Comprehensive Genome Analysis on the Novel Species Sphingomonas panacis DCY99T Reveals Insights into Iron Tolerance of Ginseng
Abstract
:1. Introduction
2. Results
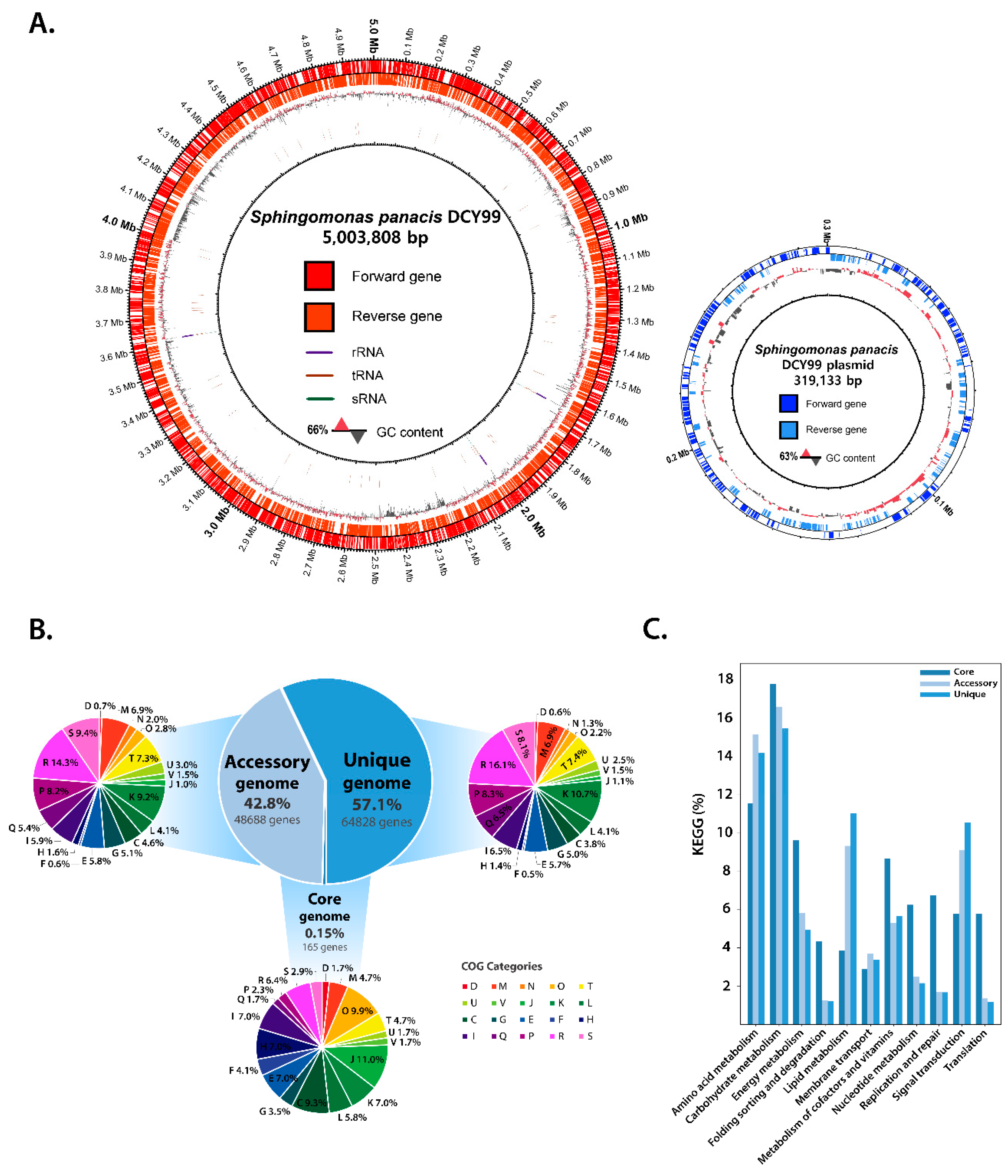
2.1. Reconstruction of the S. panacis DCY99T Genome
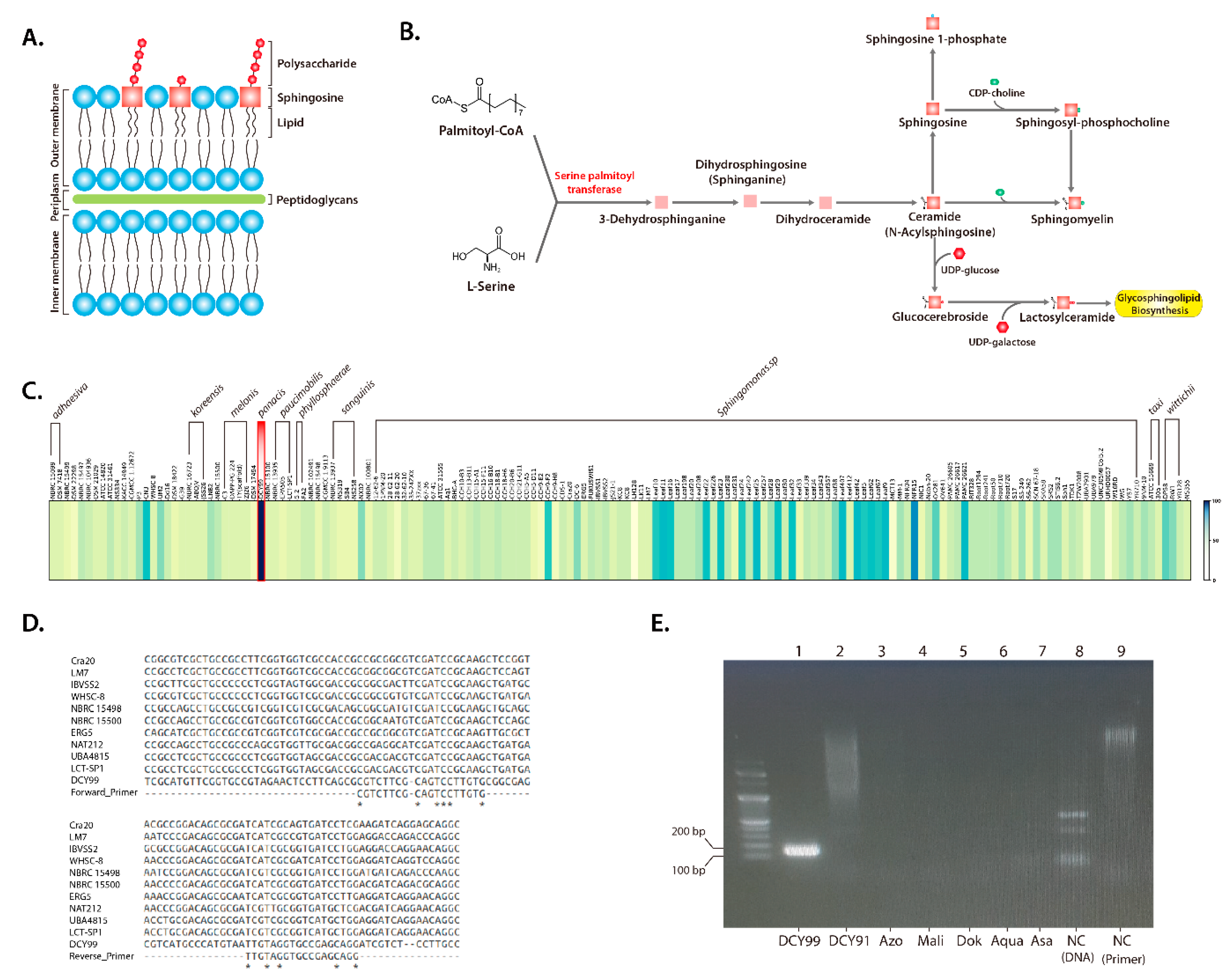
2.2. Pan-Genome and Functional Genome Analysis with the 159 Sphingomonas Genomes
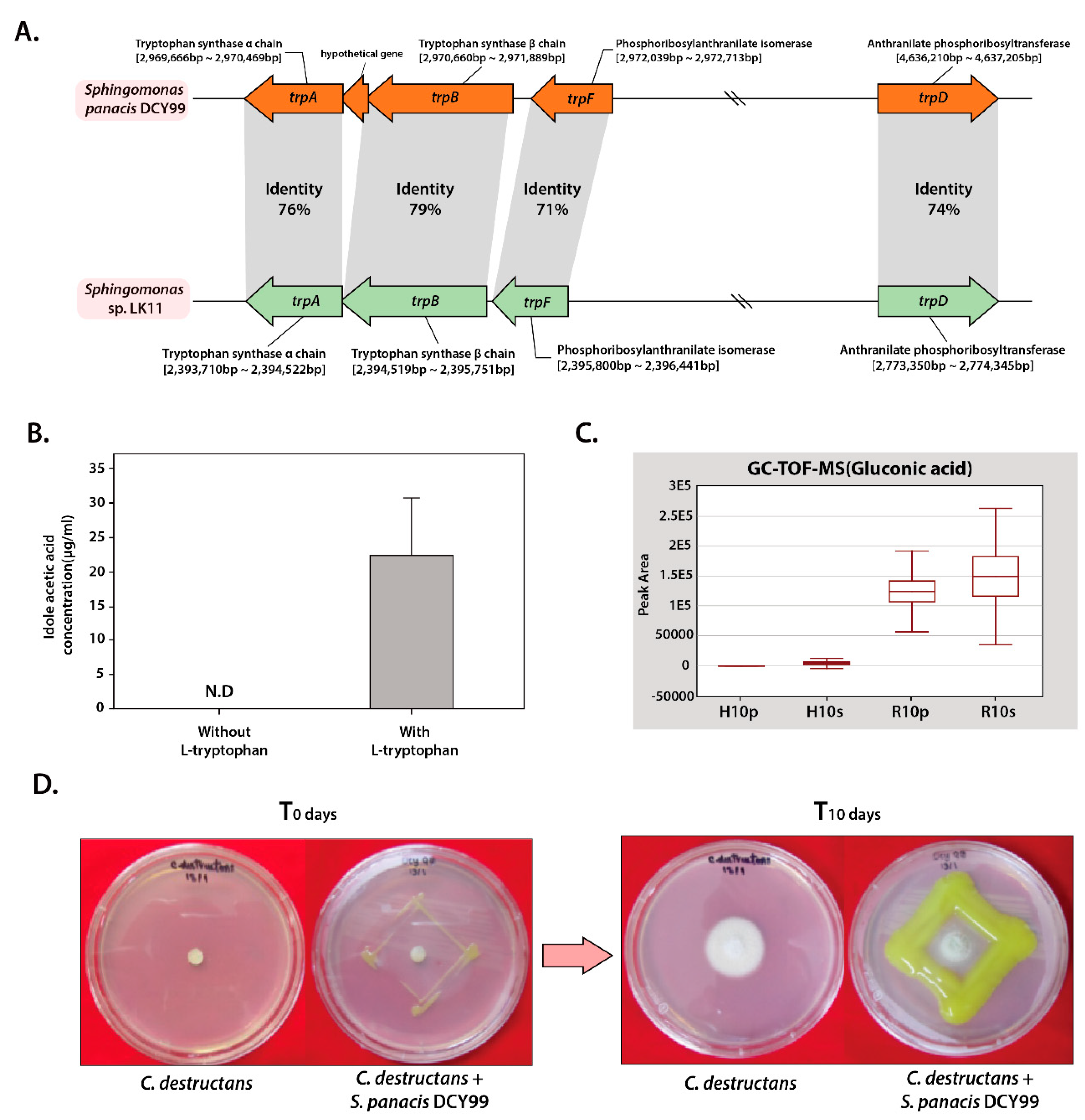
2.3. Plant Growth-Promoting Potential of S. panacis DCY99T
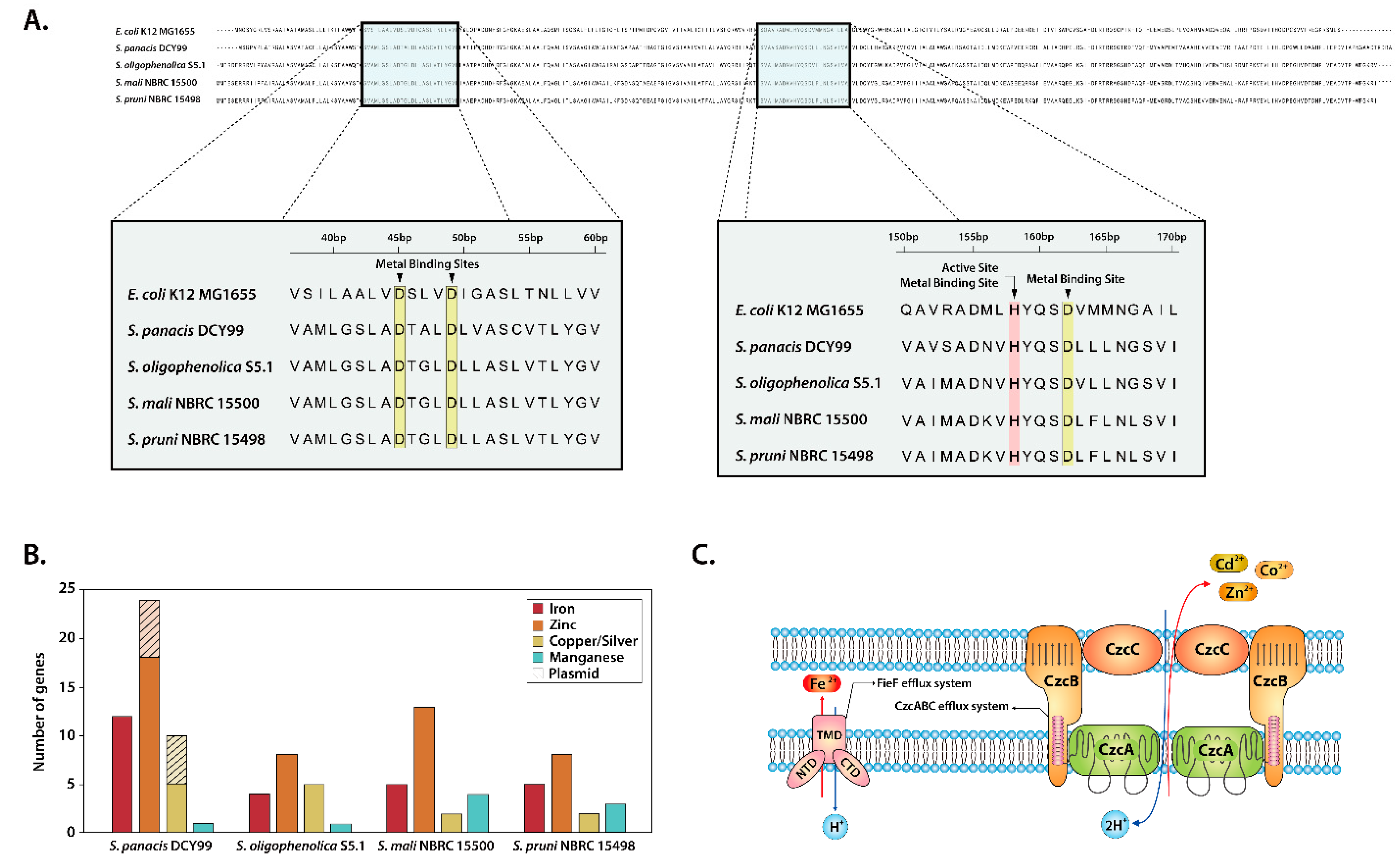
2.4. Heavy Metal Resistance of S. panacis DCY99T
2.5. Improvement of P. ginseng Meyer Growth with S. panacis DCY99T under Biotic/Abiotic Stress
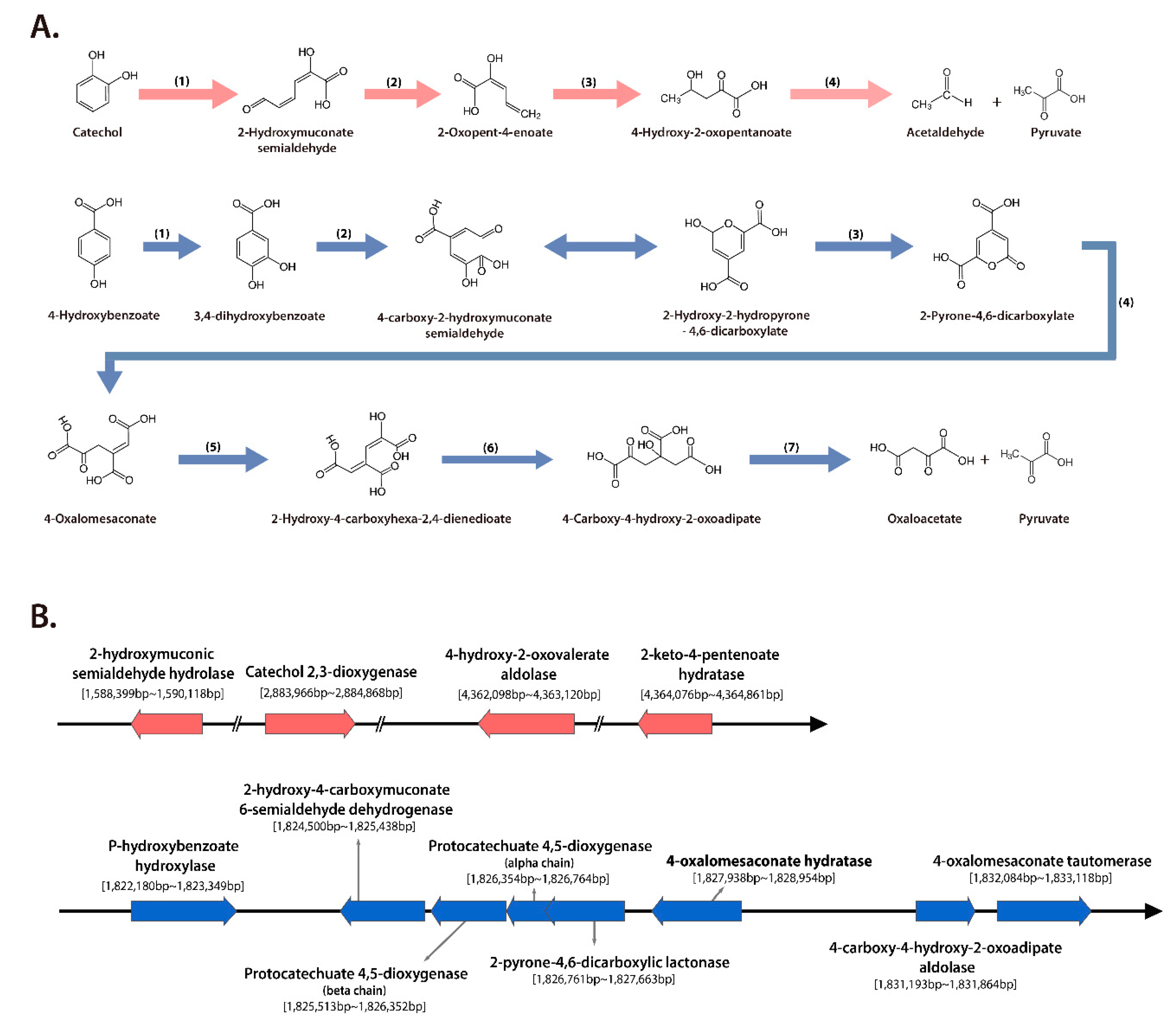
2.6. Phenolic Compounds and 3-hydroxybutanoic Acid Degradation by S. panacis DCY99T
2.7. Design of a S. panacis DCY99T Biomarker
3. Discussion
4. Materials and Methods
4.1. Sphingomonas Strains and Genomic Analysis
4.2. Plant Material and Culture Conditions
4.3. S. panacis DCY99T Genome Analysis
4.4. Clusters of Orthologous Groups (COG) Analysis
4.5. Genome Comparisons
4.6. In Vitro Plant Growth-Promotion and Product Assays
4.7. S. panacis DCY99T and P. ginseng Meyer Compatibility under Biotic /Abiotic Stress
4.8. GC-TOF-MS Analysis
4.9. Constructing the Heat Map
4.10. Primer Design
4.11. Primer Alignment
4.12. Quantification of S. panacis DCY99T Gene Expression
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–25. [Google Scholar]
- Hell, R.; Stephan, U.W. Iron uptake, trafficking and homeostasis in plants. Planta 2003, 216, 541–551. [Google Scholar] [CrossRef]
- Bodek, I. Environmental Inorganic Chemistry: Properties, Processes, and Estimation Methods; Pergamon Press: Pergamon, Turkey, 1988. [Google Scholar]
- De Dorlodot, S.; Lutts, S.; Bertin, P. Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J. Plant Nutr. 2005, 28, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Xu, C.; Sun, H.; Wang, J.; Li, L. Iron (Fe2+)-induced toxicity produces morphological and physiological changes in roots in Panax ginseng grown in hydroponics. Toxicol. Environ. Chem. 2016, 98, 630–637. [Google Scholar] [CrossRef]
- Balusamy, S.R.D.; Kim, Y.-J.; Rahimi, S.; Senthil, K.S.; Lee, O.R.; Lee, S.; Yang, D.-C. Transcript pattern of cytochrome P450, antioxidant and ginsenoside biosynthetic pathway genes under heavy metal stress in Panax ginseng Meyer. Bull. Environ. Contam. Toxicol. 2013, 90, 194–202. [Google Scholar] [CrossRef]
- Kang, K.S.; Yokozawa, T.; Kim, H.Y.; Park, J.H. Study on the nitric oxide scavenging effects of ginseng and its compounds. J. Agric. Food Chem. 2006, 54, 2558–2562. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.-N.; Jeong, H.-J.; Choi, I.-Y.; An, H.-J.; Moon, P.-D.; Kim, S.-J.; Jee, S.-Y.; Um, J.-Y.; Hong, S.-H.; Shin, S.-S. Mountain grown ginseng induces apoptosis in HL-60 cells and its mechanism have little relation with TNF-α production. Am. J. Chin. Med. 2007, 35, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.M.; Yance, D.; Wong, R. Natural health products that inhibit angiogenesis: A potential source for investigational new agents to treat cancer—Part 1. Curr. Oncol. 2006, 13, 14. [Google Scholar] [PubMed]
- Shin, B.-K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Punja, Z.K. Biochemistry of ginseng root tissues affected by rusty root symptoms. Plant Physiol. Biochem. 2005, 43, 1103–1114. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Gao, L.; Liu, W.; Liu, R.; Zhao, J.; You, J. Changes in element accumulation, phenolic metabolism, and antioxidative enzyme activities in the red-skin roots of Panax ginseng. J. Ginseng Res. 2017, 41, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Farh, M.E.-A.; Kim, Y.-J.; Kim, Y.-J.; Yang, D.-C. Cylindrocarpon destructans/Ilyonectria radicicola-species complex: Causative agent of ginseng root-rot disease and rusty symptoms. J. Ginseng Res. 2018, 42, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Sun, Y.; Guo, S.; Tian, S.; Liu, Z. Studies on the genesis of ginseng rust spots. J. Ginseng Res. 1997, 21, 69–77. [Google Scholar]
- Wang, Q.; Xu, C.; Sun, H.; Ma, L.; Li, L.; Zhang, D.; Zhang, Y. Analysis of the relationship between rusty root incidences and soil properties in Panax ginseng. In Proceedings of IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2016; Volume 41, p. 012001. [Google Scholar]
- Punja, Z.; Wan, A.; Goswami, R.; Verma, N.; Rahman, M.; Barasubiye, T.; Seifert, K.; Lévesque, C. Diversity of Fusarium species associated with discolored ginseng roots in British Columbia. Can. J. Plant Pathol. 2007, 29, 340–353. [Google Scholar] [CrossRef]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Imran, A.; Rahim, N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol. 2015, 6, 198. [Google Scholar] [CrossRef] [Green Version]
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856. [Google Scholar] [CrossRef] [Green Version]
- Raza, W.; Yousaf, S.; Rajer, F.U. Plant growth promoting activity of volatile organic compounds produced by biocontrol strains. Sci. Lett. 2016, 4, 40–43. [Google Scholar]
- Sukweenadhi, J.; Kim, Y.-J.; Choi, E.-S.; Koh, S.-C.; Lee, S.-W.; Kim, Y.-J.; Yang, D.C. Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol. Res. 2015, 172, 7–15. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Barzanti, R.; Ozino, F.; Bazzicalupo, M.; Gabbrielli, R.; Galardi, F.; Gonnelli, C.; Mengoni, A. Isolation and characterization of endophytic bacteria from the nickel hyperaccumulator plant Alyssum bertolonii. Microb. Ecol. 2007, 53, 306–316. [Google Scholar] [CrossRef]
- Belimov, A.; Hontzeas, N.; Safronova, V.; Demchinskaya, S.; Piluzza, G.; Bullitta, S.; Glick, B. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 2005, 37, 241–250. [Google Scholar] [CrossRef]
- Benmalek, Y.; Halouane, A.; Hacene, H.; Fardeau, M.-L. Resistance to heavy metals and bioaccumulation of lead and zinc by Chryseobacterium solincola strain 1YB-R12T isolated from soil. Int. J. Environ. Eng. 2014, 6, 68–77. [Google Scholar] [CrossRef]
- Dell’Amico, E.; Cavalca, L.; Andreoni, V. Analysis of rhizobacterial communities in perennial Graminaceae from polluted water meadow soil, and screening of metal-resistant, potentially plant growth-promoting bacteria. FEMS Microbiol. Ecol. 2005, 52, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, R.; Trifonova, R.; Puschenreiter, M.; Wenzel, W.W.; Sessitsch, A. Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl. Environ. Microbiol. 2004, 70, 2667–2677. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.-Y.; Sheng, X.-F.; Qian, M.; Wang, Q.-Y. Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 2008, 72, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Kuffner, M.; De Maria, S.; Puschenreiter, M.; Fallmann, K.; Wieshammer, G.; Gorfer, M.; Strauss, J.; Rivelli, A.; Sessitsch, A. Culturable bacteria from Zn-and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J. Appl. Microbiol. 2010, 108, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Al-Harrasi, A.; Lee, I.-J. Complete genome sequencing and analysis of endophytic Sphingomonas sp. LK11 and its potential in plant growth. 3 Biotech 2018, 8, 389. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, X.; Cao, Z.; Zhao, K.; Wang, S.; Chen, M.; Hu, X. Growth-promoting Sphingomonas paucimobilis ZJSH1 associated with D endrobium officinale through phytohormone production and nitrogen fixation. Microb. Biotechnol. 2014, 7, 611–620. [Google Scholar] [CrossRef]
- Story, S.; Kline, E.; Hughes, T.; Riley, M.; Hayasaka, S.S. Degradation of aromatic hydrocarbons by Sphingomonas paucimobilis strain EPA505. Arch. Environ. Contam. Toxicol. 2004, 47, 168–176. [Google Scholar] [CrossRef]
- Desai, A.M.; Autenrieth, R.L.; Dimitriou-Christidis, P.; McDonald, T.J. Biodegradation kinetics of select polycyclic aromatic hydrocarbon (PAH) mixtures by Sphingomonas paucimobilis EPA505. Biodegradation 2008, 19, 223–233. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lim, J.; Sukweenadhi, J.; Seok, J.W.; Lee, S.-W.; Park, J.C.; Taizhanova, A.; Kim, D.; Yang, D.C. Genomic Characterization of a Newly Isolated Rhizobacteria Sphingomonas panacis Reveals Plant Growth Promoting Effect to Rice. Biotechnol. Bioprocess Eng. 2019. [Google Scholar] [CrossRef]
- Jacobsen, A.; Hendriksen, R.S.; Aaresturp, F.M.; Ussery, D.W.; Friis, C. The Salmonella enterica Pan-genome. Microb. Ecol. 2011, 62, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krziwon, C.; Zähringer, U.; Kawahara, K.; Weidemann, B.; Kusumoto, S.; Rietschel, E.T.; Flad, H.-D.; Ulmer, A.J. Glycosphingolipids from Sphingomonas paucimobilis induce monokine production in human mononuclear cells. Infect. Immun. 1995, 63, 2899–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.-C.; Rutherford, D.J.; Edward, W.Y. Characterization of the multidrug efflux regulator AcrR from Escherichia coli. Biochem. Biophys. Res. Commun. 2007, 361, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddocks, S.E.; Oyston, P.C. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 2008, 154, 3609–3623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustos, S.A.; Schleif, R.F. Functional domains of the AraC protein. Proc. Natl. Acad. Sci. USA 1993, 90, 5638–5642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egler, M.; Grosse, C.; Grass, G.; Nies, D.H. Role of the extracytoplasmic function protein family sigma factor RpoE in metal resistance of Escherichia coli. J. Bacteriol. 2005, 187, 2297–2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grove, A. MarR family transcription factors. Curr. Biol. 2013, 23, R142–R143. [Google Scholar] [CrossRef] [Green Version]
- Grove, A. Regulation of metabolic pathways by MarR family transcription factors. Comput. Struct. Biotechnol. J. 2017, 15, 366–371. [Google Scholar] [CrossRef]
- Ogasawara, H.; Kori, A.; Yamada, K.; Yamamoto, K.; Ishihama, A. Regulation of the E. coli csgD gene encoding the master regulator of biofilm formation: Interplay between multiple transcription factors. In Proceedings of the 2009 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 8–11 November 2009; pp. 262–266. [Google Scholar]
- JANG, Y.-H.; KIM, H.-R.; CHOI, T.J.; NAM, S.-W.; KIM, Y.T. Overexpression of Arylsulfatase in E. coli and Its Application to Desulfatation of Agar. J. Microbiol. Biotechnol. 2004, 14, 777–782. [Google Scholar]
- Melo, M.; Feitosa, J.; Freitas, A.; De Paula, R. Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr. Polym. 2002, 49, 491–498. [Google Scholar] [CrossRef]
- Romanenko, L.A.; Uchino, M.; Frolova, G.M.; Tanaka, N.; Kalinovskaya, N.I.; Latyshev, N.; Mikhailov, V.V. Sphingomonas molluscorum sp. nov., a novel marine isolate with antimicrobial activity. Int. J. Syst. Evol. Microbiol. 2007, 57, 358–363. [Google Scholar] [CrossRef]
- Shin, S.C.; Kim, S.J.; Ahn, D.H.; Lee, J.K.; Park, H. Draft genome sequence of Sphingomonas echinoides ATCC 14820. J. Bacteriol. 2012, 194, 1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.-H.; Lee, C.-H.; Yeo, S.-H.; Oh, T.-K. Sphingopyxis baekryungensis sp. nov., an orange-pigmented bacterium isolated from sea water of the Yellow Sea in Korea. Int. J. Syst. Evol. Microbiol. 2005, 55, 1223–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.-G.; Han, B.; Duan, S.; Zhao, Z.; Wang, Y. Degradation of the endocrine-disrupting dimethyl phthalate carboxylic ester by Sphingomonas yanoikuyae DOS01 isolated from the South China Sea and the biochemical pathway. Int. Biodeterior. Biodegrad. 2009, 63, 450–455. [Google Scholar] [CrossRef]
- Eguchi, M.; Ostrowski, M.; Fegatella, F.; Bowman, J.; Nichols, D.; Nishino, T.; Cavicchioli, R. Sphingomonas alaskensis strain AFO1, an abundant oligotrophic ultramicrobacterium from the North Pacific. Appl. Environ. Microbiol. 2001, 67, 4945–4954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margesin, R.; Zhang, D.-C.; Busse, H.-J. Sphingomonas alpina sp. nov., a psychrophilic bacterium isolated from alpine soil. Int. J. Syst. Evol. Microbiol. 2012, 62, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhou, H.; Li, J.; Huang, B.; Guo, J.; Zhang, X.-L.; Gao, L.-C.; Xu, C.; Liu, C.-T. Draft genome sequence of Sphingomonas paucimobilis strain LCT-SP1 isolated from the Shenzhou X spacecraft of China. Stand. Genom. Sci. 2016, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Shin, S.C.; Lee, J.; Kim, S.J.; Kim, B.-K.; Hong, S.G.; Kim, E.H.; Park, H. Genome sequence of Sphingomonas sp. strain PAMC 26621, an Arctic-lichen-associated bacterium isolated from a Cetraria sp. J. Bacteriol. 2012, 194, 3030. [Google Scholar] [CrossRef] [Green Version]
- Hoch, J.A. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 2000, 3, 165–170. [Google Scholar] [CrossRef]
- Kinjo, Y.; Pei, B.; Bufali, S.; Raju, R.; Richardson, S.K.; Imamura, M.; Fujio, M.; Wu, D.; Khurana, A.; Kawahara, K. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem. Biol. 2008, 15, 654–664. [Google Scholar] [CrossRef] [Green Version]
- Dodd, I.; Zinovkina, N.; Safronova, V.; Belimov, A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Tank, J.G.; Pandya, R.V.; Thaker, V.S. IAA and zeatin controls cell division and endoreduplication process in quiescent center cells of Allium cepa root. Indian J. Plant Physiol. 2015, 20, 124–129. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Ouyang, J.; Shao, X.; Li, J. Indole-3-glycerol phosphate, a branchpoint of indole-3-acetic acid biosynthesis from the tryptophan biosynthetic pathway in Arabidopsis thaliana. Plant J. 2000, 24, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Tadra-Sfeir, M.; Souza, E.; Faoro, H.; Müller-Santos, M.; Baura, V.; Tuleski, T.; Rigo, L.; Yates, M.; Wassem, R.; Pedrosa, F. Naringenin regulates expression of genes involved in cell wall synthesis in Herbaspirillum seropedicae. Appl. Environ. Microbiol. 2011, 77, 2180–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Gopal, M.; Thomas, G.V.; Manikandan, V.; Gajewski, J.; Thomas, G.; Seshagiri, S.; Schuster, S.C.; Rajesh, P.; Gupta, R. Whole genome sequencing and analysis of plant growth promoting bacteria isolated from the rhizosphere of plantation crops coconut, cocoa and arecanut. PLoS ONE 2014, 9, e104259. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Halder, A.; Mishra, A.; Bhattacharyya, P.; Chakrabartty, P. Solubilization of rock phosphate by Rhizobium and Bradyrhizobium. J. Gen. Appl. Microbiol. 1990, 36, 81–92. [Google Scholar] [CrossRef]
- Sundararao, W. Phosphate dissolving organisms in the soil and rhizosphere. Indian J. Agric. Sci. 1963, 33, 272–278. [Google Scholar]
- Craven, P.A.; Hayasaka, S.S. Inorganic phosphate solubilization by rhizosphere bacteria in a Zostera marina community. Can. J. Microbiol. 1982, 28, 605–610. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017, 15, 338. [Google Scholar] [CrossRef] [PubMed]
- Bååth, E. Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut. 1989, 47, 335–379. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Hoang, V.-A.; Farh, M.E.-A.; Yang, D.-C. Sphingomonas panacis sp. nov., isolated from rhizosphere of rusty ginseng. Antonie Van Leeuwenhoek 2015, 108, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Pi, H.; Helmann, J.D. Ferrous iron efflux systems in bacteria. Metallomics 2017, 9, 840–851. [Google Scholar] [CrossRef]
- Brocklehurst, K.R.; Morby, A.P. Metal-ion tolerance in Escherichia coli: Analysis of transcriptional profiles by gene-array technology. Microbiology 2000, 146, 2277–2282. [Google Scholar] [CrossRef] [Green Version]
- Grass, G.; Otto, M.; Fricke, B.; Haney, C.J.; Rensing, C.; Nies, D.H.; Munkelt, D. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 2005, 183, 9–18. [Google Scholar] [CrossRef]
- Kunito, T.; Kusano, T.; Oyaizu, H.; Senoo, K.; Kanazawa, S.; Matsumoto, S. Cloning and sequence analysis of czc genes in Alcaligenes sp. strain CT14. Biosci. Biotechnol. Biochem. 1996, 60, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Nies, D.H. The cobalt, zinc, and cadmium efflux system CzcABC from Alcaligenes eutrophus functions as a cation-proton antiporter in Escherichia coli. J. Bacteriol. 1995, 177, 2707–2712. [Google Scholar] [CrossRef] [Green Version]
- Voloudakis, A.E.; Reignier, T.M.; Cooksey, D.A. Regulation of resistance to copper in Xanthomonas axonopodis pv. vesicatoria. Appl. Environ. Microbiol. 2005, 71, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Grass, G.; Rensing, C. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2001, 286, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Weissman, Z.; Berdicevsky, I.; Cavari, B.-Z.; Kornitzer, D. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc. Natl. Acad. Sci. USA 2000, 97, 3520–3525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanovic, M.I.; Ding, C.; Thiele, D.J.; Darwin, K.H. Copper in microbial pathogenesis: Meddling with the metal. Cell Host Microbe 2012, 11, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, C.J.; Brown, N.L.; Constantinidou, C.; Patel, M.D.; Hobman, J.L. The expression profile of Escherichia coli K-12 in response to minimal, optimal and excess copper concentrations. Microbiology 2005, 151, 1187–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmar, J.A.; Su, C.C.; Yu, E.W. CusC Heavy Metal Efflux Channel of Escherichia Coli. Encycl. Inorg. Bioinorg. Chem. 2011, 1, 1–14. [Google Scholar]
- Gudipaty, S.A.; Larsen, A.S.; Rensing, C.; McEvoy, M.M. Regulation of Cu (I)/Ag (I) efflux genes in Escherichia coli by the sensor kinase CusS. FEMS Microbiol. Lett. 2012, 330, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Lu, X.; Chen, A.J.; Luo, Y.; Hao, J.J.; Gao, W.J.M. Effects of Fusarium solani and F. oxysporum Infection on the Metabolism of Ginsenosides in American Ginseng Roots. Molecules 2015, 20, 10535–10552. [Google Scholar] [CrossRef] [Green Version]
- Farh, M.E.-A.; Kim, Y.-J.; Abbai, R.; Singh, P.; Jung, K.-H.; Kim, Y.-J.; Yang, D.-C. Pathogenesis strategies and regulation of ginsenosides by two species of Ilyonectria in Panax ginseng: Power of speciation. J. Ginseng Res. 2019, 44, 332–340. [Google Scholar] [CrossRef]
- Bi, X.; Yang, J.; Gao, W. Autotoxicity of phenolic compounds from the soil of American ginseng (Panax quinquefolium L.). Allelopath. J. 2010, 25, 115–121. [Google Scholar]
- Campeau, C.; Proctor, J.T.; Jackson, C.-J.C.; Rupasinghe, H.V. Rust-spotted North American ginseng roots: Phenolic, antioxidant, ginsenoside, and mineral nutrient content. HortScience 2003, 38, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Fredrickson, J.; Balkwill, D.; Drake, G.; Romine, M.; Ringelberg, D.; White, D. Aromatic-degrading Sphingomonas isolates from the deep subsurface. Appl. Environ. Microbiol. 1995, 61, 1917–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugulis, A.J.; McCracken, C.M. Microbial degradation of high and low molecular weight polyaromatic hydrocarbons in a two-phase partitioning bioreactor by two strains of Sphingomonas sp. Biotechnol. Lett. 2003, 25, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Leys, N.M.; Bastiaens, L.; Verstraete, W.; Springael, D. Influence of the carbon/nitrogen/phosphorus ratio on polycyclic aromatic hydrocarbon degradation by Mycobacterium and Sphingomonas in soil. Appl. Microbiol. Biotechnol. 2005, 66, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Leys, N.M.; Ryngaert, A.; Bastiaens, L.; Verstraete, W.; Top, E.M.; Springael, D. Occurrence and phylogenetic diversity of Sphingomonas strains in soils contaminated with polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 2004, 70, 1944–1955. [Google Scholar] [CrossRef] [Green Version]
- Leys, N.; Ryngaert, A.; Bastiaens, L.; Top, E.; Verstraete, W.; Springael, D. Culture independent detection of Sphingomonas sp. EPA 505 related strains in soils contaminated with polycyclic aromatic hydrocarbons (PAHs). Microb. Ecol. 2005, 49, 443–450. [Google Scholar] [CrossRef]
- White, D.C.; Sutton, S.D.; Ringelberg, D.B. The genus Sphingomonas: Physiology and ecology. Curr. Opin. Biotechnol. 1996, 7, 301–306. [Google Scholar] [CrossRef]
- Heung, L.J.; Luberto, C.; Del Poeta, M. Role of sphingolipids in microbial pathogenesis. Infect. Immun. 2006, 74, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I.; Jantzen, E. Sphingolipids in bacteria and fungi. Anaerobe 2001, 7, 103–112. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Wong, M.H.; Wong, Y.S.; Tam, N.F. Multi-factors on biodegradation kinetics of polycyclic aromatic hydrocarbons (PAHs) by Sphingomonas sp. a bacterial strain isolated from mangrove sediment. Mar. Pollut. Bull. 2008, 57, 695–702. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galperin, M.Y. Conserved ‘hypothetical’proteins: New hints and new puzzles. Int. J. Genom. 2001, 2, 14–18. [Google Scholar]
- Garg, R.; Tripathi, D.; Kant, S.; Chandra, H.; Bhatnagar, R.; Banerjee, N. The conserved hypothetical protein Rv0574c is required for cell wall integrity, stress tolerance, and virulence of Mycobacterium tuberculosis. Infect. Immun. 2015, 83, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, J.; Brostromer, E.; Liu, X.-Y.; Kristensen, O.; Su, X.-D. Bioinformatics and structural characterization of a hypothetical protein from Streptococcus mutans: Implication of antibiotic resistance. PLoS ONE 2009, 4, e7245. [Google Scholar] [CrossRef]
- Brown, T.A.; Ahn, S.-J.; Frank, R.N.; Chen, Y.-Y.M.; Lemos, J.A.; Burne, R.A. A hypothetical protein of Streptococcus mutans is critical for biofilm formation. Infect. Immun. 2005, 73, 3147–3151. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, N.M.; Gupta, V.K.; Dutta, C. BPGA-an ultra-fast pan-genome analysis pipeline. Sci. Rep. 2016, 6, 24373. [Google Scholar] [CrossRef] [Green Version]
- Lukjancenko, O.; Wassenaar, T.M.; Ussery, D.W. Comparison of 61 sequenced Escherichia coli genomes. Microb. Ecol. 2010, 60, 708–720. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-K.; Luo, H.; Zhang, Y.; Wang, B.; Gao, F. Pan-genomic analysis provides novel insights into the association of E. coli with human host and its minimal genome. Bioinformatics 2019, 35, 1987–1991. [Google Scholar] [CrossRef]
- Ryu, R.J.; Patten, C.L. Aromatic amino acid-dependent expression of indole-3-pyruvate decarboxylase is regulated by TyrR in Enterobacter cloacae UW5. J. Bacteriol. 2008, 190, 7200–7208. [Google Scholar] [CrossRef] [Green Version]
- Kochar, M.; Vaishnavi, A.; Upadhyay, A.; Srivastava, S. Bacterial biosynthesis of indole-3-acetic acid: Signal messenger service. Mol. Microb. Ecol. Rhizosphere 2013, 1, 309–326. [Google Scholar]
- Punja, Z.K. Fungal pathogens of American ginseng (Panax quinquefolium) in British Columbia. Can. J. Plant Pathol. 1997, 19, 301–306. [Google Scholar] [CrossRef]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000, 46, 237–245. [Google Scholar] [CrossRef] [PubMed]
- El-Meihy, R.M.; Abou-Aly, H.E.; Youssef, A.M.; Tewfike, T.A.; El-Alkshar, E.A. Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ. Exp. Bot. 2019, 162, 295–301. [Google Scholar] [CrossRef]
- Huo, Y.; Kang, J.P.; Ahn, J.C.; Kim, Y.J.; Piao, C.H.; Yang, D.U.; Yang, D.C. Siderophore-producing rhizobacteria reduce heavy metal-induced oxidative stress in Panax ginseng Meyer. J. Ginseng Res. 2020. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gopal, R.; Dube, B. Impact of iron stress on biomass, yield, metabolism and quality of potato (Solanum tuberosum L.). Sci. Hortic. 2006, 108, 1–6. [Google Scholar] [CrossRef]
- Ghani, A. Toxic effects of heavy metals on plant growth and metal accumulation in maize (Zea mays L.). Chemistry 2010, 3, 325–334. [Google Scholar]
- Fischer, K.; Bipp, H.-P. Removal of heavy metals from soil components and soils by natural chelating agents. Part II. Soil extraction by sugar acids. Water Air Soil Pollut. 2002, 138, 271–288. [Google Scholar] [CrossRef]
- Rodriguez, H.; Gonzalez, T.; Goire, I.; Bashan, Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 2004, 91, 552–555. [Google Scholar] [CrossRef]
- Hwangbo, H.; Park, R.D.; Kim, Y.W.; Rim, Y.S.; Park, K.H.; Kim, T.H.; Suh, J.S.; Kim, K.Y. 2-Ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr. Microbiol. 2003, 47, 0087–0092. [Google Scholar]
- Huson, D.H.; Richter, D.C.; Rausch, C.; Dezulian, T.; Franz, M.; Rupp, R. Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinform. 2007, 8, 460. [Google Scholar] [CrossRef] [Green Version]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokri, D.; Emtiazi, G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr. Microbiol. 2010, 61, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]

| Gene | Locus | Product |
|---|---|---|
| phoB | AWL63_12720 | Phosphate regulon transcriptional regulatory protein PhoB |
| phoR | AWL63_12750 | Phosphate regulon sensor protein PhoR |
| phoU | AWL63_12725 | Phosphate transport system regulatory protein PhoU |
| pstB | AWL63_12730 | Phosphate ABC transporter, ATP-binding protein PstB |
| pstA | AWL63_12735 | Phosphate ABC transporter, permease protein PstA |
| pstC | AWL63_12740 | Phosphate ABC transporter, permease protein PstC |
| pstS | AWL63_12745 | Phosphate ABC transporter, substrate-binding protein PstS |
| Metals | DCY99T | S. oligophenolica | S. mali | S. pruni |
|---|---|---|---|---|
| No metals | +++ | +++ | +++ | +++ |
| Iron | ++ | ++ | ++ | ++ |
| Zinc | ++ | ++ | ++ | w |
| Cupper | + | w | + | ++ |
| Silver | w | w | - | w |
| Manganese | ++ | ++ | ++ | ++ |
| Locus Tag | Catechol Meta Cleavage Pathway | Identity |
| BV96_03589 | Catechol 2,3-dioxygenase | 30% |
| BV96_02173 | 2-hydroxymuconic semialdehyde hydrolase | 61% |
| BV96_03586 | 2-keto-4-pentenoate hydratase | 50% |
| BV96_00245 | 4-hydroxy-2-oxovalerate aldolase | 82% |
| Locus tag | 4-hydroxybenzene Degradation Pathway | Identity |
| BV96_00588 | P-hydroxybenzoate hydroxylase | 84% |
| BV96_00594 | Protocatechuate 4,5-dioxygenase alpha chain | 80% |
| BV96_00593 | Protocatechuate 4,5-dioxygenase beta chain | 82% |
| BV96_00592 | 4-carboxy-2-hydroxymuconate-6-semialdehyde dehydrogenase | 85% |
| BV96_00600 | 2-pyrone-4,6-dicarboxylic acid hydrolase | 57% |
| BV96_00599 | 4-oxalomesaconate tautomerase | 79% |
| BV96_00595 | 4-oxalomesaconate hydratase | 82% |
| BV96_00598 | 4-carboxy-4-hydroxy-2-oxoadipate aldolase | 84% |
| Sequence Definition | Sequence Length | Probe Sequence | Genomic Position | Length | Tm | GC% |
| 1261 | TCACATCGGCGTCGAACGTCATGCCCA | 1006 | 27 | 78.8 | 59.3 | |
| Sequence Definition | Product Length | Sense/Anti-sense Primer | Genomic Position | Length | Tm | GC% |
| 149 | CGTCTTCGCAGTCCTTGTG | 907 | 19 | 67.4 | 57.9 | |
| CCTGCTCGGCACCTACAA | 1055 | 18 | 68.4 | 61.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Park, J.Y.; Balusamy, S.R.; Huo, Y.; Nong, L.K.; Thi Le, H.; Yang, D.C.; Kim, D. Comprehensive Genome Analysis on the Novel Species Sphingomonas panacis DCY99T Reveals Insights into Iron Tolerance of Ginseng. Int. J. Mol. Sci. 2020, 21, 2019. https://doi.org/10.3390/ijms21062019
Kim Y-J, Park JY, Balusamy SR, Huo Y, Nong LK, Thi Le H, Yang DC, Kim D. Comprehensive Genome Analysis on the Novel Species Sphingomonas panacis DCY99T Reveals Insights into Iron Tolerance of Ginseng. International Journal of Molecular Sciences. 2020; 21(6):2019. https://doi.org/10.3390/ijms21062019
Chicago/Turabian StyleKim, Yeon-Ju, Joon Young Park, Sri Renukadevi Balusamy, Yue Huo, Linh Khanh Nong, Hoa Thi Le, Deok Chun Yang, and Donghyuk Kim. 2020. "Comprehensive Genome Analysis on the Novel Species Sphingomonas panacis DCY99T Reveals Insights into Iron Tolerance of Ginseng" International Journal of Molecular Sciences 21, no. 6: 2019. https://doi.org/10.3390/ijms21062019





