An Approach for Examining the Impact of Food Group-Based Sources of Nutrients on Outcomes with Application to PUFAs and LDL in Youth with Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Development of Food Groups
2.3. Lipid Levels
2.4. Other Variables
2.5. Statistical Analysis
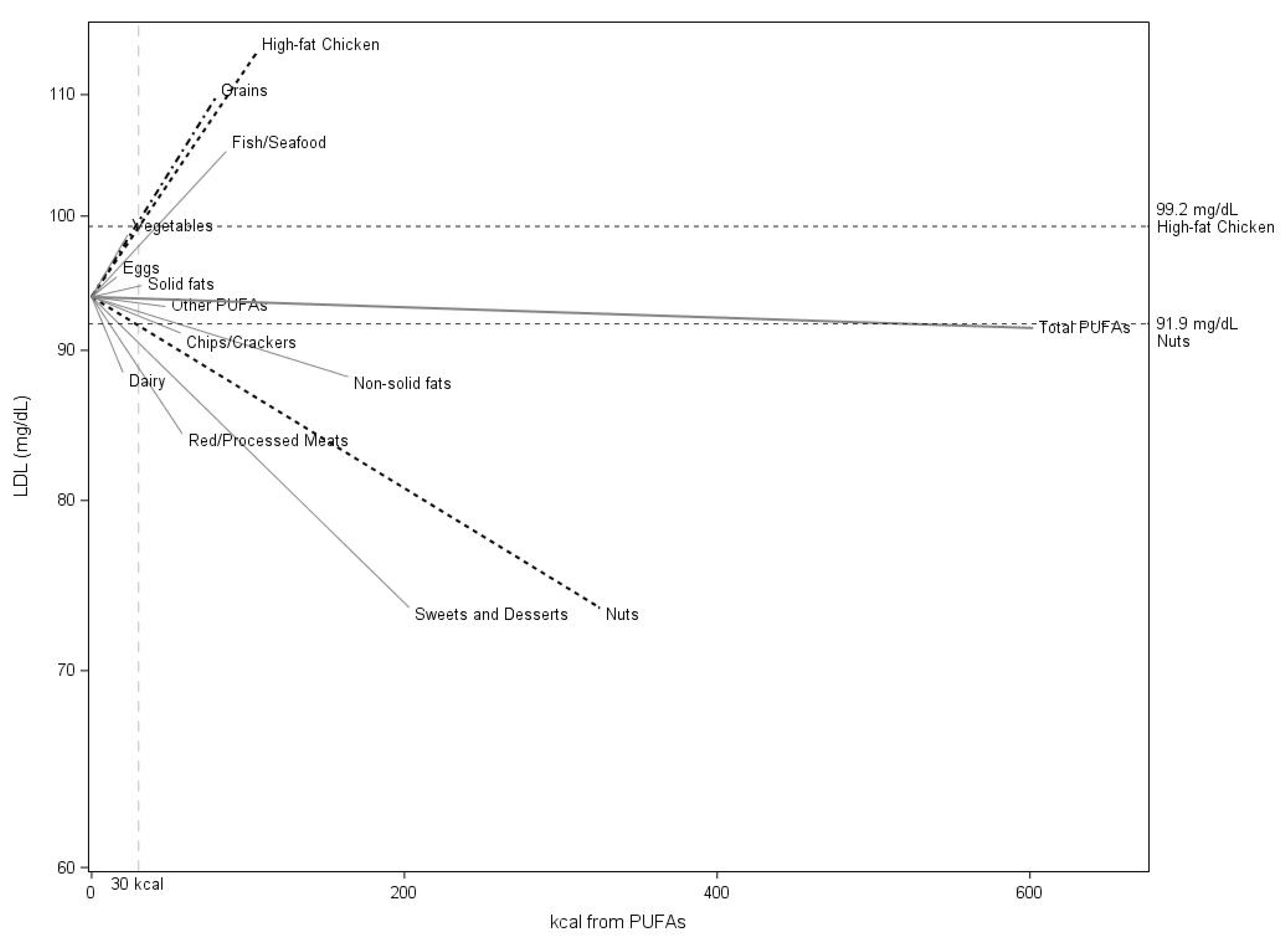
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baum, S.J.; Kris-Etherton, P.M.; Willett, W.C.; Lichtenstein, A.H.; Rudel, L.L.; Maki, K.C.; Whelan, J.; Ramsden, C.E.; Block, R.C. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipid 2012, 6, 216–234. [Google Scholar] [CrossRef]
- Czernichow, S.; Thomas, D.; Bruckert, E. N-6 fatty acids and cardiovascular health: A review of the evidence for dietary intake recommendations. Br. J. Nutr. 2010, 104, 788–796. [Google Scholar] [CrossRef]
- Clifton, P.M.; Keogh, J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1060–1080. [Google Scholar] [CrossRef]
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Haldorssoni, T.; Riserus, U.; Uusitupa, M.; Becker, W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014, 58. [Google Scholar] [CrossRef]
- Kipnis, V.; Freedman, L.S.; Brown, C.C.; Hartman, A.; Schatzkin, A.; Wacholder, S. Interpretation of energy adjustment models for nutritional epidemiology. Am. J. Epidemiol. 1993, 137, 1376–1380. [Google Scholar] [CrossRef]
- De Oliveira Otto, M.C.; Mozaffarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef]
- Praagman, J.; Beulens, J.W.; Alssema, M.; Zock, P.L.; Wanders, A.J.; Sluijs, I.; van der Schouw, Y.T. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Am. J. Clin. Nutr. 2016, 103, 356–365. [Google Scholar] [CrossRef]
- Kulldorff, M.; Sinha, R.; Chow, W.H.; Rothman, N. Comparing odds ratios for nested subsets of dietary components. Int. J. Epidemiol. 2000, 29, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, E.J.; Hirte, C.; Couper, J.J. Dietary fats do not contribute to hyperlipidemia in children and adolescents with type 1 diabetes. Diabetes Care 2003, 26, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kuratsune, H.; Nitta, H.; Kawahara, K.; Hamano, M.; Matsuda, M.; Kaku, K.; Eto, M. Plasma lipid levels and nutritional intake in childhood- and adolescence-onset young type 1 patients in Japan. Diabetes Res. Clin. Pract. 2006, 73, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Couch, S.C.; Crandell, J.L.; Peairs, A.; Liese, A.D.; Shah, A.S.; Dolan, L.M.; Tooze, J.; Crume, J.; King, I.; Mayer-Davis, E.J. Associations between long chain polyunsaturated fatty acids and cardiovascular risk factors in youth with type 1 diabetes (T1D): SEARCH Nutrition Ancillary Study. J. Diabetes Complicat. 2017, 31, 67–73. [Google Scholar] [CrossRef] [PubMed]
- SEARCH Study Group. SEARCH for Diabetes in Youth: A multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control. Clin. Trials 2004, 25, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Hamman, R.F.; Bell, R.A.; Dabelea, D.; D’Agostino, R.B., Jr.; Dolan, L.; Imperatore, G.; Lawrence, J.M.; Linder, B.; Marcovina, S.M.; Mayer-Davis, E.J.; et al. The SEARCH for Diabetes in Youth study: Rationale, findings, and future directions. Diabetes Care 2014, 37, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Nichols, M.; Liese, A.D.; Bell, R.A.; Dabelea, D.M.; Johansen, J.M.; Pihoker, C.; Rodriguez, B.L.; Thomas, J.; Williams, D.; SEARCH for Diabetes in Youth Study Group. Dietary intake among youth with diabetes: The SEARCH for Diabetes in Youth Study. J. Am. Diet. Assoc. 2006, 106, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.D.; Crandell, J.L.; Tooze, J.A.; Fangman, M.T.; Couch, S.C.; Merchant, A.T.; Bell, R.A.; Mayer-Davis, E.J. Relative validity and reliability of a food frequency questionnaire in youth with type 1 diabetes. Publ. Health Nutr. 2015, 28, 1–10. [Google Scholar]
- Hainline, A., Jr.; Miller, D.T.; Mather, A. The Coronary Drug Project. Role and methods of the central laboratory. Control. Clin. Trials 1983, 4, 377–387. [Google Scholar] [CrossRef]
- Grieco, E.M.; Cassidy, R.C. Overview of Race and Hispanic Origin: Census 2000 Brief; US Department of Commerce, Economic and Statistics Administration, US Census Bureau: Suitland-Silver Hill, MD, USA, 2001.
- Sabaté, J.; Oda, K.; Ros, E. Nut Consumption and Blood Lipid Levels: A Pooled Analysis of 25 Intervention Trials. Arch. Intern. Med. 2010, 170, 821–827. [Google Scholar] [CrossRef]
- Nutrition Data System (NDS) for Research, version 4.05/33; database 3; Nutrition Coordinating Center, University of Minnesota: Minneapolis, MN, USA, 2002.
- Sanjeevi, N.; Lipsky, L.M.; Nansel, T.R. Cardiovascular biomarkers in association with dietary intakes in a longitudinal study of youth with type 1 diabetes. Nutrients 2018, 10, 1552. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hu, F.B.; Ros, E.; Sabaté, J. The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J. Nutr. 2008, 138, 1746S–1751S. [Google Scholar] [CrossRef]
- Dobarganes, C.; Marquez-Ruiz, G. Possible adverse effects of frying with vegetable oils. Br. J. Nutr. 2015, 113 (Suppl. 2), S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Albuquerque, T.; Oliveira, M.B.; Sanches-Silva, A.; Cristina Bento, A.; Costa, H.S. The impact of cooking methods on the nutritional quality and safety of chicken breaded nuggets. Food. Funct. 2016, 7, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Juhaimi, F.A.; Ghafoor, K.; Babiker, E.E.; Özcan, M.M.; Aadiamo, O.Q.; Alsawmahi, O.N. Influence of Storage and Roasting on the Quality Properties of Kernel and Oils of Raw and Roasted Peanuts. J. Oleo. Sci. 2018, 67, 755–762. [Google Scholar] [CrossRef] [PubMed]
| Food Group | Constituents of Food Group | % PUFA Intakes from Food Group | Primary Sources of PUFAs in Food Group (% PUFAs in Food Group) 1 |
|---|---|---|---|
| Nonsolid Fats | salad dressings, canola oil, corn oil, cottonseed oil, olive oil, soybean oil, vegetable oil | 28.1 | Soybean oil (35.9%), Ranch salad dressing regular (24.8%), unknown oil (20.3%), mayonnaise or mayo type dressing (10.1%) |
| Nuts | almonds, cashews, peanut butter, peanuts, walnuts | 13.5 | Roasted peanuts (54.7%), peanut butter (31.4%), walnuts (9.7%) |
| Grains | bagel, biscuit, bread, cornbread, taco shell, tortilla, buns, cereal, egg rolls, starch, French toast, grits, hush puppies, cornstarch, flour, pancakes, pita, waffles, granola, oatmeal, pasta, noodles, rice | 11.3 | White bread (15.7%), wheat bread (10.5%), pancakes (10.3%), flour tortilla (7.4%) |
| Red and Processed Meats | beef, hamburger, chili, pork, venison, bacon, bologna, corn dog, ham, hot dogs, lunch meats, sausage | 8.9 | Pork sausage (22.0%), hamburger (10.7%), pork ribs (9.6%), bologna (7.1%), bacon (7.0%), hot dogs (7.0%), Vienna sausage (6.6%) |
| High-fat Chicken | chicken nuggets (fast food), chicken with skin | 7.5 | Fast food chicken nuggets (69.6%), chicken (light or dark) with skin (19.4%), chicken (light or dark) with skin removed (6.9%) |
| Low-fat Chicken | Chicken, breast, skin removed before cooking | 0.3 | Chicken, breast, skin removed before cooking (100%) |
| Sweets and Desserts | fruit roll ups, cinnamon buns, cakes, candy, cookies, doughnuts, high fructose corn syrup, honey, ice cream, jelly, pies, pudding, sugar, syrups, turnovers | 7.7 | Cake (28.3%), cookies (26.9%), doughnuts (21.3%), ice cream and frozen desserts (5.7%) |
| Chips and Crackers | corn chips, potato chips, snack crackers, popcorn, pretzels | 5.9 | Snack crackers (45.0%), potato chips (24.0%), corn chips (19.1%), peanut-butter filled sandwich (5.1%) |
| Solid Fats | butter, shortening, animal fats, margarine, animal gravy, lard | 3.3 | Margarine (70.0%), vegetable shortening (15.7%), poultry gravy (5.9%), pork fat (5.9%) |
| Dairy | cheese, cottage cheese, whey, milk, buttermilk, cream, yogurt | 3.2 | Cheddar cheese (35.4%), 2% or reduced fat milk (20.4%), whole milk yogurt (13.5%), whole milk (8.7%), mozzarella cheese (5.8%) |
| Vegetables | broccoli, collards, salad, spinach, turnip greens, bok choy, mustard greens, parsley, spinach, Brussels sprouts, cabbage, cauliflower, relish, green beans, peppers, yeast, okra onion, squash, celery, garlic, ginger, lettuce, mushrooms, peas, potatoes, carrots, corn, BBQ sauce, catsup, salsa, tomato, tomato sauce/paste | 2.4 | Mashed potatoes (41.2%), corn (7.2%), green peas (5.7%) |
| Fish and Seafood | fish, seafood | 2.3 | Tuna salad with mayo (no egg) (54.1%), salmon (37.1%) |
| Eggs | eggs | 1.8 | Cooked whole eggs (52.6%), Ingredient whole eggs (29.8%), ingredient egg yolk (6.8%), boiled eggs (5.5%) |
| Sports Bars | granola bars, cereal bars, power bars | 1.1 | Cereal bars (65.5%), granola bars (31.9%) |
| Meal Replacements | meal replacement products, bars and drinks | 1.0 | Breakfast bars (74.4%), energy snack bars (14.0%), low calorie meal replacement drink (11.6%) |
| Fruit | apricot, bananas, figs, grapes, mango, plantains, prune, raisins, orange, tangerine, apple, applesauce, berries, cantaloupe, fruit cocktail, melon, papaya, peach, pear, persimmon, fruit juices, vinegar | 0.9 | Plantains (32.9%), banana (10.8%), orange juice (10.7%), apple (8.4%), apple cider (7.4%) |
| Soy | soy protein concentrate, soy milk, soy sauce, Worcestershire sauce, tofu, soybeans | 0.5 | Cooked tofu (78.4%), soy milk (18.3%) |
| Legumes | baked beans, refried beans, kidney beans, garbanzo beans | 0.2 | Refried beans (68.4%), baked beans (22.0%) |
| Characteristics | (n = 1435) | |
|---|---|---|
| n | % | |
| Gender | ||
| Female | 730 | 50.9 |
| Male | 705 | 49.1 |
| Race | ||
| White | 1147 | 79.9 |
| Black | 103 | 7.2 |
| Other | 185 | 12.9 |
| Mean | SD | |
| Age (years) | 14.5 | 2.9 |
| Duration of diabetes (months) | 43.4 | 42.7 |
| non-PUFA fats, kcal/day | 508.4 | 241.1 |
| Protein, kcal/day | 279.7 | 121.2 |
| Carbohydrates, kcal/day | 839.1 | 356.9 |
| Fiber, g/1000 kcal | 7.9 | 2.4 |
| Energy, kcal/day | ||
| Female, 10–13 years | 1613 | 666 |
| Male, 10–13 years | 1824 | 682 |
| Female, 14–22 years | 1590 | 633 |
| Male, 14–22 years | 2020 | 807 |
| n-3 and n-6 PUFAs | n-3 PUFAs | n-6 PUFAs | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Nonsolid Fats | 38.0 | 24.9 | 4.3 | 2.8 | 33.8 | 22.1 |
| Nuts | 18.3 | 33.3 | 0.4 | 0.9 | 18.0 | 32.5 |
| Grains | 15.3 | 9.7 | 1.3 | 0.8 | 14.0 | 8.8 |
| Red and Processed Meats | 11.7 | 9.2 | 1.0 | 0.8 | 10.7 | 8.5 |
| Sweets and Desserts | 10.5 | 13.9 | 1.1 | 1.4 | 9.4 | 12.6 |
| High-fat Chicken | 10.0 | 12.9 | 1.0 | 1.3 | 9.0 | 11.6 |
| Chips and Crackers | 8.0 | 7.8 | 0.5 | 0.6 | 7.5 | 7.3 |
| Solid Fats | 4.5 | 4.2 | 0.4 | 0.4 | 4.1 | 3.8 |
| Dairy | 4.2 | 2.8 | 1.2 | 1.0 | 3.0 | 1.9 |
| Vegetables | 3.2 | 2.9 | 0.6 | 0.6 | 2.6 | 2.5 |
| Fish and Seafood | 3.0 | 6.2 | 0.8 | 1.7 | 2.1 | 4.5 |
| Eggs | 2.5 | 2.6 | 0.1 | 0.2 | 2.3 | 2.4 |
| Sports Bars | 1.5 | 2.7 | 0.1 | 0.2 | 1.4 | 2.5 |
| Meal Replacements | 1.3 | 2.2 | 0.2 | 0.3 | 1.2 | 1.9 |
| Fruit | 1.2 | 1.2 | 0.3 | 0.2 | 1.0 | 1.0 |
| Soy | 0.6 | 2.2 | 0.1 | 0.3 | 0.5 | 1.9 |
| Low-fat Chicken | 0.4 | 0.7 | <0.1 | 0.1 | 0.4 | 0.7 |
| Legumes | 0.2 | 0.5 | 0.1 | 0.2 | 0.1 | 0.3 |
| Beverages and Broths | <0.1 | 0.1 | 0.0 | 0.0 | <0.1 | 0.1 |
| Other1 | 5.3 | 5.6 | 0.7 | 0.7 | 4.6 | 5.0 |
| Total | 134.4 | 67.6 | 13.5 | 6.5 | 121.0 | 61.8 |
| Parameter | Estimate 2 | SE | p-Value |
|---|---|---|---|
| Intercept | 4.5418 | 0.0495 | <0.0001 |
| PUFA intakes from food group 3: | |||
| Sweets/ Desserts | −0.0120 | 0.0080 | 0.1342 |
| Grains | 0.0197 | 0.0100 | 0.0495 |
| Dairy | −0.0296 | 0.0415 | 0.4763 |
| Nuts | −0.0075 | 0.0034 | 0.0293 |
| Red/Processed meat | −0.0185 | 0.0158 | 0.2400 |
| Eggs | 0.0098 | 0.0308 | 0.7506 |
| Nonsolid fats | −0.0038 | 0.0037 | 0.3069 |
| Fats | 0.0028 | 0.0198 | 0.8883 |
| Chips/Crackers | −0.0050 | 0.0099 | 0.6156 |
| Fish/Seafood | 0.0133 | 0.0126 | 0.2913 |
| High-fat Chicken | 0.0182 | 0.0065 | 0.0053 |
| Vegetables | 0.0211 | 0.0301 | 0.4829 |
| Other | −0.0016 | 0.0146 | 0.9127 |
| Fats, non-PUFA | 0.0042 | 0.0017 | 0.0111 |
| Protein | −0.0078 | 0.0024 | 0.0014 |
| Carbohydrates | 0.0002 | 0.0004 | 0.5845 |
| Total Fiber (g/1000 kcal) | −0.0031 | 0.0038 | 0.4134 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tooze, J.A.; The, N.S.; Crandell, J.L.; Couch, S.C.; Mayer-Davis, E.J.; Koebnick, C.; Liese, A.D. An Approach for Examining the Impact of Food Group-Based Sources of Nutrients on Outcomes with Application to PUFAs and LDL in Youth with Type 1 Diabetes. Nutrients 2020, 12, 941. https://doi.org/10.3390/nu12040941
Tooze JA, The NS, Crandell JL, Couch SC, Mayer-Davis EJ, Koebnick C, Liese AD. An Approach for Examining the Impact of Food Group-Based Sources of Nutrients on Outcomes with Application to PUFAs and LDL in Youth with Type 1 Diabetes. Nutrients. 2020; 12(4):941. https://doi.org/10.3390/nu12040941
Chicago/Turabian StyleTooze, Janet A., Natalie S. The, Jamie L. Crandell, Sarah C. Couch, Elizabeth J. Mayer-Davis, Corinna Koebnick, and Angela D. Liese. 2020. "An Approach for Examining the Impact of Food Group-Based Sources of Nutrients on Outcomes with Application to PUFAs and LDL in Youth with Type 1 Diabetes" Nutrients 12, no. 4: 941. https://doi.org/10.3390/nu12040941
APA StyleTooze, J. A., The, N. S., Crandell, J. L., Couch, S. C., Mayer-Davis, E. J., Koebnick, C., & Liese, A. D. (2020). An Approach for Examining the Impact of Food Group-Based Sources of Nutrients on Outcomes with Application to PUFAs and LDL in Youth with Type 1 Diabetes. Nutrients, 12(4), 941. https://doi.org/10.3390/nu12040941





