design
4. Nervous System
4.1. Pain
4.2. Seizures
4.3. Anxiety
4.4. Depression
4.5. Addictions
4.6. Cognitive Functions
5. Neurodegeneration
6. Inflammatory and Autoimmune Diseases
7. Metabolic Diseases
7.1. Obesity
7.2. Diabetes
7.3. Hepatic Diseases
8. Cardiovascular Diseases
8.1. Hypertension
8.2. Atherosclerosis
8.3. Myocardial Dysfunctions
9. Cancer
10. Other
10.1. Respiratory Disorders
10.2. Gastroenterology
10.3. Osteology
10.4. Reproductive System
10.5. Dermatology
10.6. Genetic Disorders
11. Approved Drugs and Clinical Trials
12. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| 5-HT | 5-hydroxytryptamine (serotonin) |
| 5-HT1A | serotonin receptor 1A |
| 5-HT2B | serotonin receptor 2B |
| 5-HT3 | serotonin receptor 3 |
| 9-THC | 9-tetrahydrocannabinol |
| AA | arachidonic acid |
| ABHD6 | / hydrolase domain 6 |
| ABHD12 | / hydrolase domain 12 |
| AC | adenylyl cyclase |
| ACE | angiotensin-converting enzyme |
| AD | Alzheimer disease |
| AEA | N-arachidonoylethanolamine (anandamide) |
| AIDS | acquired immunodeficiency syndrome |
| ALS | amyotrophic lateral sclerosis |
| AM404 | N-arachidonoylaminophenol |
| AMP | adenosine monophosphate |
| AMPA | -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPK | AMP-activated protein kinase |
| AMT | anandamide membrane transporter |
| BLA | basolateral complex of amygdala |
| BP | blood pressure |
| cAMP | cyclic adenosine monophosphate |
| CB | cannabinoid |
| CB1 | cannabinoid receptor type 1 |
| CB2 | cannabinoid receptor type 2 |
| CBD | cannabidiol |
| CBDV | cannabidivarin |
| CBR | cannabinoid receptor |
| CIPN | chemotherapy-induced peripheral neuropathy |
| CNS | central nervous system |
| COX | cyclooxygenase |
| COX-2 | cyclooxygenase 2 |
| COX-3 | cyclooxygenase 3 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DA | dopamine |
| DAG | diacylglycerol |
| DAGL | diacylglycerol lipase |
| DN | diabetic neuropathy |
| DPP4 | dipeptidyl peptidase 4 |
| eCB | endocannabinoid |
| ECS | endocannabinoid system |
| ER | endoplasmic reticulum |
| ET | endotoxin tolerance |
| FAAH | fatty acid amide hydrolase |
| FABP | fatty-acid-binding protein |
| FCD | focal cortical dysplasia |
| FDA | Food and Drug Administration |
| GABA | -aminobutyric acid |
| GDP | guanosine diphosphate |
| GI | gastrointestinal |
| GIT | gastrointestinal tract |
| GLUT-2 | glucose transporter 2 |
| GPCR | G protein-coupled receptor |
| GPR18 | G protein-coupled receptor 18 |
| GPR55 | G protein-coupled receptor 55 |
| GPR119 | G protein-coupled receptor 119 |
| GTP | guanosine triphosphate |
| HAND | human immunodeficiency virus associated neurocognitive disorder |
| HER2 | human epidermal growth factor receptor 2 |
| HIV-1 | human immunodeficiency virus 1 |
| HPA | hypothalamic-pituitary-adrenal axis |
| HPO | hypothalamic-pituitary-ovarian axis |
| HSP70 | 70 kilodalton heat shock protein |
| IBD | inflammatory bowel disease |
| IL-1 | interleukin 1 |
| IL-6 | interleukin 6 |
| IL-18 | interleukin 18 |
| IMMA | indomethacin morpholinamide |
| iNOS | inducible nitric oxide synthase |
| IPAH | idiopathic pulmonary arterial hypertension |
| LHb | lateral habenula |
| LPI | lysophosphatidylinositol |
| LPS | lipopolysaccharide (endotoxin) |
| MAGL | monoacylglycerol lipase |
| MAPK | mitogen activated protein kinase |
| MCP-1 | monocyte chemoattractant protein 1 |
| MI | myocardial infraction |
| MOA | mechanism of action |
| mTORC1 | mammalian target of rapamycin complex 1 |
| NAAA | N-acylethanolamine acid amidase |
| NAFLD | nonalcoholic fatty liver disease |
| NAM | negative allosteric modulator |
| NAPE | N-acylphosphatidylethanolamine |
| NAPE-PLD | N-acylphosphatidylethanolamine-hydrolyzing phospholipase D |
| NASH | nonalcoholic steatohepatitis |
| NF-B | nuclear factor-B |
| NMDA | N-methyl-D-aspartate |
| NOS | nitric oxide synthase |
| NSAID | nonsteroidal anti-inflammatory drug |
| NSC | neural stem cell |
| OEA | oleoylethanolamine |
| OXPKOS | oxidative phosphorylation |
| PAM | positive allosteric modulator |
| PEA | palmitoylethanolamide |
| PG | prostaglandin |
| PGE2 | prostaglandin E2 |
| PPAR | peroxisome proliferator-activated receptor |
| PPAR | peroxisome proliferator-activated receptor |
| PPAR | peroxisome proliferator-activated receptor |
| PRCB | peripherally restricted cannabinoid |
| PTSD | post-traumatic stress disorder |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SCA | spinocerebellar ataxia |
| SSc | systemic sclerosis |
| T2DM | type 2 diabetes mellitus |
| TBI | traumatic brain injury |
| THC | tetrahydrocannabinol |
| TIMP-1 | tissue inhibitor of matrix metalloproteinases-1 |
| TLR3 | toll-like receptor 3 |
| TLR4 | toll-like receptor 4 |
| TNF | tumor necrosis factor |
| TNF- | tumor necrosis factor |
| TRPV1 | transient receptor potential vanilloid type 1 channel |
| WAT | white adipose tissue |
References
- Wu, J. Cannabis, cannabinoid receptors, and endocannabinoid system: Yesterday, today, and tomorrow. Acta Pharmacol. Sin. 2019, 40, 297–299. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz, F.R.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Compton, D.R.; Pertwee, R.G.; Griffin, G.; Bayewitch, M.; Barg, J.; Vogel, Z. Identification of an endogenous 2-monoglyceride, present in canine gut that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB 1 receptor signaling in the brain: Extracting specificity from ubiquity. Neuropsychopharmacology 2018, 43, 4–20. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Sam, A.H.; Salem, V.; Ghatei, M.A. Rimonabant: From RIO to ban. J. Obes. 2011, 2011, 432607. [Google Scholar] [CrossRef]
- Navarro, M.; Hernández, E.; Muñoz, R.M.; del Arco, I.; Villanúa, M.A.; Carrera, M.R.A.; de Fonseca, F.R. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport 1997, 8, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.B.; Abazia, D.T. Medicinal cannabis: History, pharmacology, and implications for the acute care setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician 2017, 20, E755–E796. [Google Scholar] [PubMed]
- Stockings, E.; Campbell, G.; Hall, W.D.; Nielsen, S.; Zagic, D.; Rahman, R.; Murnion, B.; Farrell, M.; Weier, M.; Degenhardt, L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain 2018, 159, 1932–1954. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Beyond cannabis: Plants and the endocannabinoid system. Trends Pharmacol. Sci. 2016, 37, 594–605. [Google Scholar] [CrossRef]
- Green, K.; Drvota, V.; Vesterqvist, O. Pronounced reduction of in vivo prostacyclin synthesis in humans by acetaminophen (paracetamol). Prostaglandins 1989, 37, 311–315. [Google Scholar] [CrossRef]
- Botting, R.M. Mechanism of action of acetaminophen: Is there a cyclooxygenase 3? Clin. Infect. Dis. 2000, 31, S202–S210. [Google Scholar] [CrossRef] [Green Version]
- Ouellet, M.; Percival, M.D. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch. Biochem. Biophys. 2001, 387, 273–280. [Google Scholar] [CrossRef]
- Botting, R.; Ayoub, S.S. COX-3 and the mechanism of action of paracetamol/acetaminophen. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 85–87. [Google Scholar] [CrossRef]
- Alloui, A.; Chassaing, C.; Schmidt, J.; Ardid, D.; Dubray, C.; Cloarec, A.; Eschalier, A. Paracetamol exerts a spinal, tropisetron-reversible, antinociceptive effect in an inflammatory pain model in rats. Eur. J. Pharmacol. 2002, 443, 71–77. [Google Scholar] [CrossRef]
- Pickering, G.; Loriot, M.A.; Libert, F.; Eschalier, A.; Beaune, P.; Dubray, C. Analgesic effect of acetaminophen in humans: First, evidence of a central serotonergic mechanism. Clin. Pharmacol. Ther. 2006, 79, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.; Bailey, I.; Toms, N.J.; Clarke, G.D.; Kitchen, I.; Hourani, S.M. Paracetamol inhibits nitric oxide synthesis in murine spinal cord slices. Eur. J. Pharmacol. 2007, 562, 68–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottani, A.; Leone, S.; Sandrini, M.; Ferrari, A.; Bertolini, A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur. J. Pharmacol. 2006, 531, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.V.; Long, J.H.; Shah, S.; Rahman, J.; Perrett, D.; Ayoub, S.S.; Mehta, V. First, evidence of the conversion of paracetamol to AM404 in human cerebrospinal fluid. J. Pain Res. 2017, 10, 2703–2709. [Google Scholar] [CrossRef] [Green Version]
- Beltramo, M.; Stella, N.; Calignano, A.; Lin, S.; Makriyannis, A.; Piomelli, D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 1997, 277, 1094–1097. [Google Scholar] [CrossRef] [Green Version]
- Sinning, C.; Watzer, B.; Coste, O.; Nusing, R.M.; Ott, I.; Ligresti, A.; Marzo, V.D.; Imming, P. New analgesics synthetically derived from the paracetamol metabolite N-(4-hydroxyphenyl)-(5 Z, 8 Z, 11 Z, 14 Z)-icosatetra-5, 8, 11, 14-enamide. J. Med. Chem. 2008, 51, 7800–7805. [Google Scholar] [CrossRef]
- Stueber, T.; Meyer, S.; Jangra, A.; Hage, A.; Eberhardt, M.; Leffler, A. Activation of the capsaicin-receptor TRPV1 by the acetaminophen metabolite N-arachidonoylaminophenol results in cytotoxicity. Life Sci. 2018, 194, 67–74. [Google Scholar] [CrossRef]
- Rogosch, T.; Sinning, C.; Podlewski, A.; Watzer, B.; Schlosburg, J.; Lichtman, A.H.; Cascio, M.G.; Bisogno, T.; Di Marzo, V.; Nüsing, R.; et al. Novel bioactive metabolites of dipyrone (metamizol). Bioorganic Med. Chem. 2012, 20, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Crunfli, F.; Vilela, F.C.; Giusti-Paiva, A. Cannabinoid CB1 receptors mediate the effects of dipyrone. Clin. Exp. Pharmacol. Physiol. 2015, 42, 246–255. [Google Scholar] [CrossRef]
- Priestley, R.S.; Nickolls, S.A.; Alexander, S.P.; Kendall, D.A. A potential role for cannabinoid receptors in the therapeutic action of fenofibrate. FASEB J. 2015, 29, 1446–1455. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal structure of the human cannabinoid receptor CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Qu, L.; et al. Crystal structure of the human cannabinoid receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2017, 22, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, related compounds and their metabolic routes. Molecules 2014, 19, 17078–17106. [Google Scholar] [CrossRef]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Maccarrone, M. Metabolism of the endocannabinoid anandamide: Open questions after 25 years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Δ9-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424. [Google Scholar] [CrossRef] [Green Version]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Godlewski, G.; Offertáler, L.; Wagner, J.A.; Kunos, G. Receptors for acylethanolamides—GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009, 89, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Tortora, C.; Punzo, F.; Bellini, G.; Argenziano, M.; Di Paola, A.; Torella, M.; Perrotta, S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 1919. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar]
- Kind, L.; Kursula, P. Structural properties and role of the endocannabinoid lipases ABHD6 and ABHD12 in lipid signaling and disease. Amino Acids 2019, 51, 151–174. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Al-Zoubi, R.; Morales, P.; Reggio, P.H. Structural insights into CB1 receptor biased signaling. Int. J. Mol. Sci. 2019, 20, 1837. [Google Scholar] [CrossRef] [Green Version]
- Bouaboula, M.; Perrachon, S.; Milligan, L.; Canat, X.; Rinaldi-Carmona, M.; Portier, M.; Barth, F.; Calandra, B.; Pecceu, F.; Lupker, J.; et al. A Selective Inverse Agonist for Central Cannabinoid Receptor Inhibits Mitogen-activated Protein Kinase Activation Stimulated by Insulin or Insulin-like Growth Factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997, 272, 22330–22339. [Google Scholar] [CrossRef] [Green Version]
- Console-Bram, L.; Marcu, J.; Abood, M.E. Cannabinoid receptors: Nomenclature and pharmacological principles. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Meye, F.; Trezza, V.; Vanderschuren, L.J.; Ramakers, G.; Adan, R. Neutral antagonism at the cannabinoid 1 receptor: A safer treatment for obesity. Mol. Psychiatry 2013, 18, 1294–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosier, B.; Muccioli, G.G.; Hermans, E.; Lambert, D.M. Functionally selective cannabinoid receptor signaling: Therapeutic implications and opportunities. Biochem. Pharmacol. 2010, 80, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB1 and CB2 receptor signaling and bias. Cannabis Cannabinoid Res. 2017, 2, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, signaling, and physiological functions of G-proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [Green Version]
- Ford, B.M.; Franks, L.N.; Tai, S.; Fantegrossi, W.E.; Stahl, E.L.; Berquist, M.D.; Cabanlong, C.V.; Wilson, C.D.; Penthala, N.R.; Crooks, P.A.; et al. Characterization of structurally novel G protein biased CB1 agonists: Implications for drug development. Pharmacol. Res. 2017, 125, 161–177. [Google Scholar] [CrossRef]
- Shore, D.M.; Baillie, G.L.; Hurst, D.H.; Navas, F.; Seltzman, H.H.; Marcu, J.P.; Abood, M.E.; Ross, R.A.; Reggio, P.H. Allosteric modulation of a cannabinoid G protein-coupled receptor binding site elucidation and relationship to G protein signaling. J. Biol. Chem. 2014, 289, 5828–5845. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Yan, W.; Chapman, K.; Ramesh, K.; Ferrell, A.J.; Yin, J.; Wang, X.; Xu, Q.; Rosenbaum, D.M. Structure of an allosteric modulator bound to the CB1 cannabinoid receptor. Nat. Chem. Biol. 2019, 15, 1199–1205. [Google Scholar] [CrossRef]
- Pandey, P.; Roy, K.K.; Doerksen, R.J. Negative allosteric modulators of cannabinoid receptor 2: Protein modeling, binding site identification and molecular dynamics simulations in the presence of an orthosteric agonist. J. Biomol. Struct. Dyn. 2020, 38, 32–47. [Google Scholar] [CrossRef]
- Kenakin, T.; Miller, L.J. Seven transmembrane receptors as shapeshifting proteins: The impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010, 62, 265–304. [Google Scholar] [CrossRef] [Green Version]
- May, L.T.; Leach, K.; Sexton, P.M.; Christopoulos, A. Allosteric modulation of G protein–coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 1–51. [Google Scholar] [CrossRef]
- Conn, P.J.; Christopoulos, A.; Lindsley, C.W. Allosteric modulators of GPCRs: A novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009, 8, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Li, J.X.; Thomas, B.F.; Wiley, J.L.; Kenakin, T.P.; Zhang, Y. Allosteric modulation: An alternate approach targeting the cannabinoid CB1 receptor. Med. Res. Rev. 2017, 37, 441–474. [Google Scholar] [CrossRef] [Green Version]
- Gado, F.; Meini, S.; Bertini, S.; Digiacomo, M.; Macchia, M.; Manera, C. Allosteric modulators targeting cannabinoid cb1 and cb2 receptors: Implications for drug discovery. Future Med. Chem. 2019, 11, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Mahmoud, M.M.; Shim, J.Y.; Kendall, D.A. Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J. Biol. Chem. 2013, 288, 9790–9800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurana, L.; Mackie, K.; Piomelli, D.; Kendall, D.A. Modulation of CB1 cannabinoid receptor by allosteric ligands: Pharmacology and therapeutic opportunities. Neuropharmacology 2017, 124, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Ordóñez, A.; Martín-Fontecha, M.; Ortega-Gutiérrez, S.; López-Rodríguez, M.L. Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem. Pharmacol. 2018, 157, 18–32. [Google Scholar] [CrossRef]
- Tripathi, R.K.P. A perspective review on fatty acid amide hydrolase (FAAH) inhibitors as potential therapeutic agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Blankman, J.L.; Long, J.Z.; Nomura, D.K.; Pan, B.; Kinsey, S.G.; Nguyen, P.T.; Ramesh, D.; Booker, L.; Burston, J.J.; et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010, 13, 1113–1119. [Google Scholar] [CrossRef] [Green Version]
- Dainese, E.; Oddi, S.; Simonetti, M.; Sabatucci, A.; Angelucci, C.B.; Ballone, A.; Dufrusine, B.; Fezza, F.; De Fabritiis, G.; Maccarrone, M. The endocannabinoid hydrolase FAAH is an allosteric enzyme. Sci. Rep. 2020, 10, 2292. [Google Scholar] [CrossRef] [Green Version]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Diaz-Franulic, I.; Poblete, H.; Miño-Galaz, G.; González, C.; Latorre, R. Allosterism and structure in thermally activated transient receptor potential channels. Annu. Rev. Biophys. 2016, 45, 371–398. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Julian, M.; Carrascosa, A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Donvito, G.; Nass, S.R.; Wilkerson, J.L.; Curry, Z.A.; Schurman, L.D.; Kinsey, S.G.; Lichtman, A.H. The endogenous cannabinoid system: A budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology 2018, 43, 52–79. [Google Scholar] [CrossRef] [Green Version]
- Puighermanal, E.; Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos. Trans. R. Soc. B: Biol. Sci. 2012, 367, 3254–3263. [Google Scholar] [CrossRef] [Green Version]
- Wiskerke, J.; Pattij, T.; Schoffelmeer, A.N.; De Vries, T.J. The role of CB1 receptors in psychostimulant addiction. Addict. Biol. 2008, 13, 225–238. [Google Scholar] [CrossRef]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signaling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef] [Green Version]
- Mulpuri, Y.; Marty, V.N.; Munier, J.J.; Mackie, K.; Schmidt, B.L.; Seltzman, H.H.; Spigelman, I. Synthetic peripherally-restricted cannabinoid suppresses chemotherapy-induced peripheral neuropathy pain symptoms by CB1 receptor activation. Neuropharmacology 2018, 139, 85–97. [Google Scholar] [CrossRef]
- Slivicki, R.A.; Xu, Z.; Kulkarni, P.M.; Pertwee, R.G.; Mackie, K.; Thakur, G.A.; Hohmann, A.G. Positive allosteric modulation of cannabinoid receptor type 1 suppresses pathological pain without producing tolerance or dependence. Biol. Psychiatry 2018, 84, 722–733. [Google Scholar] [CrossRef] [Green Version]
- Nent, E.; Nozaki, C.; Schmöle, A.C.; Otte, D.; Zimmer, A. CB2 receptor deletion on myeloid cells enhanced mechanical allodynia in a mouse model of neuropathic pain. Sci. Rep. 2019, 9, 7468. [Google Scholar] [CrossRef]
- Gado, F.; Di Cesare Mannelli, L.; Lucarini, E.; Bertini, S.; Cappelli, E.; Digiacomo, M.; Stevenson, L.A.; Macchia, M.; Tuccinardi, T.; Ghelardini, C.; et al. Identification of the first synthetic allosteric modulator of the CB2 receptors and evidence of its efficacy for neuropathic pain relief. J. Med. Chem. 2019, 62, 276–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapper, J.R.; Henry, C.L.; Niphakis, M.J.; Knize, A.M.; Coppola, A.R.; Simon, G.M.; Ngo, N.; Herbst, R.A.; Herbst, D.M.; Reed, A.W.; et al. Monoacylglycerol lipase inhibition in human and rodent systems supports clinical evaluation of endocannabinoid modulators. J. Pharmacol. Exp. Ther. 2018, 367, 494–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brindisi, M.; Borrelli, G.; Brogi, S.; Grillo, A.; Maramai, S.; Paolino, M.; Benedusi, M.; Pecorelli, A.; Valacchi, G.; Di Cesare Mannelli, L.; et al. Development of Potent Inhibitors of Fatty Acid Amide Hydrolase Useful for the Treatment of Neuropathic Pain. ChemMedChem 2018, 13, 2090–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, N.; Starowicz, K. Dual-acting compounds targeting endocannabinoid and endovanilloid systems—A novel treatment option for chronic pain management. Front. Pharmacol. 2016, 7, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Fonseca Pacheco, D.; Romero, T.R.L.; Duarte, I.D.G. Ketamine induces central antinociception mediated by endogenous cannabinoids and activation of CB1 receptors. Neurosci. Lett. 2019, 699, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Wen, J.; Selvaraj, P.; Tanaka, M.; Moran, S.; Zhang, Y. Therapeutic effect of the substrate-selective COX-2 inhibitor IMMA in the animal model of chronic constriction injury. Front. Pharmacol. 2018, 9, 1481. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Santos, A.F.; Ferreira, R.C.; Duarte, I.D.; Aguiar, D.C.; Romero, T.R.; Moreira, F.A. The antipsychotic aripiprazole induces antinociceptive effects: Possible role of peripheral dopamine D2 and serotonin 5-HT1A receptors. Eur. J. Pharmacol. 2015, 765, 300–306. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Almeida-Santos, A.F.; Duarte, I.D.; Aguiar, D.C.; Moreira, F.A.; Romero, T.R. Role of Endocannabinoid System in the Peripheral Antinociceptive Action of Aripiprazole. Anesth. Analg. 2019, 129, 263–268. [Google Scholar] [CrossRef]
- Fei, L.; Abrardi, L.; Mediati, R.D. Unexpected effect of aripiprazole on nociceptive pain. Ther. Adv. Psychopharmacol. 2012, 2, 211–212. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B. Cannabis and epilepsy: An ancient treatment returns to the fore. Epilepsy Behav. 2017, 70, 292–297. [Google Scholar] [CrossRef]
- Colangeli, R.; Di Maio, R.; Pierucci, M.; Deidda, G.; Casarrubea, M.; Di Giovanni, G. Synergistic action of CB1 and 5-HT2B receptors in preventing pilocarpine-induced status epilepticus in rats. Neurobiol. Dis. 2019, 125, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zareie, P.; Sadegh, M.; Palizvan, M.R.; Moradi-Chameh, H. Anticonvulsive effects of endocannabinoids; an investigation to determine the role of regulatory components of endocannabinoid metabolism in the Pentylenetetrazol induced tonic-clonic seizures. Metab. Brain Dis. 2018, 33, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.S.; Umathe, S.N. Involvement of transient receptor potential vanilloid type 1 channels in the pro-convulsant effect of anandamide in pentylenetetrazole-induced seizures. Epilepsy Res. 2012, 100, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Makuch, W.; Korostynski, M.; Malek, N.; Slezak, M.; Zychowska, M.; Petrosino, S.; De Petrocellis, L.; Cristino, L.; Przewlocka, B.; et al. Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS ONE 2013, 8, e60040. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhang, J.; Jia, M.; Yang, C.; Pan, Y.; Li, S.; Luo, Y.; Zheng, J.; Ji, J.; Chen, J.; et al. α/β-Hydrolase domain-containing 6 (ABHD6) negatively regulates the surface delivery and synaptic function of AMPA receptors. Proc. Natl. Acad. Sci. USA 2016, 113, E2695–E2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, L.S.; DeLorenzo, R.J. Acetaminophen inhibits status epilepticus in cultured hippocampal neurons. Neuroreport 2011, 22, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Suemaru, K.; Yoshikawa, M.; Tanaka, A.; Araki, H.; Aso, H.; Watanabe, M. Anticonvulsant effects of acetaminophen in mice: Comparison with the effects of nonsteroidal anti-inflammatory drugs. Epilepsy Res. 2018, 140, 22–28. [Google Scholar] [CrossRef]
- Suemaru, K.; Yoshikawa, M.; Aso, H.; Watanabe, M. TRPV1 mediates the anticonvulsant effects of acetaminophen in mice. Epilepsy Res. 2018, 145, 153–159. [Google Scholar] [CrossRef]
- Whalley, B.J.; Lin, H.; Bell, L.; Hill, T.; Patel, A.; Gray, R.A.; Roberts, C.E.; Devinsky, O.; Bazelot, M.; Williams, C.M.; et al. Species-specific susceptibility to cannabis-induced convulsions. Br. J. Pharmacol. 2019, 176, 1506–1523. [Google Scholar] [CrossRef] [Green Version]
- Greenwich Biosciences. EPIDIOLEX (cannabidiol) [drug label]. Revised June 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf (accessed on 18 February 2020).
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.; Bagher, A.; Kelly, M.; Denovan-Wright, E. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bih, C.I.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef]
- Hill, M.N.; Gorzalka, B.B. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol. Disord.-Drug Targets 2009, 8, 451–458. [Google Scholar] [CrossRef]
- Jacob, W.; Yassouridis, A.; Marsicano, G.; Monory, K.; Lutz, B.; Wotjak, C. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: Role of glutamatergic transmission. Genes Brain Behav. 2009, 8, 685–698. [Google Scholar] [CrossRef]
- Moreira, F.A.; Grieb, M.; Lutz, B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: Focus on anxiety and depression. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 133–144. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and negative effects of cannabis and cannabinoids on health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147. [Google Scholar] [CrossRef]
- Viana, T.G.; Bastos, J.R.; Costa, R.B.; Hott, S.C.; Mansur, F.S.; Coimbra, C.C.; Resstel, L.B.; Aguiar, D.C.; Moreira, F.A. Hypothalamic endocannabinoid signaling modulates aversive responses related to panic attacks. Neuropharmacology 2019, 148, 284–290. [Google Scholar] [CrossRef]
- Zimmermann, T.; Bartsch, J.C.; Beer, A.; Lomazzo, E.; Guggenhuber, S.; Lange, M.D.; Bindila, L.; Pape, H.C.; Lutz, B. Impaired anandamide/palmitoylethanolamide signaling in hippocampal glutamatergic neurons alters synaptic plasticity, learning, and emotional responses. Neuropsychopharmacology 2019, 44, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.N.; Hohman, Z.P.; Campbell, C.M.; King, K.S.; Tucker, C.A. FAAH genotype, CRFR1 genotype, and cortisol interact to predict anxiety in an aging, rural Hispanic population: A Project FRONTIER study. Neurobiol. Stress 2019, 10, 100154. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, A.; Dörfel, D.; Diers, K.; Witt, S.H.; Strobel, A.; Brocke, B. Impact of FAAH genetic variation on fronto-amygdala function during emotional processing. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Danandeh, A.; Vozella, V.; Lim, J.; Oveisi, F.; Ramirez, G.L.; Mears, D.; Wynn, G.; Piomelli, D. Effects of fatty acid amide hydrolase inhibitor URB597 in a rat model of trauma-induced long-term anxiety. Psychopharmacology 2018, 235, 3211–3221. [Google Scholar] [CrossRef]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Morena, M.; Haataja, R.; Hammar, V.; Augier, G.; Hill, M.N.; Heilig, M. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase (FAAH): A randomized, controlled experimental medicine trial. Biol. Psychiatry 2019, 87, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Morena, M.; Aukema, R.J.; Leitl, K.D.; Rashid, A.J.; Vecchiarelli, H.A.; Josselyn, S.A.; Hill, M.N. Upregulation of Anandamide Hydrolysis in the Basolateral Complex of Amygdala Reduces Fear Memory Expression and Indices of Stress and Anxiety. J. Neurosci. 2019, 39, 1275–1292. [Google Scholar] [CrossRef]
- Surkin, P.N.; Gallino, S.L.; Luce, V.; Correa, F.; Fernandez-Solari, J.; De Laurentiis, A. Pharmacological augmentation of endocannabinoid signaling reduces the neuroendocrine response to stress. Psychoneuroendocrinology 2018, 87, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Berger, A.L.; Henricks, A.M.; Lugo, J.M.; Wright, H.R.; Warrick, C.R.; Sticht, M.A.; Morena, M.; Bonilla, I.; Laredo, S.A.; Craft, R.M.; et al. The lateral habenula directs coping styles under conditions of stress via recruitment of the endocannabinoid system. Biol. Psychiatry 2018, 84, 611–623. [Google Scholar] [CrossRef]
- Poleszak, E.; Wośko, S.; Sławińska, K.; Szopa, A.; Wróbel, A.; Serefko, A. Cannabinoids in depressive disorders. Life Sci. 2018, 213, 18–24. [Google Scholar] [CrossRef]
- Burstein, O.; Shoshan, N.; Doron, R.; Akirav, I. Cannabinoids prevent depressive-like symptoms and alterations in BDNF expression in a rat model of PTSD. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 129–139. [Google Scholar] [CrossRef]
- Liu, Q.R.; Canseco-Alba, A.; Zhang, H.Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Escosteguy-Neto, J.C.; Ishiguro, H.; et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 2017, 7, 17410. [Google Scholar] [CrossRef] [PubMed]
- Khakpai, F.; Ebrahimi-Ghiri, M.; Alijanpour, S.; Zarrindast, M.R. Ketamine-induced antidepressant like effects in mice: A possible involvement of cannabinoid system. Biomed. Pharmacother. 2019, 112, 108717. [Google Scholar] [CrossRef] [PubMed]
- Shearman, L.; Rosko, K.; Fleischer, R.; Wang, J.; Xu, S.; Tong, X.; Rocha, B.A. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav. Pharmacol. 2003, 14, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.E.; Dwyer, J.M.; Piesla, M.J.; Platt, B.J.; Shen, R.; Rahman, Z.; Chan, K.; Manners, M.T.; Samad, T.A.; Kennedy, J.D.; et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol. Dis. 2010, 39, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X. FAAH inhibition produces antidepressant-like efforts of mice to acute stress via synaptic long-term depression. Behav. Brain Res. 2017, 324, 138–145. [Google Scholar] [CrossRef]
- Panlilio, L.V.; Goldberg, S.R.; Justinova, Z. Cannabinoid abuse and addiction: Clinical and preclinical findings. Clin. Pharmacol. Ther. 2015, 97, 616–627. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, R.; Berrendero, F.; Ozaita, A.; Robledo, P. Neurochemical basis of cannabis addiction. Neuroscience 2011, 181, 1–17. [Google Scholar] [CrossRef]
- He, X.h.; Jordan, C.J.; Vemuri, K.; Bi, G.h.; Zhan, J.; Gardner, E.L.; Makriyannis, A.; Wang, Y.l.; Xi, Z.X. Cannabinoid CB 1 receptor neutral antagonist AM4113 inhibits heroin self-administration without depressive side effects in rats. Acta Pharmacol. Sin. 2019, 40, 365–373. [Google Scholar] [CrossRef]
- Balla, A.; Dong, B.; Shilpa, B.M.; Vemuri, K.; Makriyannis, A.; Pandey, S.C.; Sershen, H.; Suckow, R.F.; Vinod, K.Y. Cannabinoid-1 receptor neutral antagonist reduces binge-like alcohol consumption and alcohol-induced accumbal dopaminergic signaling. Neuropharmacology 2018, 131, 200–208. [Google Scholar] [CrossRef]
- Jing, L.; Qiu, Y.; Zhang, Y.; Li, J.X. Effects of the cannabinoid CB1 receptor allosteric modulator ORG 27569 on reinstatement of cocaine-and methamphetamine-seeking behavior in rats. Drug Alcohol Depend. 2014, 143, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Gamage, T.F.; Ignatowska-Jankowska, B.M.; Wiley, J.L.; Abdelrahman, M.; Trembleau, L.; Greig, I.R.; Thakur, G.A.; Tichkule, R.; Poklis, J.; Ross, R.A.; et al. In-Vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav. Pharmacol. 2014, 25, 182–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godlewski, G.; Cinar, R.; Coffey, N.J.; Liu, J.; Jourdan, T.; Mukhopadhyay, B.; Chedester, L.; Liu, Z.; Osei-Hyiaman, D.; Iyer, M.R.; et al. Targeting Peripheral CB1 Receptors Reduces Ethanol Intake via a Gut-Brain Axis. Cell Metab. 2019, 29, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.V.; Chaves, G.A.; Marino, B.L.; Sousa, K.P.; Souza, L.R.; Brito, M.F.; Teixeira, H.R.; da Silva, C.H.; Santos, C.B.; Hage-Melim, L.I. Cannabinoid type 1 receptor (CB1) ligands with therapeutic potential for withdrawal syndrome in chemical dependents of Cannabis sativa. ChemMedChem 2017, 12, 1408–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trexler, K.; Eckard, M.; Kinsey, S. CB1 positive allosteric modulation attenuates Δ9-THC withdrawal and NSAID-induced gastric inflammation. Pharmacol. Biochem. Behav. 2019, 177, 27–33. [Google Scholar] [CrossRef]
- Centanni, S.W.; Morris, B.D.; Luchsinger, J.R.; Bedse, G.; Fetterly, T.L.; Patel, S.; Winder, D.G. Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology 2019, 44, 526–537. [Google Scholar] [CrossRef] [Green Version]
- Raichlen, D.A.; Foster, A.D.; Gerdeman, G.L.; Seillier, A.; Giuffrida, A. Wired to run: Exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J. Exp. Biol. 2012, 215, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Stone, N.L.; Millar, S.A.; Herrod, P.J.; Barrett, D.A.; Ortori, C.A.; Mellon, V.A.; O’Sullivan, S.E. An analysis of endocannabinoid concentrations and mood following singing and exercise in healthy volunteers. Front. Behav. Neurosci. 2018, 12, 269. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; GONG, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Gao, M.; Liu, Q.R.; Bi, G.H.; Li, X.; Yang, H.J.; Gardner, E.L.; Wu, J.; Xi, Z.X. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E5007–E5015. [Google Scholar] [CrossRef] [Green Version]
- Navarrete, F.; García-Gutiérrez, M.S.; Manzanares, J. Pharmacological regulation of cannabinoid CB2 receptor modulates the reinforcing and motivational actions of ethanol. Biochem. Pharmacol. 2018, 157, 227–234. [Google Scholar] [CrossRef]
- Gobira, P.H.; Oliveira, A.C.; Gomes, J.S.; da Silveira, V.T.; Asth, L.; Bastos, J.R.; Batista, E.M.; Issy, A.C.; Okine, B.N.; de Oliveira, A.C.; et al. Opposing roles of CB1 and CB2 cannabinoid receptors in the stimulant and rewarding effects of cocaine. Br. J. Pharmacol. 2019, 176, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, A.; Warnault, V.; Montagud-Romero, S.; Pastor, A.; Mondragón, N.; De La Torre, R.; Valverde, O. Alcohol-induced conditioned place preference is modulated by CB2 cannabinoid receptors and modifies levels of endocannabinoids in the mesocorticolimbic system. Pharmacol. Biochem. Behav. 2019, 183, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.; Hamaoui, A.; Bieniek, D.; Korte, F. Effects of (–)Δ 9-trans-Tetrahydrocannabinol and a Synthetic Derivative on Maze Performance of Rats. Pharmacology 1970, 4, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Terranova, J.P.; Storme, J.J.; Lafon, N.; Perio, A.; Rinaldi-Carmona, M.; Le Fur, G.; Soubrie, P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology 1996, 126, 165–172. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, S.; Xu, E.; Wu, X.; Zhao, R. Inhibition of CB1 receptor ameliorates spatial learning and memory impairment in mice with traumatic brain injury. Neurosci. Lett. 2019, 696, 127–131. [Google Scholar] [CrossRef]
- Rabbani, M.; Vaseghi, G.; Hajhashemi, V. AM281, Cannabinoid Antagonist/Inverse agonist, ameliorates scopolamine-induced cognitive deficit. Iran. J. Basic Med. Sci. 2012, 15, 1106–1110. [Google Scholar]
- Navarro-Romero, A.; Vázquez-Oliver, A.; Gomis-González, M.; Garzón-Montesinos, C.; Falcón-Moya, R.; Pastor, A.; Martín-García, E.; Pizarro, N.; Busquets-Garcia, A.; Revest, J.M.; et al. Cannabinoid type-1 receptor blockade restores neurological phenotypes in two models for Down syndrome. Neurobiol. Dis. 2019, 125, 92–106. [Google Scholar] [CrossRef] [Green Version]
- Vallée, M.; Vitiello, S.; Bellocchio, L.; Hébert-Chatelain, E.; Monlezun, S.; Martin-Garcia, E.; Kasanetz, F.; Baillie, G.L.; Panin, F.; Cathala, A.; et al. Pregnenolone can protect the brain from cannabis intoxication. Science 2014, 343, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Bilkei-Gorzo, A.; Albayram, O.; Draffehn, A.; Michel, K.; Piyanova, A.; Oppenheimer, H.; Dvir-Ginzberg, M.; Rácz, I.; Ulas, T.; Imbeault, S.; et al. A chronic low dose of Δ 9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat. Med. 2017, 23, 782–787. [Google Scholar] [CrossRef]
- Gruber, S.A.; Sagar, K.A.; Dahlgren, M.K.; Gonenc, A.; Smith, R.T.; Lambros, A.M.; Cabrera, K.B.; Lukas, S.E. The grass might be greener: Medical marijuana patients exhibit altered brain activity and improved executive function after 3 months of treatment. Front. Pharmacol. 2018, 8, 983. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Dong, R.; Xu, X.; Yang, X.; Peng, M. Activation of cannabinoid receptor type 2 attenuates surgery-induced cognitive impairment in mice through anti-inflammatory activity. J. Neuroinflamm. 2017, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, P.; Morena, M.; Scaccianoce, S.; Trezza, V.; Chiarotti, F.; Schelling, G.; Cuomo, V.; Roozendaal, B. Novelty-induced emotional arousal modulates cannabinoid effects on recognition memory and adrenocortical activity. Neuropsychopharmacology 2013, 38, 1276–1286. [Google Scholar] [CrossRef] [Green Version]
- Morena, M.; Roozendaal, B.; Trezza, V.; Ratano, P.; Peloso, A.; Hauer, D.; Atsak, P.; Trabace, L.; Cuomo, V.; McGaugh, J.L.; et al. Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc. Natl. Acad. Sci. USA 2014, 111, 18333–18338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morena, M.; Campolongo, P. The endocannabinoid system: An emotional buffer in the modulation of memory function. Neurobiol. Learn. Mem. 2014, 112, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Ratano, P.; Petrella, C.; Forti, F.; Passeri, P.P.; Morena, M.; Palmery, M.; Trezza, V.; Severini, C.; Campolongo, P. Pharmacological inhibition of 2-arachidonoilglycerol hydrolysis enhances memory consolidation in rats through CB2 receptor activation and mTOR signaling modulation. Neuropharmacology 2018, 138, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; del Mar Fernández-Arjona, M.; Silva-Peña, D.; Blanco, E.; Vargas, A.; López-Ávalos, M.D.; Grondona, J.M.; Serrano, A.; Pavón, F.J.; de Fonseca, F.R.; et al. Pharmacological blockade of fatty acid amide hydrolase (FAAH) by URB597 improves memory and changes the phenotype of hippocampal microglia despite ethanol exposure. Biochem. Pharmacol. 2018, 157, 244–257. [Google Scholar] [CrossRef]
- Contarini, G.; Ferretti, V.; Papaleo, F. Acute administration of URB597 fatty acid amide hydrolase (FAAH) inhibitor prevents attentional impairments by distractors in adolescent mice. Front. Pharmacol. 2019, 10, 787. [Google Scholar] [CrossRef] [Green Version]
- Brown, W.; Leff, R.L.; Griffin, A.; Hossack, S.; Aubray, R.; Walker, P.; Chiche, D.A. Safety, pharmacokinetics, and pharmacodynamics study in healthy subjects of oral NEO6860, a modality selective transient receptor potential vanilloid subtype 1 antagonist. J. Pain 2017, 18, 726–738. [Google Scholar] [CrossRef]
- Zhong, P.; Wang, W.; Pan, B.; Liu, X.; Zhang, Z.; Long, J.Z.; Zhang, H.T.; Cravatt, B.F.; Liu, Q.S. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology 2014, 39, 1763–1776. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Arencibia, M.; Molina-Holgado, E.; Molina-Holgado, F. Effect of endocannabinoid signaling on cell fate: Life, death, differentiation and proliferation of brain cells. Br. J. Pharmacol. 2019, 176, 1361–1369. [Google Scholar] [CrossRef]
- Hill, J.D.; Zuluaga-Ramirez, V.; Gajghate, S.; Winfield, M.; Persidsky, Y. Activation of GPR55 increases neural stem cell proliferation and promotes early adult hippocampal neurogenesis. Br. J. Pharmacol. 2018, 175, 3407–3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, T.; Maroso, M.; Beer, A.; Baddenhausen, S.; Ludewig, S.; Fan, W.; Vennin, C.; Loch, S.; Berninger, B.; Hofmann, C.; et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb. Cortex 2018, 28, 4454–4471. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.W.; Oliveira, C.L.; Guimarães, F.S.; Campos, A.C. Cannabinoid signaling in embryonic and adult neurogenesis: Possible implications for psychiatric and neurological disorders. Acta Neuropsychiatr. 2019, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, M.A.; Gomez, O.; Esteban, P.F.; Garcia-Ovejero, D.; Molina-Holgado, E. The endocannabinoid 2-arachidonoylglycerol regulates oligodendrocyte progenitor cell migration. Biochem. Pharmacol. 2018, 157, 180–188. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Albayram, O.; Ativie, F.; Chasan, S.; Zimmer, T.; Bach, K.; Zimmer, A. Cannabinoid 1 receptor signaling on GABAergic neurons influences astrocytes in the ageing brain. PLoS ONE 2018, 13, e0202566. [Google Scholar] [CrossRef] [PubMed]
- Ativie, F.; Komorowska, J.A.; Beins, E.; Albayram, Ö.; Zimmer, T.; Zimmer, A.; Tejera, D.; Heneka, M.; Bilkei-Gorzo, A. Cannabinoid 1 Receptor Signaling on Hippocampal GABAergic Neurons Influences Microglial Activity. Front. Mol. Neurosci. 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannery, L.E.; Kerr, D.M.; Finn, D.P.; Roche, M. FAAH inhibition attenuates TLR3-mediated hyperthermia, nociceptive-and anxiety-like behaviour in female rats. Behav. Brain Res. 2018, 353, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Aguilera-Portillo, G.; Rangel-López, E.; Villeda-Hernández, J.; Chavarría, A.; Castellanos, P.; Elmazoglu, Z.; Karasu, Ç.; Túnez, I.; Pedraza, G.; Königsberg, M.; et al. The pharmacological inhibition of fatty acid amide hydrolase prevents excitotoxic damage in the rat striatum: Possible involvement of CB1 receptors regulation. Mol. Neurobiol. 2019, 56, 844–856. [Google Scholar] [CrossRef]
- Saliba, S.W.; Jauch, H.; Gargouri, B.; Keil, A.; Hurrle, T.; Volz, N.; Mohr, F.; van der Stelt, M.; Bräse, S.; Fiebich, B.L. Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J. Neuroinflamm. 2018, 15, 322. [Google Scholar] [CrossRef]
- Aso, E.; Andrés-Benito, P.; Ferrer, I. Genetic deletion of CB1 cannabinoid receptors exacerbates the Alzheimer-like symptoms in a transgenic animal model. Biochem. Pharmacol. 2018, 157, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Crunfli, F.; Vrechi, T.A.; Costa, A.P.; Torrão, A.S. Cannabinoid Receptor Type 1 Agonist ACEA Improves Cognitive Deficit on STZ-Induced Neurotoxicity Through Apoptosis Pathway and NO Modulation. Neurotox. Res. 2019, 35, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, F.A.; Ferreira, J.; de Lima, O.M.; Duarte, F.S.; Bento, A.F.; Forner, S.; Villarinho, J.G.; Bellocchio, L.; Wotjak, C.T.; Lerner, R.; et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 21134–21139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Ruiz, J.; Romero, J.; Velasco, G.; Tolón, R.M.; Ramos, J.A.; Guzmán, M. Cannabinoid CB2 receptor: A new target for controlling neural cell survival? Trends Pharmacol. Sci. 2007, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Resina, I.; Navarro, G.; Aguinaga, D.; Canela, E.I.; Schoeder, C.T.; Załuski, M.; Kieć-Kononowicz, K.; Saura, C.A.; Müller, C.E.; Franco, R. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relevance in neurodegenerative diseases. Biochem. Pharmacol. 2018, 157, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Yan, Y.; Chen, T.; Zhang, L.; Gao, X.; Du, C.; Du, H. Forsythiaside prevents β-amyloid-induced hippocampal slice injury by upregulating 2-arachidonoylglycerol via cannabinoid receptor 1-dependent NF-κB pathway. Neurochem. Int. 2019, 125, 57–66. [Google Scholar] [CrossRef]
- Balleza-Tapia, H.; Crux, S.; Andrade-Talavera, Y.; Dolz-Gaiton, P.; Papadia, D.; Chen, G.; Johansson, J.; Fisahn, A. TrpV1 receptor activation rescues neuronal function and network gamma oscillations from Aβ-induced impairment in mouse hippocampus in vitro. eLife 2018, 7, e37703. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Aguinaga, D.; Navarro, G.; Rico, A.J.; Oyarzábal, J.; Sánchez-Arias, J.A.; Lanciego, J.L.; Franco, R. Targeting CB 1 and GPR55 Endocannabinoid Receptors as a Potential Neuroprotective Approach for Parkinson’s Disease. Mol. Neurobiol. 2019, 56, 5900–5910. [Google Scholar] [CrossRef]
- Viveros-Paredes, J.; Gonzalez-Castañeda, R.; Escalante-Castañeda, A.; Tejeda-Martínez, A.; Castañeda-Achutiguí, F.; Flores-Soto, M. Effect of inhibition of fatty acid amide hydrolase on MPTP-induced dopaminergic neuronal damage. Neurología 2019, 34, 143–152. [Google Scholar] [CrossRef]
- Horne, E.A.; Coy, J.; Swinney, K.; Fung, S.; Cherry, A.E.; Marrs, W.R.; Naydenov, A.V.; Lin, Y.H.; Sun, X.; Dirk Keene, C.; et al. Downregulation of cannabinoid receptor 1 from neuropeptide Y interneurons in the basal ganglia of patients with Huntington’s disease and mouse models. Eur. J. Neurosci. 2013, 37, 429–440. [Google Scholar] [CrossRef]
- Holley, S.M.; Galvan, L.; Kamdjou, T.; Dong, A.; Levine, M.S.; Cepeda, C. Major contribution of somatostatin-expressing interneurons and cannabinoid receptors to increased GABA synaptic activity in the striatum of Huntington’s disease mice. Front. Synaptic Neurosci. 2019, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Laprairie, R.B.; Bagher, A.M.; Rourke, J.L.; Zrein, A.; Cairns, E.A.; Kelly, M.E.; Sinal, C.J.; Kulkarni, P.M.; Thakur, G.A.; Denovan-Wright, E.M. Positive allosteric modulation of the type 1 cannabinoid receptor reduces the signs and symptoms of Huntington’s disease in the R6/2 mouse model. Neuropharmacology 2019, 151, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Covey, D.P.; Dantrassy, H.M.; Yohn, S.E.; Castro, A.; Conn, P.J.; Mateo, Y.; Cheer, J.F. Inhibition of endocannabinoid degradation rectifies motivational and dopaminergic deficits in the Q175 mouse model of Huntington’s disease. Neuropsychopharmacology 2018, 43, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Moro, M.A.; Martínez-Orgado, J. Cannabinoids in neurodegenerative disorders and stroke/brain trauma: From preclinical models to clinical applications. Neurotherapeutics 2015, 12, 793–806. [Google Scholar] [CrossRef] [Green Version]
- DeSanty, K.; Dar, M.S. Cannabinoid-induced motor incoordination through the cerebellar CB1 receptor in mice. Pharmacol. Biochem. Behav. 2001, 69, 251–259. [Google Scholar] [CrossRef]
- Patel, S.; Hillard, C.J. Cannabinoid CB1 receptor agonists produce cerebellar dysfunction in mice. J. Pharmacol. Exp. Ther. 2001, 297, 629–637. [Google Scholar]
- Gómez-Ruiz, M.; Rodriguez-Cueto, C.; Luna-Piñel, E.; Hernández-Gálvez, M.; Fernández-Ruiz, J. Endocannabinoid system in spinocerebellar ataxia type-3 and other autosomal-dominant cerebellar ataxias: Potential role in pathogenesis and expected relevance as neuroprotective targets. Front. Mol. Neurosci. 2019, 12, 94. [Google Scholar] [CrossRef]
- Hermes, D.J.; Xu, C.; Poklis, J.L.; Niphakis, M.J.; Cravatt, B.F.; Mackie, K.; Lichtman, A.H.; Ignatowska-Jankowska, B.M.; Fitting, S. Neuroprotective effects of fatty acid amide hydrolase catabolic enzyme inhibition in a HIV-1 Tat model of neuroAIDS. Neuropharmacology 2018, 141, 55–65. [Google Scholar] [CrossRef] [PubMed]
- García-Rincón, D.; Díaz-Alonso, J.; Ortega, Z.; de Salas-Quiroga, A.; Paraíso-Luna, J.; Aguareles, J.; Jou, C.; De Prada, I.; Martinez Cerdeño, V.; Aronica, E.; et al. Contribution of altered endocannabinoid system to overactive mTORC1 signaling in focal cortical dysplasia. Front. Pharmacol. 2018, 9, 1508. [Google Scholar] [CrossRef] [Green Version]
- Parlar, A.; Arslan, S.O.; Doğan, M.F.; Çam, S.A.; Yalçin, A.; Elibol, E.; Özer, M.K.; Üçkardeş, F.; Kara, H. The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp. Ther. Med. 2018, 16, 4900–4908. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, E.D.; Dutra, R.C. Cannabinoid receptors as therapeutic targets for autoimmune diseases: Where do we stand? Drug Discov. Today 2019, 24, 1845–1853. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB 2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [Green Version]
- Vara, D.; Salazar, M.; Olea-Herrero, N.; Guzman, M.; Velasco, G.; Diaz-Laviada, I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011, 18, 1099–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, G.R.; Schertzer, J.D. AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: Implications for diabetes and cardiovascular disease. Immunol. Cell Biol. 2014, 92, 340–345. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, G.; Mabin, T.; Engelbrecht, A.M. Anti-inflammatory mechanisms of cannabinoids: An immunometabolic perspective. Inflammopharmacology 2019, 27, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Toguri, J.; Leishman, E.; Szczesniak, A.; Laprairie, R.; Oehler, O.; Straiker, A.; Kelly, M.; Bradshaw, H. Inflammation and CB2 signaling drive novel changes in the ocular lipidome and regulate immune cell activity in the eye. Prostaglandins Other Lipid Mediat. 2018, 139, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Riquer, Z.P.; Ibarra-Sánchez, A.; Vibhushan, S.; Bratti, M.; Charles, N.; Blank, U.; Rodríguez-Manzo, G.; González-Espinosa, C. TLR4 Receptor Induces 2-AG–Dependent Tolerance to Lipopolysaccharide and Trafficking of CB2 Receptor in Mast Cells. J. Immunol. 2019, 202, 2360–2371. [Google Scholar] [CrossRef]
- Buisseret, B.; Alhouayek, M.; Guillemot-Legris, O.; Muccioli, G.G. Endocannabinoid and Prostanoid Crosstalk in Pain. Trends Mol. Med. 2019, 25, 882–896. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; uczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Nam, G.; Jeong, S.K.; Park, B.M.; Lee, S.H.; Kim, H.J.; Hong, S.P.; Kim, B.; Kim, B.W. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann. Dermatol. 2016, 28, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani, M.R.; Shamsizadeh, A.; Moghadam-Ahmadi, A.; Bazmandegan, G.; Allahtavakoli, M. JZL184, as a monoacylglycerol lipase inhibitor, down-regulates inflammation in a cannabinoid pathway dependent manner. Biomed. Pharmacother. 2018, 103, 1720–1726. [Google Scholar] [CrossRef]
- Chen, H.J.C.; Spiers, J.G.; Sernia, C.; Lavidis, N.A. Inhibition of fatty acid amide hydrolase by PF-3845 alleviates the nitrergic and proinflammatory response in rat hippocampus following acute stress. Int. J. Neuropsychopharmacol. 2018, 21, 786–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Yagyu, K.; Sackett, S.; Zhang, Y. Anti-Inflammatory Effects by Pharmacological Inhibition or Knockdown of Fatty Acid Amide Hydrolase in BV2 Microglial Cells. Cells 2019, 8, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H. The Emerging Role of TRPV1 in Airway Inflammation. Allergy Asthma Immunol. Res. 2018, 10, 187–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, F.; Aono, H. Role of transient receptor potential vanilloid 1 in inflammation and autoimmune diseases. Pharmaceuticals 2012, 5, 837–852. [Google Scholar] [CrossRef] [Green Version]
- Cruz, S.L.; Sánchez-Miranda, E.; Castillo-Arellano, J.I.; Cervantes-Villagrana, R.D.; Ibarra-Sánchez, A.; González-Espinosa, C. Anandamide inhibits FcεRI-dependent degranulation and cytokine synthesis in mast cells through CB2 and GPR55 receptor activation. Possible involvement of CB2-GPR55 heteromers. Int. Immunopharmacol. 2018, 64, 298–307. [Google Scholar] [CrossRef]
- Motwani, M.P.; Bennett, F.; Norris, P.C.; Maini, A.A.; George, M.J.; Newson, J.; Henderson, A.; Hobbs, A.J.; Tepper, M.; White, B.; et al. Potent anti-inflammatory and pro-resolving effects of anabasum in a human model of self-resolving acute inflammation. Clin. Pharmacol. Ther. 2018, 104, 675–686. [Google Scholar] [CrossRef]
- García-Martín, A.; Garrido-Rodríguez, M.; Navarrete, C.; Caprioglio, D.; Palomares, B.; DeMesa, J.; Rollland, A.; Appendino, G.; Muñoz, E. Cannabinoid derivatives acting as dual PPARγ/CB2 agonists as therapeutic agents for systemic sclerosis. Biochem. Pharmacol. 2019, 163, 321–334. [Google Scholar] [CrossRef]
- del Rio, C.; Cantarero, I.; Palomares, B.; Gómez-Cañas, M.; Fernández-Ruiz, J.; Pavicic, C.; García-Martín, A.; Luz Bellido, M.; Ortega-Castro, R.; Pérez-Sánchez, C.; et al. VCE-004.3, a cannabidiol aminoquinone derivative, prevents bleomycin-induced skin fibrosis and inflammation through PPARγ-and CB2 receptor-dependent pathways. Br. J. Pharmacol. 2018, 175, 3813–3831. [Google Scholar] [CrossRef] [Green Version]
- Cinar, R.; Gochuico, B.R.; Iyer, M.R.; Jourdan, T.; Yokoyama, T.; Park, J.K.; Coffey, N.J.; Pri-Chen, H.; Szanda, G.; Liu, Z.; et al. Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight 2017, 2, 92281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, S.; Yang, P.; Tian, Y.; Feng, Z.; Xie, X.Q.; Liu, Y. Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int. 2018, 94, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of cannabinoids in obesity. Int. J. Mol. Sci. 2018, 19, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Azua, I.R.; Lutz, B. Multiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissues. Cell. Mol. Life Sci. 2019, 76, 1341–1363. [Google Scholar] [CrossRef]
- Ogden, S.B.; Malamas, M.S.; Makriyannis, A.; Eckel, L.A. The novel cannabinoid 1 receptor agonist AM11101 increases food intake in female rats. Br. J. Pharmacol. 2019, 176, 3972–3982. [Google Scholar] [CrossRef]
- Scheen, A.J.; Finer, N.; Hollander, P.; Jensen, M.D.; Van Gaal, L.F.; RIO-Diabetes Study Group. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: A randomised controlled study. Lancet 2006, 368, 1660–1672. [Google Scholar] [CrossRef]
- Chorvat, R.J. Peripherally restricted CB1 receptor blockers. Bioorganic Med. Chem. Lett. 2013, 23, 4751–4760. [Google Scholar] [CrossRef]
- Han, J.H.; Shin, H.; Park, J.Y.; Rho, J.G.; Son, D.H.; Kim, K.W.; Seong, J.K.; Yoon, S.H.; Kim, W. A novel peripheral cannabinoid 1 receptor antagonist, AJ5012, improves metabolic outcomes and suppresses adipose tissue inflammation in obese mice. FASEB J. 2019, 33, 4314–4326. [Google Scholar] [CrossRef]
- Han, J.H.; Shin, H.; Rho, J.G.; Kim, J.E.; Son, D.H.; Yoon, J.; Lee, Y.J.; Park, J.H.; Song, B.J.; Choi, C.S.; et al. Peripheral cannabinoid 1 receptor blockade mitigates adipose tissue inflammation via NLRP3 inflammasome in mouse models of obesity. Diabetes Obes. Metab. 2018, 20, 2179–2189. [Google Scholar] [CrossRef]
- Seltzman, H.H.; Maitra, R.; Bortoff, K.; Henson, J.; Reggio, P.H.; Wesley, D.; Tam, J. Metabolic profiling of CB1 neutral antagonists. Methods Enzymol. 2017, 593, 199–215. [Google Scholar]
- Horswill, J.; Bali, U.; Shaaban, S.; Keily, J.; Jeevaratnam, P.; Babbs, A.; Reynet, C.; Wong Kai In, P. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br. J. Pharmacol. 2007, 152, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Qiu, Y.; Jing, L.; Thorn, D.A.; Zhang, Y.; Li, J.X. Behavioral effects of the cannabinoid CB1 receptor allosteric modulator ORG27569 in rats. Pharmacol. Res. Perspect. 2014, 2, e00069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipina, C.; Walsh, S.K.; Mitchell, S.E.; Speakman, J.R.; Wainwright, C.L.; Hundal, H.S. GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues. FASEB J. 2019, 33, 1299–1312. [Google Scholar] [CrossRef] [Green Version]
- Simcocks, A.; Jenkin, K.; O’keefe, L.; Samuel, C.; Mathai, M.; McAinch, A.; Hryciw, D. Atypical cannabinoid ligands O-1602 and O-1918 administered chronically in diet-induced obesity. Endocr. Connect. 2019, 8, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 2008, 51, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- Gruden, G.; Barutta, F.; Kunos, G.; Pacher, P. Role of the endocannabinoid system in diabetes and diabetic complications. Br. J. Pharmacol. 2016, 173, 1116–1127. [Google Scholar] [CrossRef]
- Grunewald, Z.I.; Lee, S.; Kirkland, R.; Ross, M.; Claire, B. Cannabinoid receptor type-1 partially mediates metabolic endotoxemia-induced inflammation and insulin resistance. Physiol. Behav. 2019, 199, 282–291. [Google Scholar] [CrossRef]
- González-Mariscal, I.; Egan, J.M. Endocannabinoids in the Islets of Langerhans: The ugly, the bad, and the good facts. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E174–E179. [Google Scholar] [CrossRef]
- Hinden, L.; Udi, S.; Drori, A.; Gammal, A.; Nemirovski, A.; Hadar, R.; Baraghithy, S.; Permyakova, A.; Geron, M.; Cohen, M.; et al. Modulation of renal GLUT2 by the cannabinoid-1 receptor: Implications for the treatment of diabetic nephropathy. J. Am. Soc. Nephrol. 2018, 29, 434–448. [Google Scholar] [CrossRef] [Green Version]
- Hinden, L.; Tam, J. Do Endocannabinoids Regulate Glucose Reabsorption in the Kidney? Nephron 2019, 143, 24–27. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Mastrocola, R.; Gambino, R.; Piscitelli, F.; di Marzo, V.; Corbetta, B.; Vemuri, V.; Makriyannis, A.; Annaratone, L.; et al. Reversal of albuminuria by combined AM6545 and perindopril therapy in experimental diabetic nephropathy. Br. J. Pharmacol. 2018, 175, 4371–4385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, C.W.; Ho, C.; Hsu, Y.C.; Huang, S.C.; Shih, Y.H.; Lin, C.L. MicroRNA-29a attenuates diabetic glomerular injury through modulating cannabinoid receptor 1 signaling. Molecules 2019, 24, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumawat, V.S.; Kaur, G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019, 862, 172628. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Hu, P.; Lin, J.; Xia, W.; Zhang, R. Activating cannabinoid receptor 2 protects against diabetic cardiomyopathy through autophagy induction. Front. Pharmacol. 2018, 9, 1292. [Google Scholar] [CrossRef] [PubMed]
- Vong, C.T.; Tseng, H.H.L.; Kwan, Y.W.; Lee, S.M.Y.; Hoi, M.P.M. Novel protective effect of O-1602 and abnormal cannabidiol, GPR55 agonists, on ER stress-induced apoptosis in pancreatic β-cells. Biomed. Pharmacother. 2019, 111, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.i.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef]
- Chu, Z.L.; Carroll, C.; Alfonso, J.; Gutierrez, V.; He, H.; Lucman, A.; Pedraza, M.; Mondala, H.; Gao, H.; Bagnol, D.; et al. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology 2008, 149, 2038–2047. [Google Scholar] [CrossRef] [Green Version]
- Gendaszewska-Darmach, E.; Drzazga, A.; Koziołkiewicz, M. Targeting GPCRs Activated by Fatty Acid-Derived Lipids in Type 2 Diabetes. Trends Mol. Med. 2019, 25, 915–929. [Google Scholar] [CrossRef] [Green Version]
- Huan, Y.; Jiang, Q.; Li, G.; Bai, G.; Zhou, T.; Liu, S.; Li, C.; Liu, Q.; Sun, S.; Yang, M.; et al. The dual DPP4 inhibitor and GPR119 agonist HBK001 regulates glycemic control and beta cell function ex and in vivo. Sci. Rep. 2017, 7, 4351. [Google Scholar] [CrossRef] [Green Version]
- Bazwinsky-Wutschke, I.; Zipprich, A.; Dehghani, F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int. J. Mol. Sci. 2019, 20, 2516. [Google Scholar] [CrossRef] [Green Version]
- Jorgačević, B.; Vučević, D.; Vesković, M.; Mladenović, D.; Vukićević, D.; Vukićević, R.J.; Todorović, V.; Radosavljević, T. The effect of cannabinoid receptor 1 blockade on adipokine and proinflammatory cytokine concentration in adipose and hepatic tissue in mice with nonalcoholic fatty liver disease. Can. J. Physiol. Pharmacol. 2019, 97, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, D.H.; Yang, H.; Lee, D.H.; Bae, S.H.; Park, C.Y. CB1 receptor blockade ameliorates hepatic fat infiltration and inflammation and increases Nrf2-AMPK pathway in a rat model of severely uncontrolled diabetes. PLoS ONE 2018, 13, e0206152. [Google Scholar] [CrossRef] [PubMed]
- Bahirat, U.A.; Shenoy, R.R.; Goel, R.N.; Nemmani, K.V. APD668, a G protein-coupled receptor 119 agonist improves fat tolerance and attenuates fatty liver in high-trans fat diet induced steatohepatitis model in C57BL/6 mice. Eur. J. Pharmacol. 2017, 801, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.A.; Awan, A.; Karl, M.; Santini, A. Cardiovascular effects of cannabis (marijuana): A timely update. Phytother. Res. PTR 2019, 33, 1592–1594. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Luquin, N.; Navarro-Otano, J. The endocannabinoid system in cardiovascular function: Novel insights and clinical implications. Clin. Auton. Res. 2018, 28, 35–52. [Google Scholar] [CrossRef]
- Malinowska, B.; Toczek, M.; Pędzińska-Betiuk, A.; Schlicker, E. Cannabinoids in arterial, pulmonary and portal hypertension–mechanisms of action and potential therapeutic significance. Br. J. Pharmacol. 2019, 176, 1395–1411. [Google Scholar] [CrossRef]
- Bermudez, A.M.; Visina, J.M.; Walker, L.A. Differential effects of cannabinoid receptor stimulation in smooth muscle. FASEB J. 2017, 31, 690–694. [Google Scholar]
- Giménez, V.M.M.; Díaz-Rodríguez, P.; Sanz, R.L.; Vivero-Lopez, M.; Concheiro, A.; Diez, E.; Prado, N.; Kassuha, D.E.; Alvarez-Lorenzo, C.; Manucha, W. Anandamide-nanoformulation obtained by electrospraying for cardiovascular therapy. Int. J. Pharm. 2019, 566, 1–10. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Li, N.; Poklis, J.L.; Li, P.L.; Ritter, J.K. Modulation of mean arterial pressure and diuresis by renomedullary infusion of a selective inhibitor of fatty acid amide hydrolase. Am. J. Physiol.-Ren. Physiol. 2018, 315, F967–F976. [Google Scholar] [CrossRef]
- Toczek, M.; Baranowska-Kuczko, M.; Grzęda, E.; Pędzińska-Betiuk, A.; Weresa, J.; Malinowska, B. Age-specific influences of chronic administration of the fatty acid amide hydrolase inhibitor URB597 on cardiovascular parameters and organ hypertrophy in DOCA-salt hypertensive rats. Pharmacol. Rep. 2016, 68, 363–369. [Google Scholar] [CrossRef]
- Biernacki, M.; Ambrożewicz, E.; Gęgotek, A.; Toczek, M.; Bielawska, K.; Skrzydlewska, E. Redox system and phospholipid metabolism in the kidney of hypertensive rats after FAAH inhibitor URB597 administration. Redox Biol. 2018, 15, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lagatta, D.C.; Kuntze, L.B.; Ferreira-Junior, N.C.; Resstel, L.B. Medial prefrontal cortex TRPV1 and CB1 receptors modulate cardiac baroreflex activity by regulating the NMDA receptor/nitric oxide pathway. Pflügers Arch.-Eur. J. Physiol. 2018, 470, 1521–1542. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ayon, R.J.; Yamamura, A.; Yamamura, H.; Dash, S.; Babicheva, A.; Tang, H.; Sun, X.; Cordery, A.G.; Khalpey, Z.; et al. Capsaicin-induced Ca2+ signaling is enhanced via upregulated TRPV1 channels in pulmonary artery smooth muscle cells from patients with idiopathic PAH. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, L309–L325. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R.; Rami, M.; Herzig, S.; Steffens, S. Endocannabinoid signaling in atherosclerosis and related metabolic complications. Thromb. Haemost. 2019, 119, 567–575. [Google Scholar]
- Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Mackie, K.; Pacher, P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and-independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 2010, 160, 688–700. [Google Scholar] [CrossRef] [Green Version]
- Sugamura, K.; Sugiyama, S.; Fujiwara, Y.; Matsubara, J.; Akiyama, E.; Maeda, H.; Ohba, K.; Matsuzawa, Y.; Konishi, M.; Nozaki, T.; et al. Cannabinoid 1 receptor blockade reduces atherosclerosis with enhances reverse cholesterol transport. J. Atheroscler. Thromb. 2010, 17, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Prats, R.G.; Rami, M.; Ring, L.; Rinne, P.; Lauer, E.; Lenglet, S.; Thomas, A.; Pagano, S.; Vuilleumier, N.; Cravatt, B.F.; et al. Deficiency of monoacylglycerol lipase enhances IgM plasma levels and limits atherogenesis in a CB2-dependent manner. Thromb. Haemost. 2019, 119, 348–351. [Google Scholar]
- Rinne, P.; Guillamat-Prats, R.; Rami, M.; Bindila, L.; Ring, L.; Lyytikäinen, L.P.; Raitoharju, E.; Oksala, N.; Lehtimäki, T.; Weber, C.; et al. Palmitoylethanolamide promotes a proresolving macrophage phenotype and attenuates atherosclerotic plaque formation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2562–2575. [Google Scholar] [CrossRef]
- Montecucco, F.; Bondarenko, A.I.; Lenglet, S.; Burger, F.; Piscitelli, F.; Carbone, F.; Roth, A.; Liberale, L.; Dallegri, F.; Brandt, K.J.; et al. Treatment with the GPR55 antagonist CID16020046 increases neutrophil activation in mouse atherogenesis. Thromb. Haemost. 2016, 116, 987–997. [Google Scholar]
- Schloss, M.J.; Horckmans, M.; Guillamat-Prats, R.; Hering, D.; Lauer, E.; Lenglet, S.; Weber, C.; Thomas, A.; Steffens, S. 2-Arachidonoylglycerol mobilizes myeloid cells and worsens heart function after acute myocardial infarction. Cardiovasc. Res. 2019, 115, 602–613. [Google Scholar] [CrossRef] [Green Version]
- Duerr, G.D.; Heinemann, J.C.; Kley, J.; Eichhorn, L.; Frede, S.; Weisheit, C.; Wehner, S.; Bindila, L.; Lutz, B.; Zimmer, A.; et al. Myocardial maladaptation to pressure overload in CB2 receptor-deficient mice. J. Mol. Cell. Cardiol. 2019, 133, 86–98. [Google Scholar] [CrossRef]
- Hai, K.; Chen, G.; Gou, X.; Jang, H.; Gong, D.; Cheng, Y.; Gong, C.; Li, X.; Liu, Y.; Li, H.; et al. Monoacylglycerol Lipase Inactivation by Using URB602 Mitigates Myocardial Damage in a Rat Model of Cardiac Arrest. Crit. Care Med. 2019, 47, e144–e151. [Google Scholar] [CrossRef]
- Vago, R.; Bettiga, A.; Salonia, A.; Ciuffreda, P.; Ottria, R. Development of new inhibitors for N-acylethanolamine-hydrolyzing acid amidase as promising tool against bladder cancer. Bioorganic Med. Chem. 2017, 25, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Coke, C.J.; Scarlett, K.A.; Chetram, M.A.; Jones, K.J.; Sandifer, B.J.; Davis, A.S.; Marcus, A.I.; Hinton, C.V. Simultaneous activation of induced heterodimerization between CXCR4 chemokine receptor and cannabinoid receptor 2 (CB2) reveals a mechanism for regulation of tumor progression. J. Biol. Chem. 2016, 291, 9991–10005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela Campos, E.I. The endocannabinoid system as a target in cancer diseases: Are we there yet? Front. Pharmacol. 2019, 10, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, P.; Jagerovic, N. Antitumor cannabinoid chemotypes: Structural insights. Front. Pharmacol. 2019, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Mattei, V.; Martellucci, S.; Manganelli, V.; Saccomanni, G.; Garofalo, T.; Sorice, M.; Manera, C.; Misasi, R. Anti-Proliferative Properties and Proapoptotic Function of New CB2 Selective Cannabinoid Receptor Agonist in Jurkat Leukemia Cells. Int. J. Mol. Sci. 2018, 19, 1958. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Sobocińska, A.A.; Brodaczewska, K.K.; Zielniok, K.; Gajewska, M.; Kieda, C.; Czarnecka, A.M.; Szczylik, C. Involvement of the CB 2 cannabinoid receptor in cell growth inhibition and G0/G1 cell cycle arrest via the cannabinoid agonist WIN 55,212–2 in renal cell carcinoma. BMC Cancer 2018, 18, 583. [Google Scholar] [CrossRef] [Green Version]
- Youssif, B.G.; Mohamed, A.M.; Osman, E.E.A.; Abou-Ghadir, O.F.; Elnaggar, D.H.; Abdelrahman, M.H.; Treamblu, L.; Gomaa, H.A. 5-Chlorobenzofuran-2-carboxamides: From allosteric CB1 modulators to potential apoptotic antitumor agents. Eur. J. Med. Chem. 2019, 177, 1–11. [Google Scholar] [CrossRef]
- Carpi, S.; Fogli, S.; Polini, B.; Montagnani, V.; Podestà, A.; Breschi, M.C.; Romanini, A.; Stecca, B.; Nieri, P. Tumor-promoting effects of cannabinoid receptor type 1 in human melanoma cells. Toxicol. Vitr. 2017, 40, 272–279. [Google Scholar] [CrossRef]
- Yang, J.; Tian, Y.; Zheng, R.; Li, L.; Qiu, F. Endocannabinoid system and the expression of endogenous ceramides in human hepatocellular carcinoma. Oncol. Lett. 2019, 18, 1530–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, K.; Ramer, R.; Dithmer, S.; Ivanov, I.; Merkord, J.; Hinz, B. Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells. Oncotarget 2016, 7, 15047–15064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, E.; Van Dross, R. Anandamide-induced endoplasmic reticulum stress and apoptosis are mediated by oxidative stress in non-melanoma skin cancer: Receptor-independent endocannabinoid signaling. Mol. Carcinog. 2016, 55, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-da Silva, G.; Teixeira, N. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Yang, L.; Wang, B.; Cui, L.; Li, C.; Zhuo, Y.; Zhang, L.; Zhang, S.; Zhang, Q.; Wang, X. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108952. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Funakoshi, T.; Mori, M.; Emoto, K.; Masugi, Y.; Ekmekcioglu, S.; Amagai, M.; Tanese, K. Expression of monoacylglycerol lipase as a marker of tumour invasion and progression in malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 2038–2045. [Google Scholar] [CrossRef]
- Hu, W.R.; Lian, Y.F.; Peng, L.X.; Lei, J.J.; Deng, C.C.; Xu, M.; Feng, Q.S.; Chen, L.Z.; Bei, J.X.; Zeng, Y.X. Monoacylglycerol lipase promotes metastases in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 3704–3713. [Google Scholar]
- Li, X.; Gao, S.; Li, W.; Liu, Z.; Shi, Z.; Qiu, C.; Jiang, J. Effect of monoacylglycerol lipase on the tumor growth in endometrial cancer. J. Obstet. Gynaecol. Res. 2019, 45, 2043–2054. [Google Scholar] [CrossRef] [Green Version]
- Mulvihill, M.M.; Nomura, D.K. Therapeutic potential of monoacylglycerol lipase inhibitors. Life Sci. 2013, 92, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Granchi, C.; Lapillo, M.; Glasmacher, S.; Bononi, G.; Licari, C.; Poli, G.; El Boustani, M.; Caligiuri, I.; Rizzolio, F.; Gertsch, J.; et al. Optimization of a benzoylpiperidine class identifies a highly potent and selective reversible monoacylglycerol lipase (MAGL) inhibitor. J. Med. Chem. 2019, 62, 1932–1958. [Google Scholar] [CrossRef]
- Ma, M.; Bai, J.; Ling, Y.; Chang, W.; Xie, G.; Li, R.; Wang, G.; Tao, K. Monoacylglycerol lipase inhibitor JZL184 regulates apoptosis and migration of colorectal cancer cells. Mol. Med. Rep. 2016, 13, 2850–2856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, S.; de Ridder, D.; Bishop, R.T.; Renema, N.; Ponzetti, M.; Sophocleous, A.; Capulli, M.; Aljeffery, A.; Carrasco, G.; Gens, M.D.; et al. Paradoxical effects of JZL184, an inhibitor of monoacylglycerol lipase, on bone remodelling in healthy and cancer-bearing mice. EBioMedicine 2019, 44, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Borrelli, F.; Orlando, P.; Romano, B.; Monti, M.; Morbidelli, L.; Aviello, G.; Imperatore, R.; Capasso, R.; Piscitelli, F.; et al. Pharmacological inhibition of MAGL attenuates experimental colon carcinogenesis. Pharmacol. Res. 2017, 119, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the Endocannabinoid System as a Potential Anticancer Strategy. Front. Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, L.; Loiodice, F.; Piemontese, L.; Tortorella, P.; Laghezza, A. New Approaches to Cancer Therapy: Combining Fatty Acid Amide Hydrolase (FAAH) Inhibition with Peroxisome Proliferator-Activated Receptors (PPARs) Activation: Miniperspective. J. Med. Chem. 2019, 62, 10995–11003. [Google Scholar] [CrossRef]
- Chicca, A.; Arena, C.; Bertini, S.; Gado, F.; Ciaglia, E.; Abate, M.; Digiacomo, M.; Lapillo, M.; Poli, G.; Bifulco, M.; et al. Polypharmacological profile of 1, 2-dihydro-2-oxo-pyridine-3-carboxamides in the endocannabinoid system. Eur. J. Med. Chem. 2018, 154, 155–171. [Google Scholar] [CrossRef]
- Kargl, J.; Andersen, L.; Hasenöhrl, C.; Feuersinger, D.; Stančić, A.; Fauland, A.; Magnes, C.; El-Heliebi, A.; Lax, S.; Uranitsch, S.; et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br. J. Pharmacol. 2016, 173, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Hasenoehrl, C.; Feuersinger, D.; Sturm, E.M.; Bärnthaler, T.; Heitzer, E.; Graf, R.; Grill, M.; Pichler, M.; Beck, S.; Butcher, L.; et al. G protein-coupled receptor GPR55 promotes colorectal cancer and has opposing effects to cannabinoid receptor 1. Int. J. Cancer 2018, 142, 121–132. [Google Scholar] [CrossRef]
- Zhou, X.l.; Guo, X.; Song, Y.p.; Zhu, C.y.; Zou, W. The LPI/GPR55 axis enhances human breast cancer cell migration via HBXIP and p-MLC signaling. Acta Pharmacol. Sin. 2018, 39, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Andradas, C.; Blasco-Benito, S.; Castillo-Lluva, S.; Dillenburg-Pilla, P.; Diez-Alarcia, R.; Juanes-García, A.; García-Taboada, E.; Hernando-Llorente, R.; Soriano, J.; Hamann, S.; et al. Activation of the orphan receptor GPR55 by lysophosphatidylinositol promotes metastasis in triple-negative breast cancer. Oncotarget 2016, 7, 47565–47575. [Google Scholar] [CrossRef]
- Brown, A.J.; Castellano-Pellicena, I.; Haslam, C.P.; Nichols, P.L.; Dowell, S.J. Structure-Activity Relationship of the GPR55 Antagonist, CID16020046. Pharmacology 2018, 102, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Ramer, R. Anti-tumour actions of cannabinoids. Br. J. Pharmacol. 2019, 176, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Liu, Y.; Su, M.; Ling, X.; Liu, F.; Ge, Y.; Bai, M. Combined CB2 receptor agonist and photodynamic therapy synergistically inhibit tumor growth in triple negative breast cancer. Photodiagnosis Photodyn. Ther. 2018, 24, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Chatkin, J.; Zani-Silva, L.; Ferreira, I.; Zamel, N. Cannabis-associated asthma and allergies. Clin. Rev. Allergy Immunol. 2019, 56, 196–206. [Google Scholar] [CrossRef]
- Ashton, J.C.; Hancox, R.J. The Case for Cannabinoid CB1 Receptors as a Target for Bronchodilator Therapy for β-agonist Resistant Asthma. Curr. Drug Targets 2018, 19, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, T.E.; Kaya, Y.; Durlu-Kandilci, N.T.; Onder, S.; Sahin-Erdemli, I. The effect of cannabinoids on dinitrofluorobenzene-induced experimental asthma in mice. Respir. Physiol. Neurobiol. 2016, 231, 7–13. [Google Scholar] [CrossRef]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [Google Scholar] [CrossRef]
- Park, J.; Yokoyama, T.; Jourdan, T.; Malicdan, M.; Coffey, N.; Gahl, W.; Kunos, G.; Cinar, R. Cannabinoid Receptor 1 (cb1r) Activation Induces Alveolar Macrophages To Acquire A Proinflammatory In addition, Profibrotic Phenotype In Pulmonary Fibrosis. In American Thoracic Society International Conference Abstracts; American Thoracic Society: Washington, DC, USA, 2017; p. A7065. [Google Scholar]
- Coffey, N.; Park, J.; Jourdan, T.; Yokoyama, T.; Gahl, W.; Malicdan, M.; Cinar, R.; Kunos, G. Deletion of Cannabinoid Receptor 1 (CB1R) in Myeloid Cells Prevents the Progression of Bleomycin-Induced Pulmonary Fibrosis in Mice. In American Thoracic Society International Conference Abstracts; American Thoracic Society: San Diego, CA, USA, 2018; p. A1051. [Google Scholar]
- Cinar, R.; Yokoyama, T.; Coffey, N.; Park, J.; Iyer, M.; Gochuico, B.; Gahl, W.; Malicdan, M.; Kunos, G. The Endocannabinoid/CB1R System Is Overactive in Bleomycin-Induced Pulmonary Fibrosis in Pale Ear (HPS1) Mice. In American Thoracic Society International Conference Abstracts; American Thoracic Society: San Diego, CA, USA, 2018; p. A5757. [Google Scholar]
- Wawryk-Gawda, E.; Chłapek, K.; Zarobkiewicz, M.K.; Lis-Sochocka, M.; Chylińska-Wrzos, P.; Boguszewska-Czubara, A.; Sławiński, M.A.; Franczak, A.; Jodłowska-Jędrych, B.; Biała, G. CB2R agonist prevents nicotine induced lung fibrosis. Exp. Lung Res. 2018, 44, 344–351. [Google Scholar] [CrossRef]
- Pesce, M.; D’Alessandro, A.; Borrelli, O.; Gigli, S.; Seguella, L.; Cuomo, R.; Esposito, G.; Sarnelli, G. Endocannabinoid-related compounds in gastrointestinal diseases. J. Cell. Mol. Med. 2018, 22, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci. Rep. 2019, 9, 2358. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, T.; Simmons, A. Cannabis, cannabinoids, and the endocannabinoid system—is there therapeutic potential for inflammatory bowel disease? J. Crohn’s Colitis 2019, 13, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Salaga, M.; Binienda, A.; Tichkule, R.B.; Thakur, G.A.; Makriyannis, A.; Storr, M.; Fichna, J. The novel peripherally active cannabinoid type 1 and serotonin type 3 receptor agonist AM9405 inhibits gastrointestinal motility and reduces abdominal pain in mouse models mimicking irritable bowel syndrome. Eur. J. Pharmacol. 2018, 836, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Verga, M.C.; De Maria, N.; Villa, E.; Pini, L.A.; Guerzoni, S. Nabilone administration in refractory chronic diarrhea: A case series. BMC Gastroenterol. 2019, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Darmani, N.A.; Parker, L.A. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur. J. Pharmacol. 2014, 722, 134–146. [Google Scholar] [CrossRef] [Green Version]
- May, M.B.; Glode, A.E. Dronabinol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics. Cancer Manag. Res. 2016, 8, 49–55. [Google Scholar]
- Darmani, N.A.; Chebolu, S.; Zhong, W.; Trinh, C.; McClanahan, B.; Brar, R.S. Additive antiemetic efficacy of low-doses of the cannabinoid CB1/2 receptor agonist Δ9-THC with ultralow-doses of the vanilloid TRPV1 receptor agonist resiniferatoxin in the least shrew (Cryptotis parva). Eur. J. Pharmacol. 2014, 722, 147–155. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, Y.; Valverde, P.; Murray, D.; Tang, J.; Yao, Q.; Han, Q.; Zhang, J.; Zhang, L.; Sui, L.; et al. Central adiponectin induces trabecular bone mass partly through epigenetic downregulation of cannabinoid receptor CB1. J. Cell. Physiol. 2019, 234, 7062–7069. [Google Scholar] [CrossRef]
- Samir, S.; Malek, H. Effect of cannabinoid receptors 1 modulation on osteoporosis in a rat model of different ages. J. Physiol. Pharmacol. 2014, 65, 687–694. [Google Scholar]
- Smoum, R.; Baraghithy, S.; Chourasia, M.; Breuer, A.; Mussai, N.; Attar-Namdar, M.; Kogan, N.M.; Raphael, B.; Bolognini, D.; Cascio, M.G.; et al. CB2 cannabinoid receptor agonist enantiomers HU-433 and HU-308: An inverse relationship between binding affinity and biological potency. Proc. Natl. Acad. Sci. USA 2015, 112, 8774–8779. [Google Scholar] [CrossRef] [Green Version]
- More, K.N.; Lee, Y.J.; Kim, K.J.; Suh, Y.G.; Son, Y.J.; Chang, D.J. Effect of TRPV1 Antagonist SC0030, a Potent Painkiller, on RANKL-mediated Osteoclast Differentiation Involved in Bone Resorption. Bull. Korean Chem. Soc. 2020. [Google Scholar] [CrossRef]
- Walker, O.S.; Holloway, A.C.; Raha, S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Feng, X.; Zhao, Z.; Zhang, J.; Xu, Y.; Wang, L.; Hao, G. Restored plasma anandamide and endometrial expression of fatty acid amide hydrolase in women with polycystic ovary syndrome by the combination use of Diane-35 and metformin. Clin. Ther. 2017, 39, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Duan, H.; Wang, S.; Gan, L.; Xu, Q.; Li, J.J. Decreased Expression of Cannabinoid Receptors in the Eutopic and Ectopic Endometrium of Patients with Adenomyosis. BioMed Res. Int. 2019, 2019, 5468954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemenza, S.; Sorbi, F.; Noci, I.; Capezzuoli, T.; Turrini, I.; Carriero, C.; Buffi, N.; Fambrini, M.; Petraglia, F. From pathogenesis to clinical practice: Emerging medical treatments for endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 92–101. [Google Scholar] [CrossRef]
- Gratzke, C.; Christ, G.J.; Stief, C.G.; Andersson, K.E.; Hedlund, P. Localization and function of cannabinoid receptors in the corpus cavernosum: Basis for modulation of nitric oxide synthase nerve activity. Eur. Urol. 2010, 57, 342–348. [Google Scholar] [CrossRef]
- Shamloul, R.; Bella, A.J. Impact of cannabis use on male sexual health. J. Sex. Med. 2011, 8, 971–975. [Google Scholar] [CrossRef]
- Castelli, M.; Piras, A.; Melis, T.; Succu, S.; Sanna, F.; Melis, M.; Collu, S.; Ennas, M.G.; Diaz, G.; Mackie, K.; et al. Cannabinoid CB1 receptors in the paraventricular nucleus and central control of penile erection: Immunocytochemistry, autoradiography and behavioral studies. Neuroscience 2007, 147, 197–206. [Google Scholar] [CrossRef]
- Milando, R.; Friedman, A. Cannabinoids: Potential Role in Inflammatory and Neoplastic Skin Diseases. Am. J. Clin. Dermatol. 2019, 20, 167–180. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(ut)annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef] [Green Version]
- Zákány, N.; Oláh, A.; Markovics, A.; Takács, E.; Aranyász, A.; Nicolussi, S.; Piscitelli, F.; Allarà, M.; Pór, Á.; Kovács, I.; et al. Endocannabinoid Tone Regulates Human Sebocyte Biology. J. Investig. Dermatol. 2018, 138, 1699–1706. [Google Scholar] [CrossRef] [Green Version]
- Iannotti, F.A.; Pagano, E.; Guardiola, O.; Adinolfi, S.; Saccone, V.; Consalvi, S.; Piscitelli, F.; Gazzerro, E.; Busetto, G.; Carrella, D.; et al. Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in Duchenne muscular dystrophy. Nat. Commun. 2018, 9, 3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannotti, F.A. Pharmacological Actions and Potential Therapeutic Use of Cannabinoids in Duchenne’s Muscular Dystrophy. In Muscular Dystrophies; Sakuma, K., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Beal, J.E.; Olson, R.; Laubenstein, L.; Morales, J.O.; Bellman, P.; Yangco, B.; Lefkowitz, L.; Plasse, T.F.; Shepard, K.V. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manag. 1995, 10, 89–97. [Google Scholar] [CrossRef]
- AbbVie. MARINOL (dronabinol) [drug label]. US Food and Drug Administration. Revised August 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf (accessed on 18 February 2020).
- Badowski, M.E.; Yanful, P.K. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther. Clin. Risk Manag. 2018, 14, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeant Pharmaceuticals International. Cesamet (nabilone) [drug label]. US Food and Drug Administration. Revised May 2006. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf (accessed on 18 February 2020).
- Pergolizzi, J.V.; Taylor, R.; LeQuang, J.A.; Zampogna, G.; Raffa, R.B. Concise review of the management of iatrogenic emesis using cannabinoids: Emphasis on nabilone for chemotherapy-induced nausea and vomiting. Cancer Chemother. Pharmacol. 2017, 79, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Badowski, M.E. A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: A focus on pharmacokinetic variability and pharmacodynamics. Cancer Chemother. Pharmacol. 2017, 80, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englund, A.; Morrison, P.D.; Nottage, J.; Hague, D.; Kane, F.; Bonaccorso, S.; Stone, J.M.; Reichenberg, A.; Brenneisen, R.; Holt, D.; et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 2013, 27, 19–27. [Google Scholar] [CrossRef]
- Schubart, C.D.; Sommer, I.E.; van Gastel, W.A.; Goetgebuer, R.L.; Kahn, R.S.; Boks, M.P. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr. Res. 2011, 130, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Robson, P. Abuse potential and psychoactive effects of δ-9-tetrahydrocannabinol and cannabidiol oromucosal spray (Sativex), a new cannabinoid medicine. Expert Opin. Drug Saf. 2011, 10, 675–685. [Google Scholar] [CrossRef]
- Podda, G.; Constantinescu, C.S. Nabiximols in the treatment of spasticity, pain and urinary symptoms due to multiple sclerosis. Expert Opin. Biol. Ther. 2012, 12, 1517–1531. [Google Scholar] [CrossRef]
- Werth, V.; Oddis, C.V.; Lundberg, I.E.; Fiorentino, D.; Cornwall, C.; Dgetluck, N.; Constantine, S.; White, B. SAT0303 design of phase 3 study of lenabasum for the treatment of dermatomyositis. Ann. Rheum. Dis. 2019, 78, 1228. [Google Scholar]
- Corbus Pharmaceuticals. Lenabasum [Corbus Pharmaceuticals Pipeline]. Corbus Pharmaceuticals. Available online: https://www.corbuspharma.com/our-science/lenabasum (accessed on 18 February 2020).
- Arena Pharmaceuticals. Olorinab (APD371) [Arena Pharmaceuticals Pipeline]. Arena Pharmaceuticals. Available online: https://www.arenapharm.com/pipeline/apd371/ (accessed on 18 February 2020).
- Artelo Biosciences. ART27.13 [Artelo Development Pipeline]. Artelo Development. Available online: Http://artelobio.com/pipeline (accessed on 18 February 2020).
- Kale, V.P.; Gibbs, S.; Taylor, J.A.; Zmarowski, A.; Novak, J.; Patton, K.; Sparrow, B.; Gorospe, J.; Anand, S.; Cinar, R.; et al. Preclinical toxicity evaluation of JD5037, a peripherally restricted CB1 receptor inverse agonist, in rats and dogs for treatment of nonalcoholic steatohepatitis. Regul. Toxicol. Pharmacol. 2019, 109, 104483. [Google Scholar] [CrossRef] [PubMed]
- Corbus Pharmaceuticals. CRB-4001 [Corbus Pharmaceuticals Pipeline]. Corbus Pharmaceuticals. Available online: https://www.corbuspharma.com/our-science/crb-4001 (accessed on 18 February 2020).
- Deng, H.; Li, W. Monoacylglycerol Lipase Inhibitors: Modulators for Lipid Metabolism in Cancer Malignancy, Neurological and Metabolic Disorders. Acta Pharm. Sin. B 2019. [Google Scholar] [CrossRef]
- Huggins, J.P.; Smart, T.S.; Langman, S.; Taylor, L.; Young, T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012, 153, 1837–1846. [Google Scholar] [PubMed]
- Postnov, A.; Schmidt, M.E.; Pemberton, D.J.; de Hoon, J.; Van Hecken, A.; van den Boer, M.; Zannikos, P.; van der Ark, P.; Palmer, J.A.; Rassnick, S.; et al. Fatty acid amide hydrolase inhibition by JNJ-42165279: A multiple-ascending dose and a positron emission tomography study in healthy volunteers. Clin. Transl. Sci. 2018, 11, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, A.; Ferré, J.C.; Fillatre, P.; Ronzière, T.; Vannier, S.; Carsin-Nicol, B.; Lavoué, S.; Vérin, M.; Gauvrit, J.Y.; Le Tulzo, Y.; et al. Acute neurologic disorder from an inhibitor of fatty acid amide hydrolase. New Engl. J. Med. 2016, 375, 1717–1725. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, P.; Chiche, D.; Brown, W.; Miller, J.; Treister, R.; Leff, R.; Walker, P.; Katz, N. NEO6860, modality-selective TRPV1 antagonist: A randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain. Pain Rep. 2018, 3, e696. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Treanor, J.J.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef]
- Werth, V.; Hejazi, E.; Pena, S.; Haber, J.; Okawa, J.; Feng, R.; Gabre, K.; Concha, J.; Cornwall, C.; Dgetluck, N.; et al. FRI0470 A phase 2 study of safety and efficacy of lenabasum (JBT-101), a cannabinoid receptor type 2 agonist, in refractory skin-predominant dermatomyositis. Ann. Rheum. Dis. 2018, 77, 763–764. [Google Scholar]
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.; Domsic, R.; Hsu, V.; Furst, D.; Gordon, J.; Mayes, M.; Simms, R.; et al. OP0006 Safety and efficacy of lenabasum (JBT-101) in diffuse cutaneous systemic sclerosis subjects treated for one year in an open-label extension of trial jbt101-ssc-001. Ann. Rheum. Dis. 2018, 77, 52. [Google Scholar]
- Marcellino, D.; Carriba, P.; Filip, M.; Borgkvist, A.; Frankowska, M.; Bellido, I.; Tanganelli, S.; Müller, C.E.; Fisone, G.; Lluis, C.; et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology 2008, 54, 815–823. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, M.; Sánchez-Blázquez, P.; Merlos, M.; Garzón-Niño, J. Endocannabinoid control of glutamate NMDA receptors: The therapeutic potential and consequences of dysfunction. Oncotarget 2016, 7, 55840–55862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musella, A.; Fresegna, D.; Rizzo, F.; Gentile, A.; Bullitta, S.; De Vito, F.; Guadalupi, L.; Centonze, D.; Mandolesi, G. A novel crosstalk within the endocannabinoid system controls GABA transmission in the striatum. Sci. Rep. 2017, 7, 7363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haj-Dahmane, S.; Shen, R.Y. Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology 2011, 61, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Befort, K. Interactions of the opioid and cannabinoid systems in reward: Insights from knockout studies. Front. Pharmacol. 2015, 6, 6. [Google Scholar] [PubMed] [Green Version]
- Carvalho, A.F.; Van Bockstaele, E.J. Cannabinoid modulation of noradrenergic circuits: Implications for psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
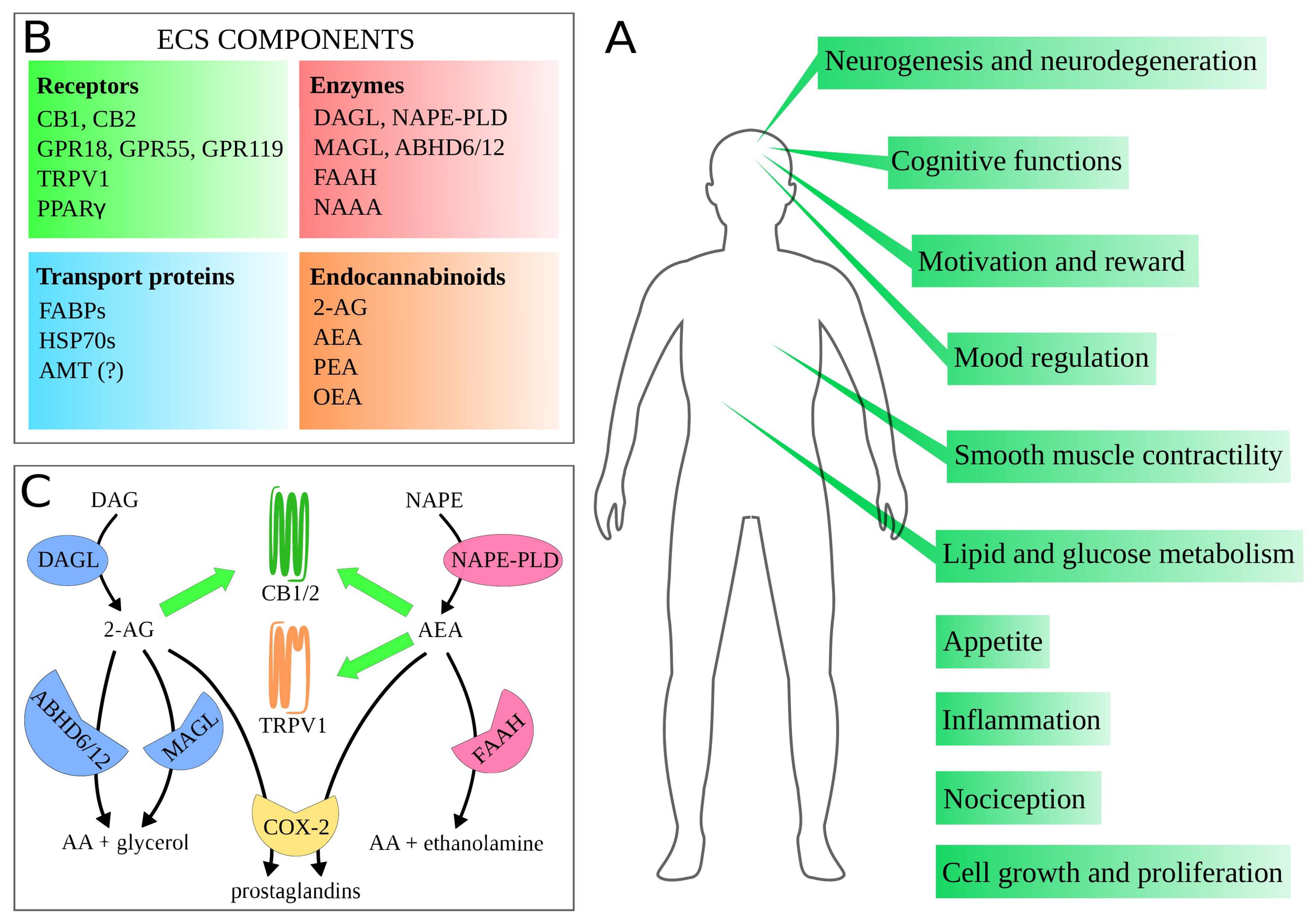
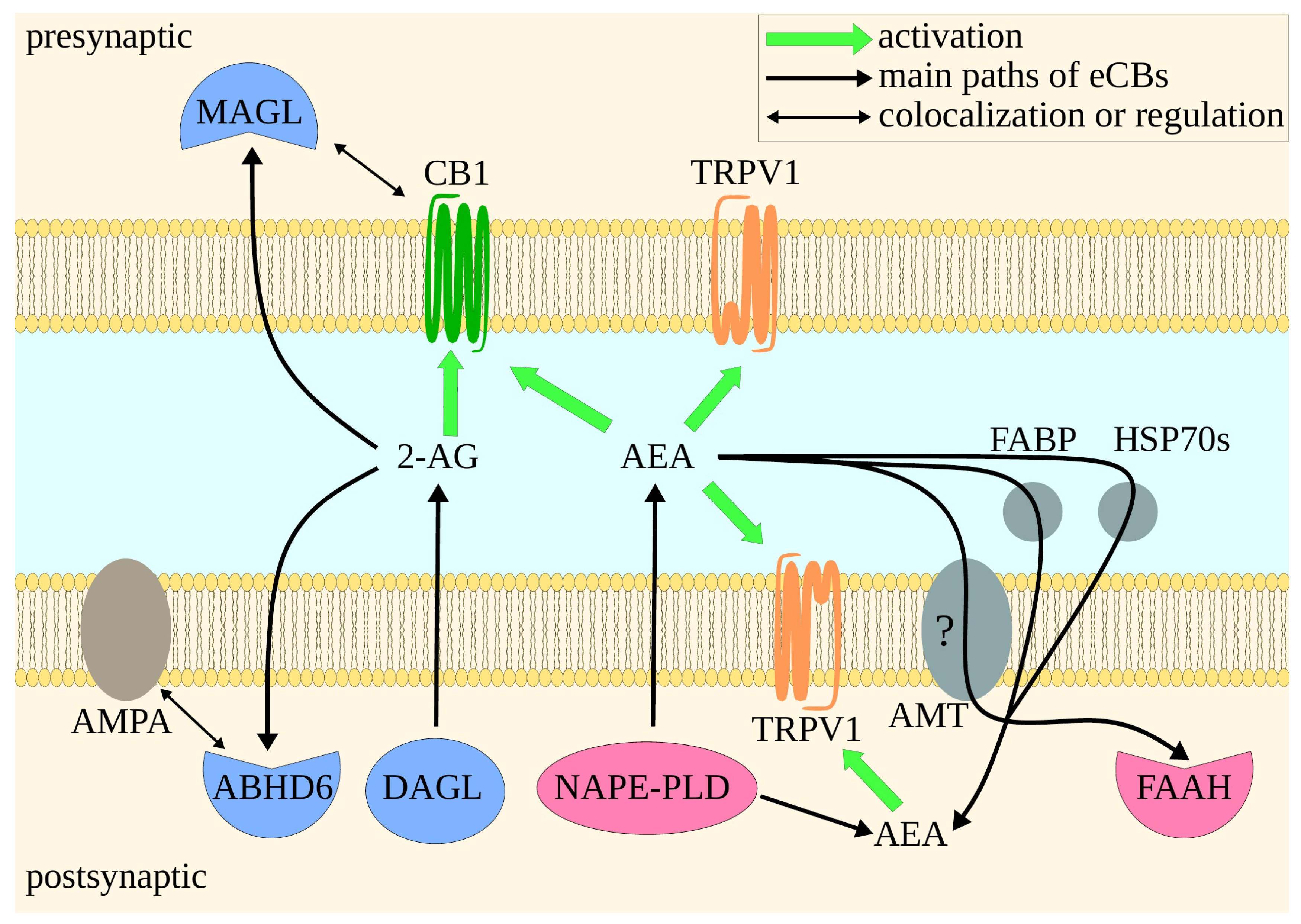
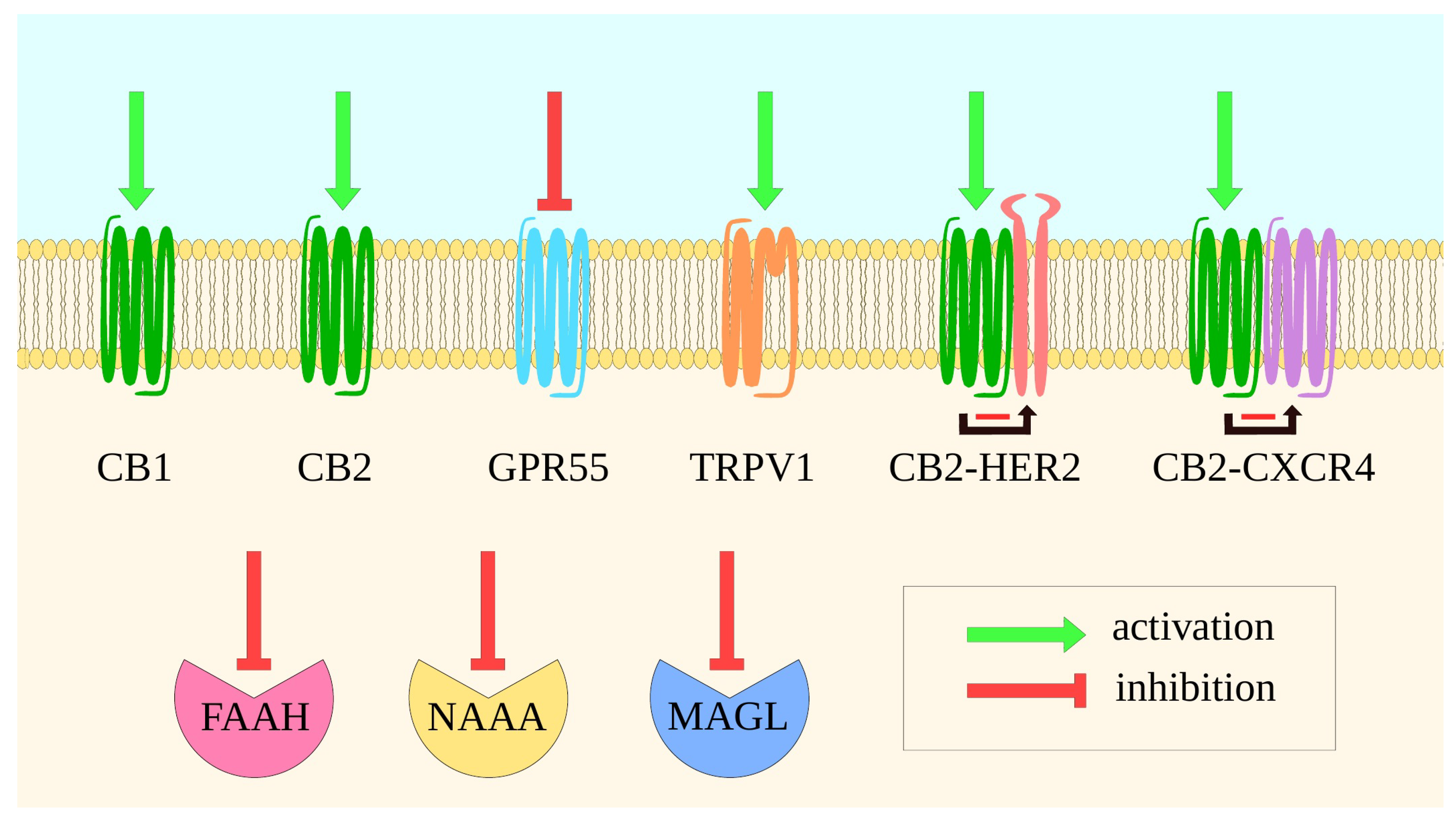
| Protein | Ligand Type | Remarks | Evidence | References |
|---|---|---|---|---|
| Pain | ||||
| CB1 | Agonist | Preferable CB1 peripheral agonists or CB1 PAMs | Well grounded | [78,79] |
| CB2 | Agonist | In addition, CB2 PAMs | [80,81] | |
| MAGL | Inhibitor | [82,84] | ||
| FAAH | Inhibitor | [83] | ||
| TRPV1 | Antagonist | [159] | ||
| AEA reuptake proteins | Inhibitor | [85] | ||
| Seizures | ||||
| CB1 | Agonist | Well grounded | [91] | |
| MAGL | Inhibitor | [92] | ||
| TRPV1 | Antagonist | [92,93] | ||
| ABHD6 | Inhibitor | [92] | ||
| AEA reuptake proteins | Inhibitor | [92] | ||
| TRPV1 | Agonist | Limited evidence | [98] | |
| Anxiety | ||||
| CB1 | Agonist | Well grounded | [111,115,118] | |
| CB2 | Agonist | [111,118] | ||
| MAGL | Inhibitor | [111] | ||
| FAAH | Inhibitor | [115,116] | ||
| TRPV1 | Agonist | [118] | ||
| ine CB1 | Antagonist | CB1 in lateral habenula | Limited evidence | [119] |
| FAAH | Enhancer | FAAH in basolateral complex of amygdala | [117] | |
| Depression | ||||
| CB1 | Agonist | Well grounded | [121] | |
| MAGL | Inhibitor | [160] | ||
| FAAH | Inhibitor | [121,126] | ||
| CB1 | Antagonist | Short-term | Limited evidence | [123,124] |
| CB2 | Agonist | [121] | ||
| CB2 | Antagonist | [123] | ||
| Addiction | ||||
| CB1 | Antagonist | Preferable neutral antagonist or peripheral antagonist/inverse agonist | Well grounded | [129,130] |
| CB2 | Agonist | [141,143] | ||
| ine CB1 | Agonist | CB1 in insula; systemic in withdrawal syndrome | Limited evidence | [134,136] |
| CB2 | Antagonist | [143] | ||
| MAGL | Inhibitor | MAGL in insula | [136] | |
| Cognitive functions | ||||
| CB1 | Agonist | Very complex topic, more reasearch needed | [154,157] | |
| CB1 | Antagonist | [146,147,148] | ||
| CB2 | Agonist | [156,157] | ||
| MAGL | Inhibitor | [156] | ||
| FAAH | Inhibitor | [158] | ||
| Name of the Active Ingredient | Mechanism of Action | Indications | Status | Remarks | References Clinical Trial IDs |
|---|---|---|---|---|---|
| Dronabinol (THC) | CB1 and CB2 partial agonist | Nausea and emesis associated with cancer chemotherapy Anorexia associated with weight loss in AIDS | FDA approved | Problems with therapeutic window | [309,327,328,329] |
| Nabilone | CB1 and CB2 partial agonist | Nausea and emesis associated with cancer chemotherapy | FDA approved | Problems with therapeutic window | [330,331] |
| CBD | CB1 negative allosteric modulator, TRPV1 agonist, GPR55 antagonist, 5-HT1A agonist, interacts with multiple other proteins | Lennox-Gastaut syndrome and Dravet syndrome (forms of epilepsy) | FDA approved | [40,100,101,102,103] | |
| THC + CBD | See: dronabinol and CBD | Spasticity and pain in multiple sclerosis Cancer pain | Approved in multiple countries | [335,336] | |
| Rimonabant | CB1 inverse agonist | Obesity | Withdrawn | Psychiatric adverse effects including depression and anxiety; subsequent suicides | [10] |
| JBT-101 (Lenabasum) | CB2 agonist | Diffuse cutaneous systemic sclerosis Dermatomyositis | Phase III trials | [338] NCT03398837 NCT03813160 | |
| Cystic fibrosis Systemic sclerosis Systemic lupus erythematosus | Phase II trials | [349,350] NCT02465450 NCT02465437 NCT04043455 | |||
| APD371 (Olorinab) | Peripheral CB2 agonist | Abdominal pain in Crohn’s disease Irritable bowel syndrome | Phase II trials | [339] NCT03155945 NCT04043455 | |
| NEO1940 (ART27.13) | Peripheral CB1 and CB2 agonist | Cancer related anorexia | Phase II trial planned | Phase I trials showed desired weight gain | [340] |
| CRB-4001 | Peripheral CB1 inverse agonist | Nonalcoholic steatohepatitis | Phase I trials planned | [341,342] | |
| ABX-1431 | MAGL inhibitor | Tourette syndrome Chronic motor tic disorder | Phase II trial | [343] NCT03625453 | |
| NEO6860 | TRPV1 antagonist | Osteoarthritis pain | Phase II trial | Phase I trials showed analgesic activity without hyperthermia; not effective in phase II trial | [159,347] NCT02712957 NCT02337543 |
| Protein | Ligand Type | Indication | Risks | References |
|---|---|---|---|---|
| CB1 | Agonist | Pain | Addiction Cognitive impairment Weight gain Erectile dysfunction | [78,79] |
| Seizures | [91] | |||
| Anxiety | [111,115,118] | |||
| Depression | [121] | |||
| Withdrawal syndrome | [134] | |||
| Neurodegenerative disorders | [165,172,178] | |||
| Spasticity in multiple sclerosis | [336] | |||
| Hypertension | [246,247] | |||
| Cancer | [269,270] | |||
| Asthma | [296] | |||
| Emesis and nausea | [308,309] | |||
| Anorexia and weight loss | [327] | |||
| Duchenne muscular dystrophy | [325] | |||
| Antagonist | Addiction | Anxiety Depression Nausea | [129,130] | |
| Cognitive impairment | [146,147,148] | |||
| Systemic sclerosis | [211] | |||
| Pulmonary fibrosis | [212] | |||
| Obesity | [215,216,218,219,221] | |||
| Diabetes | [230,231] | |||
| Nonalcoholic steatohepatitis | [243] | |||
| Atherosclerosis | [257] | |||
| CB2 | Agonist | Pain | [80,81] | |
| Anxiety | [111,118] | |||
| Addiction | [141,143] | |||
| Neurodegenerative disorders | [165] | |||
| Inflammation | [192,196,208] | |||
| Rheumatoid arthritis | [192] | |||
| Atherosclerosis | [255] | |||
| Systemic sclerosis | [210,211] | |||
| Obesity | [77] | |||
| Diabetes | [234,235] | |||
| Cancer | [268,269] | |||
| Inflammatory bowel disease | [305] | |||
| Emesis and nausea | [308,310] | |||
| Osteoporosis | [44] | |||
| Antagonist | Immunoparalysis | [197] | ||
| Renal fibrosis | [213] | |||
| MAGL | Inhibitor | Pain | [82,84] | |
| Seizures | [92] | |||
| Tourette syndrome | [343] | |||
| Anxiety | [111] | |||
| Depression | [160] | |||
| Cognitive impairment | [156] | |||
| Neurodegenerative disorders | [176,183] | |||
| Cancer | [279,281,282,283] | |||
| Inflammatory bowel disease | [305] | |||
| FAAH | Inhibitor | Pain | Seizures Neurological disorder Disbalance in the kidney redox system Disbalance in phospholipid metabolism | [83] |
| Anxiety | [115,116] | |||
| Depression | [121,126] | |||
| Cognitive impairment | [158] | |||
| Neurodegenerative disorders | [179] | |||
| Inflammation | [168,204,205] | |||
| Hypertension | [250,251] | |||
| Cancer | [285,286] | |||
| Inflammatory bowel disease | [305] | |||
| TRPV1 | Agonist | Anxiety | Seizures Aggravating pulmonary arterial hypertension | [118] |
| Neurodegenerative disorders | [177] | |||
| Hypertension | [253,254] | |||
| Cancer | [275,285] | |||
| Emesis and nausea | [310] | |||
| Osteoporosis | [44] | |||
| Antagonist | Pain | Hyperthermia | [84,159] | |
| Seizures | [92,93] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiulewicz, A.; Znajdek, K.; Grudzień, M.; Pawiński, T.; Sulkowska, J.I. A Guide to Targeting the Endocannabinoid System in Drug Design. Int. J. Mol. Sci. 2020, 21, 2778. https://doi.org/10.3390/ijms21082778
Stasiulewicz A, Znajdek K, Grudzień M, Pawiński T, Sulkowska JI. A Guide to Targeting the Endocannabinoid System in Drug Design. International Journal of Molecular Sciences. 2020; 21(8):2778. https://doi.org/10.3390/ijms21082778
Chicago/Turabian StyleStasiulewicz, Adam, Katarzyna Znajdek, Monika Grudzień, Tomasz Pawiński, and Joanna I. Sulkowska. 2020. "A Guide to Targeting the Endocannabinoid System in Drug Design" International Journal of Molecular Sciences 21, no. 8: 2778. https://doi.org/10.3390/ijms21082778
APA StyleStasiulewicz, A., Znajdek, K., Grudzień, M., Pawiński, T., & Sulkowska, J. I. (2020). A Guide to Targeting the Endocannabinoid System in Drug Design. International Journal of Molecular Sciences, 21(8), 2778. https://doi.org/10.3390/ijms21082778





