Platinum Group Elements Recovery from Used Catalytic Converters by Acidic Fusion and Leaching
Abstract
1. Introduction
2. Method, Material, and Instrumentation
2.1. Material and Instrumentation
- CE PGE concentration in liquid phase after fusion and leaching (μg/mL)
- Co PGE content in catalytic converters (μg/g)
- m mass of catalytic converters used in leaching (g)
- V leaching agent volume (mL)
2.2. Method
3. Results and Discussion
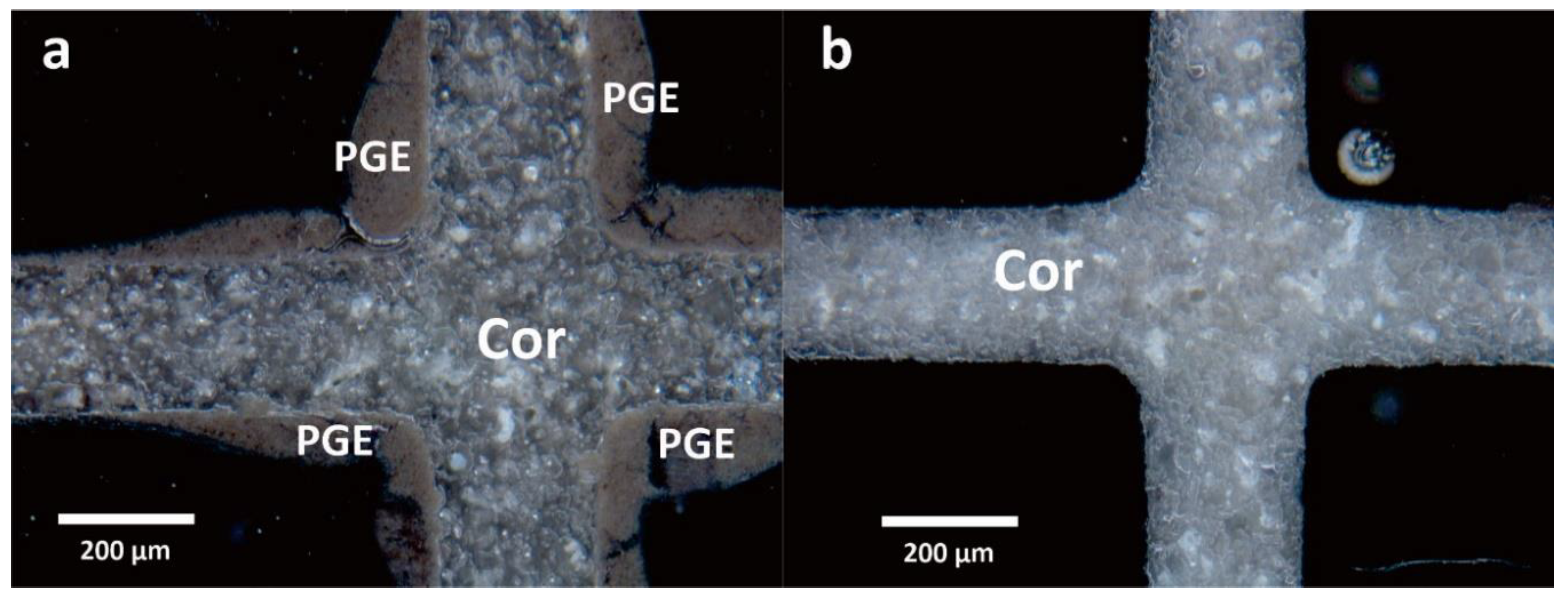
3.1. Characterization Results
3.2. Acidic Fusion Using KHSO4
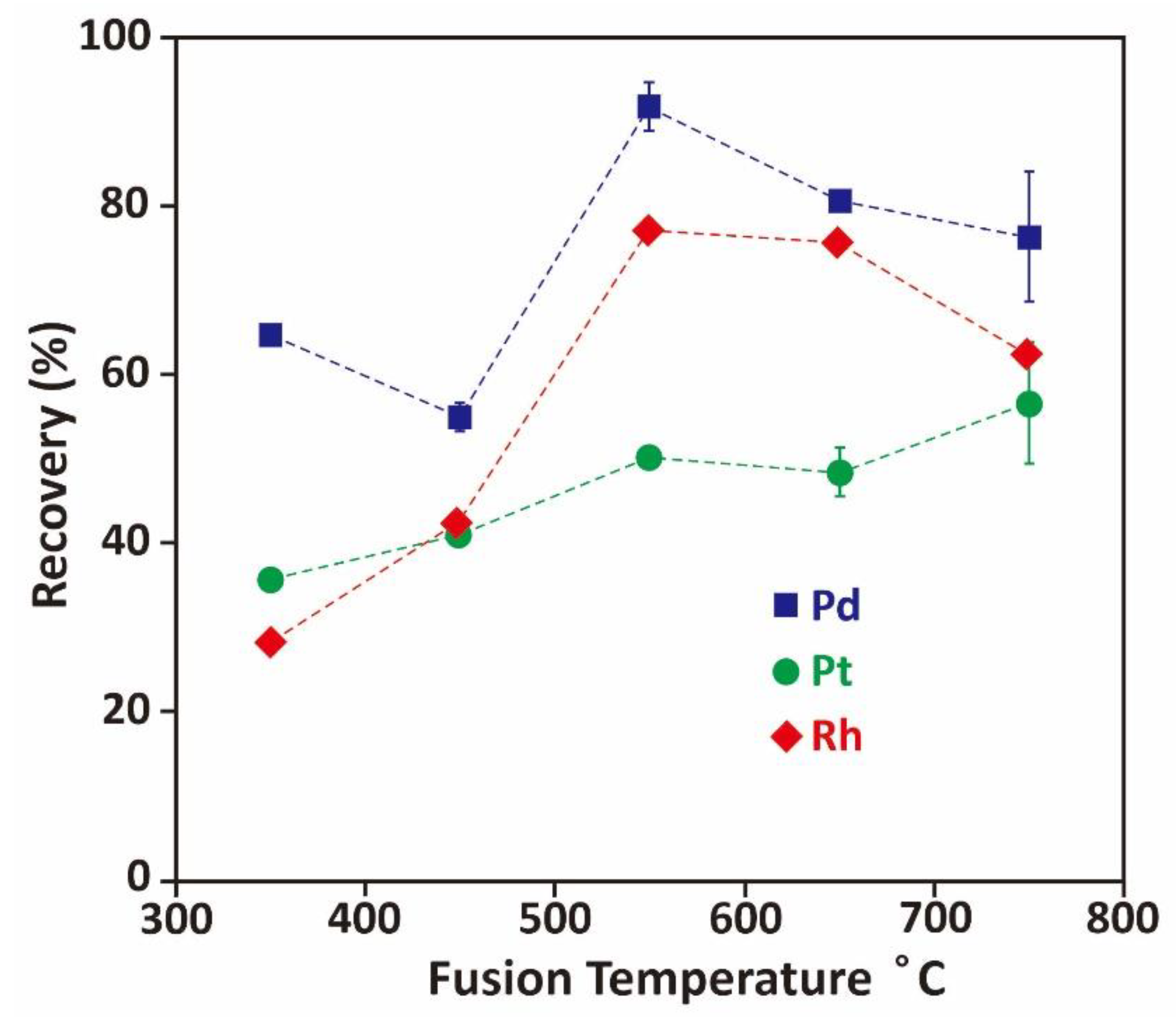
3.2.1. Effect of Fusion Temperature
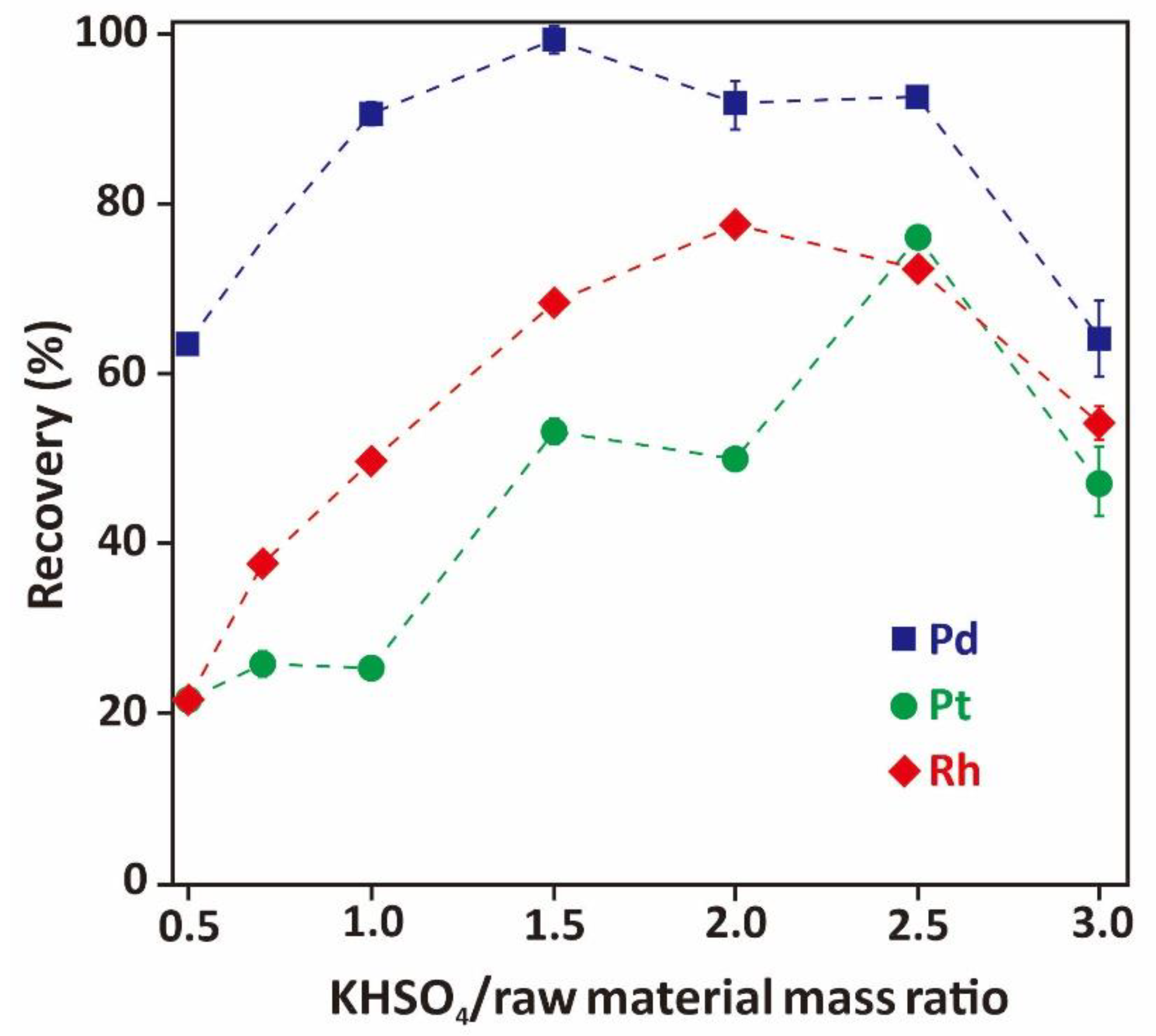
3.2.2. Effect of Mass Ratio
3.2.3. Effect of Fusion Time
3.3. Acidic Leaching using Hydrochloric Acid after KHSO4 Fusion
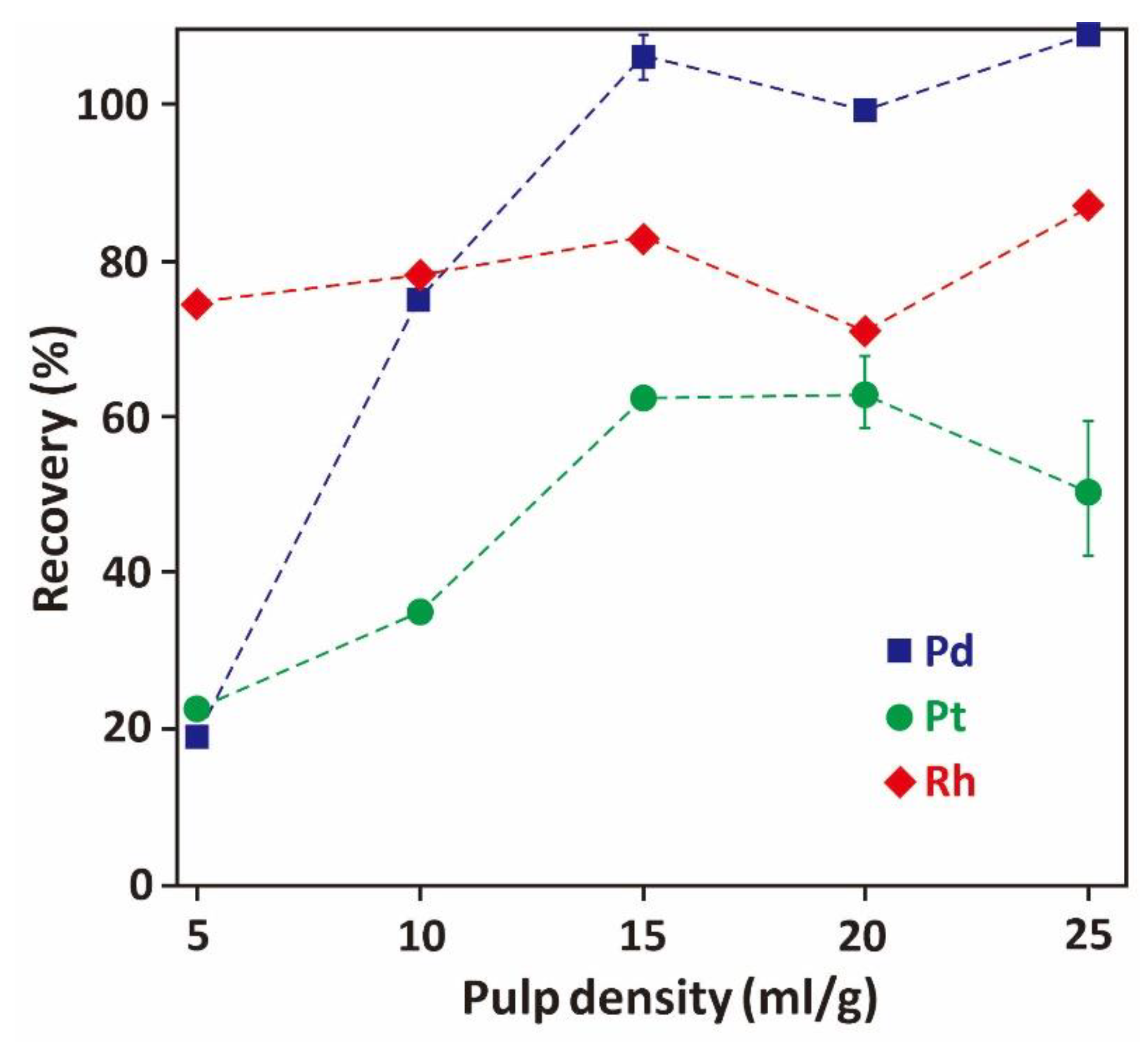
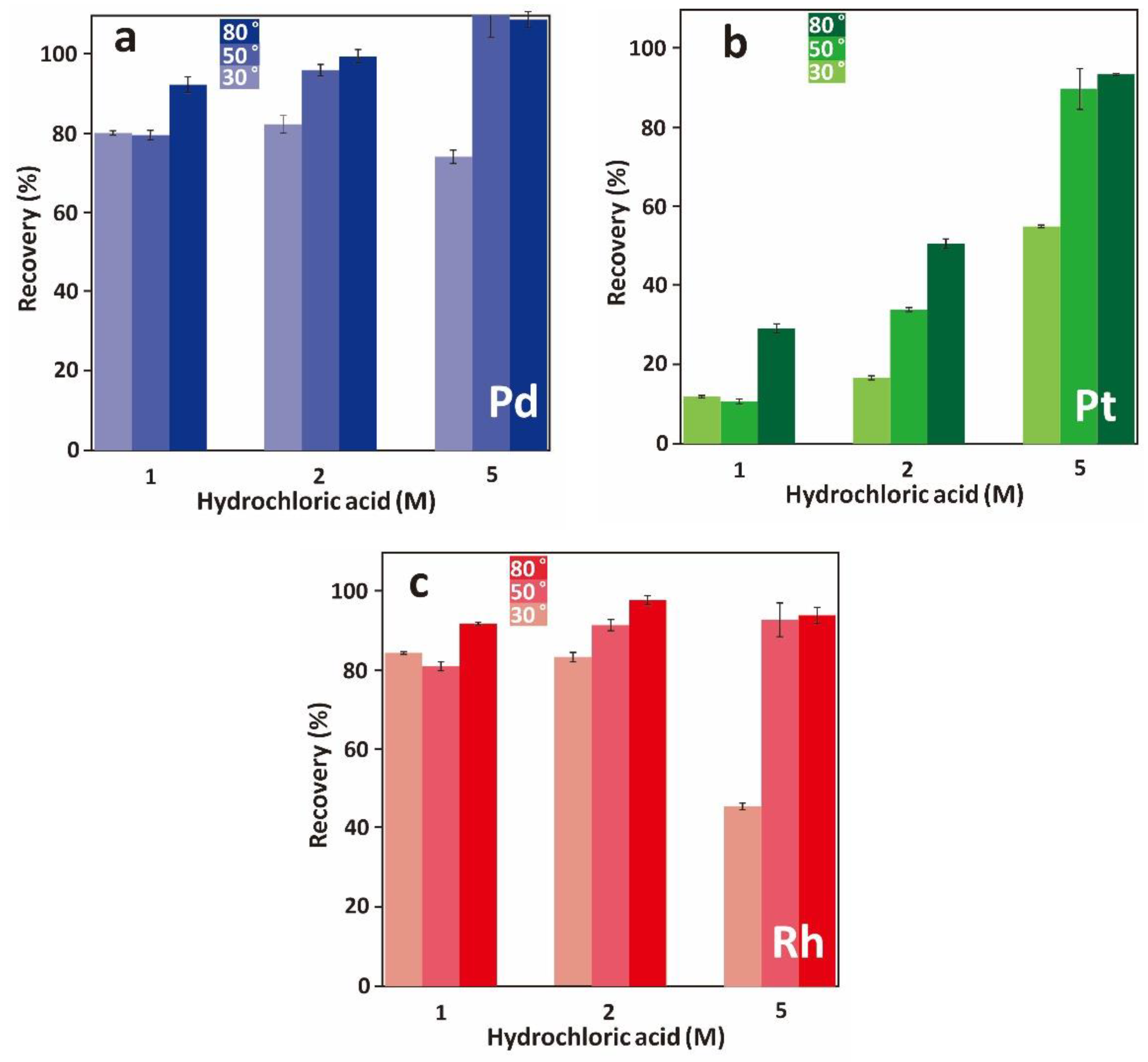
3.3.1. Effect of Leaching Pulp Density
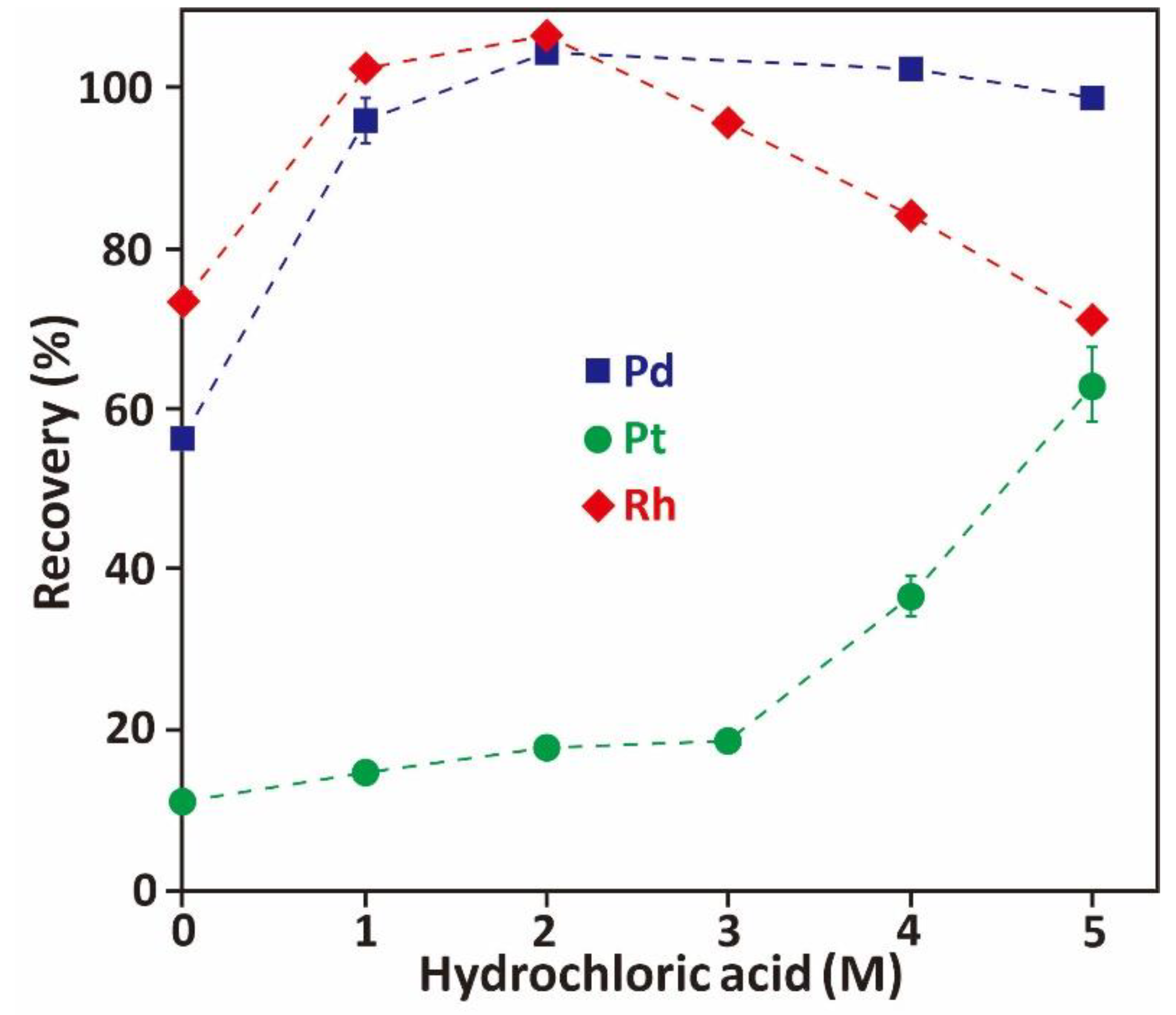
3.3.2. Effect of Hydrochloric Acid Concentration
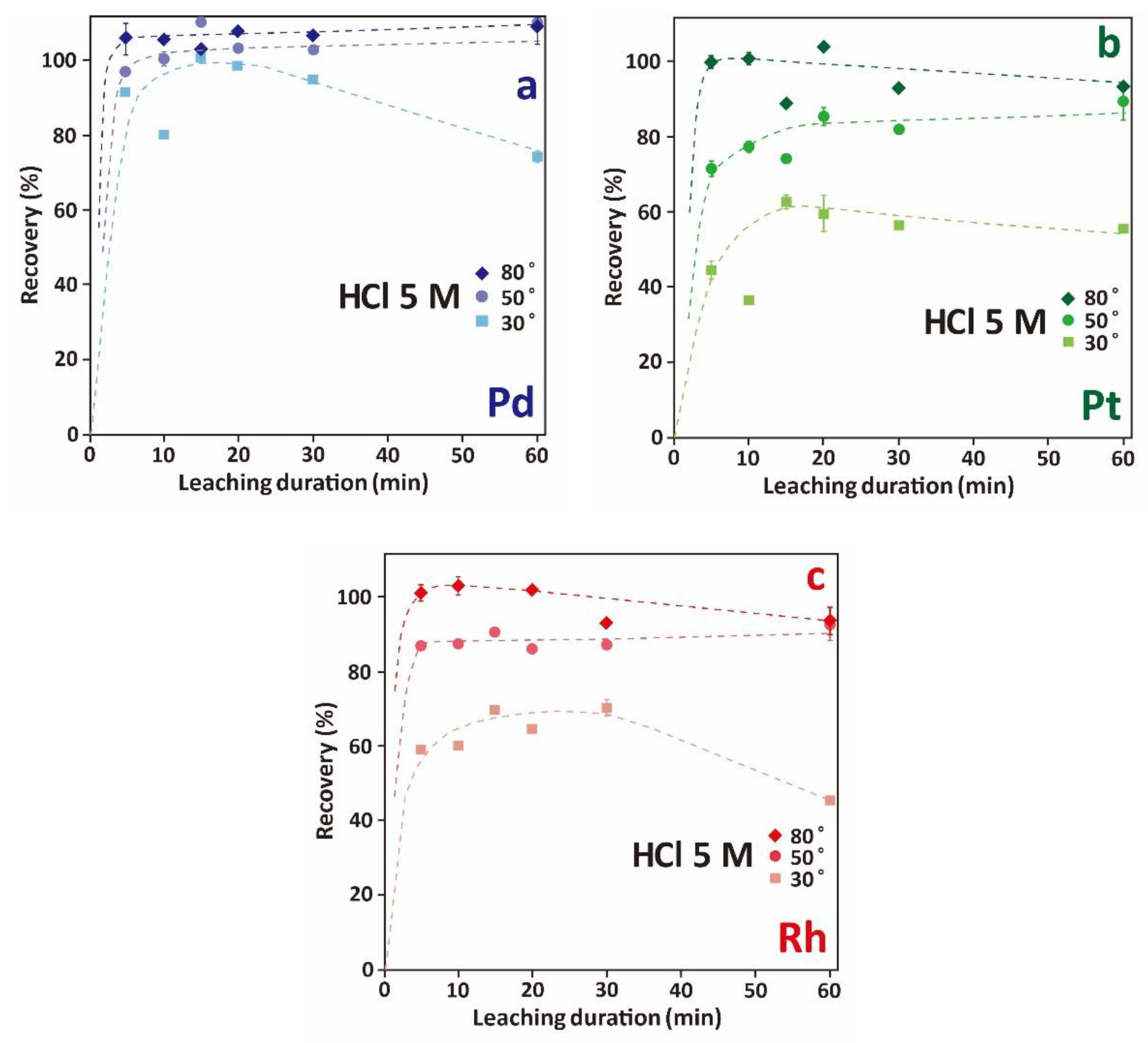
3.3.3. Effect of Leaching Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mudd, G.M.; Jowitt, S.M.; Werner, T.T. Global platinum group element resources, reserves and mining—A critical assessment. Sci. Total Environ. 2018, 622, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Loferski, P.C.; Ghalayini, Z.T.; Singerling, S.A. Platinum-Group Metals—2016; ADVANCE RELEASE; US Geological Survey: Reston, VA, USA, 2016.
- Dodson, J.R.; Hunt, A.J.; Parker, H.L.; Yang, Y.; Clark, J.H. Elemental sustainability: Towards the total recovery of scarce metals. Chem. Eng. Process. Process. Intensif. 2012, 51, 69–78. [Google Scholar] [CrossRef]
- Saguru, C.; Ndlovu, S.; Moropeng, D. A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometallurgy 2018, 182, 44–56. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Z.; Lin, X.; Tang, H.; Ye, L.; Ma, Y.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recovery of platinum group metals from spent catalysts. JOM 2017, 69, 1553–1562. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Q.; Liu, Y.; Xu, Z. Novel approach for recovery of palladium in spent catalyst from automobile by a capture technology of eutectic copper. J. Clean. Prod. 2019, 239, 118093. [Google Scholar] [CrossRef]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recycl. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Kolliopoulos, G.; Balomenos, E.; Giannopoulou, I.; Yakoumis, I.; Panias, D. Behavior of platinum group during their pyrometallurgical recovery from spent automotive catalysts. OALib 2014, 1, 1–9. [Google Scholar] [CrossRef]
- Fujima, K.; Morimoto, T.; Itoigawa, H.; Becchaku, D. Development of a process for recycling platinum group metals using molten alkali metal chlorides. J. Jpn. Inst. Met. Mater. 2017, 81, 168–177. [Google Scholar] [CrossRef]
- Yoshimura, A.; Matsuno, Y. A fundamental study of platinum recovery from spent auto catalyst using dry Aqua Regia. J. Jpn. Inst. Met. Mater. 2019, 83, 23–29. [Google Scholar] [CrossRef]
- Ding, Y.; Zheng, H.; Zhang, S.; Liu, B.; Wu, B.; Jian, Z. Highly efficient recovery of platinum, palladium, and rhodium from spent automotive catalysts via iron melting collection. Resour. Conserv. Recycl. 2020, 155, 104644. [Google Scholar] [CrossRef]
- Kim, C.H.; Woo, S.I.; Jeon, S.H. Recovery of platinum-group metals from recycled automotive catalytic converters by carbochlorination. Ind. Eng. Chem. Res. 2000, 39, 1185–1192. [Google Scholar] [CrossRef]
- Marinho, R.S.; da Silva, C.N.; Afonso, J.C.; da Cunha, J.W.S.D. Recovery of platinum, tin and indium from spent catalysts in chloride medium using strong basic anion exchange resins. J. Hazard. Mater. 2011, 192, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Harjanto, S.; Cao, Y.; Shibayama, A.; Naitoh, I.; Nanami, T.; Kasahara, K.; Okumura, Y.; Liu, K.; Fujita, T. Leaching of Pt, Pd and Rh from automotive catalyst residue in various chloride based solutions. Mater. Trans. 2006, 47, 129–135. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Lee, J.c.; Kim, E.y.; Kim, M.s.; Kim, B.S.; Kumar, V. Leaching of platinum group metals (PGMs) from spent automotive catalyst using electro-generated chlorine in HCl solution. J. Chem. Technol. Biotechnol. 2013, 88, 1991–1999. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.W.; Lee, J.C.; Choubey, P.K. A novel zero emission concept for electrogenerated chlorine leaching and its application to extraction of platinum group metals from spent automotive catalyst. Hydrometallurgy 2016, 159, 19–27. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Paiva, A.P.; Oliveira, P.C.; Costa, M.C.; da Costa, A.M.R. Oxidative leaching process with cupric ion in hydrochloric acid media for recovery of Pd and Rh from spent catalytic converters. J. Hazard. Mater. 2014, 278, 82–90. [Google Scholar] [CrossRef]
- Chen, J.; Huang, K. A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometallurgy 2006, 82, 164–171. [Google Scholar] [CrossRef]
- Shams, K.; Beiggy, M.R.; Shirazi, A.G. Platinum recovery from a spent industrial dehydrogenation catalyst using cyanide leaching followed by ion exchange. Appl. Catal. A Gen. 2004, 258, 227–234. [Google Scholar] [CrossRef]
- Mishra, R.K. PGM recoveries by atmospheric and autoclave leaching of alumina bead catalyst. Precious Met. 1987, 1987, 177–195. [Google Scholar]
- Yang, M.C.; Sun, E.T.; Zhou, Y.J.; He, X.K. A new process for recovery of Pt and Al from Pt-containing waste catalyst. Precious Met. 1996, 17, 20–24. [Google Scholar]
- Batista, S.G.; Afonso, J.C. Processing of spent platinum-based catalysts via fusion with potassium hydrogenosulfate. J. Hazard. Mater. 2010, 184, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, S.; Zhang, L.; Peng, J. Optimization of the microwave roasting extraction of palladium and rhodium from spent automobile catalysts using response surface analysis. Int. J. Miner. Process. 2015, 143, 18–24. [Google Scholar] [CrossRef]
- Wang, S.; Chen, A.; Zhang, Z.; Peng, J. Leaching of palladium and rhodium from spent automobile catalysts by microwave roasting. Environ. Prog. Sustain. Energy 2014, 33, 913–917. [Google Scholar] [CrossRef]
- Spooren, J.; Atia, T.A. Combined microwave assisted roasting and leaching to recover platinum group metals from spent automotive catalysts. Miner. Eng. 2020, 146, 106153. [Google Scholar] [CrossRef]
- Gaines, P. Samples Containing Titanium. 2020. Available online: https://www.inorganicventures.com/sample-preparation-guide/samples-containing-titanium (accessed on 9 March 2020).
- Pley, M.; Wickleder, M.S. Pt2(HSO4)2(SO4)2, the first binary sulfate of platinum. J. Inorg. Normal Chem. 2004, 630, 1036–1039. [Google Scholar]
- Bruns, J.; Klüner, T.; Wickleder, M.S. Oxidizing elemental platinum with oleum under harsh conditions: The unique tris(disulfato)platinate(IV) [Pt(S2O7)3] 2-anion. Chem. A Eur. J. 2014, 20, 7222–7227. [Google Scholar] [CrossRef]
- Schwarzer, S.; Betke, A.; Logemann, C.; Wickleder, M.S. Oxidizing Rhodium with Sulfuric Acid: The Sulfates Rh2(SO4)3 and Rh2(SO4)3·2H2O. Eur. J. Inorg. Chem. 2017, 2017, 752–758. [Google Scholar] [CrossRef]
- Hinds, J.I.D. Inorganic Chemistry: With the Elements of Physical and Theoretical Chemistry; John Wiley & Sons: New York, NY, USA, 1908. [Google Scholar]
- Smith, G.F.; Gring, J.L. The Separation and Determination of the Alkali Metals Using Perchloric Acid. V. Perchloric Acid and Chloroplatinic Acid in the Determination of Small Amounts of Potassium in the Presence of Large Amounts of Sodium. J. Am. Chem. Soc. 1933, 55, 3957–3961. [Google Scholar] [CrossRef]
- Williams, M.L. CRC Handbook of Chemistry and Physics, 76th edition. Occup. Environ. Med. 1996, 53, 504. [Google Scholar] [CrossRef]



| Elements | Pd | Pt | Rh |
|---|---|---|---|
| Content (μg/g) | 112.0 ± 1.7 | 1299.4 ± 3.5 | 316.0 ± 6.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasetyo, E.; Anderson, C. Platinum Group Elements Recovery from Used Catalytic Converters by Acidic Fusion and Leaching. Metals 2020, 10, 485. https://doi.org/10.3390/met10040485
Prasetyo E, Anderson C. Platinum Group Elements Recovery from Used Catalytic Converters by Acidic Fusion and Leaching. Metals. 2020; 10(4):485. https://doi.org/10.3390/met10040485
Chicago/Turabian StylePrasetyo, Erik, and Corby Anderson. 2020. "Platinum Group Elements Recovery from Used Catalytic Converters by Acidic Fusion and Leaching" Metals 10, no. 4: 485. https://doi.org/10.3390/met10040485
APA StylePrasetyo, E., & Anderson, C. (2020). Platinum Group Elements Recovery from Used Catalytic Converters by Acidic Fusion and Leaching. Metals, 10(4), 485. https://doi.org/10.3390/met10040485





