Lysophosphatidic Acid and Hematopoiesis: From Microenvironmental Effects to Intracellular Signaling
Abstract
1. Introduction
2. Signaling Pathways of LPA and Its Receptors
2.1. LPA Generation
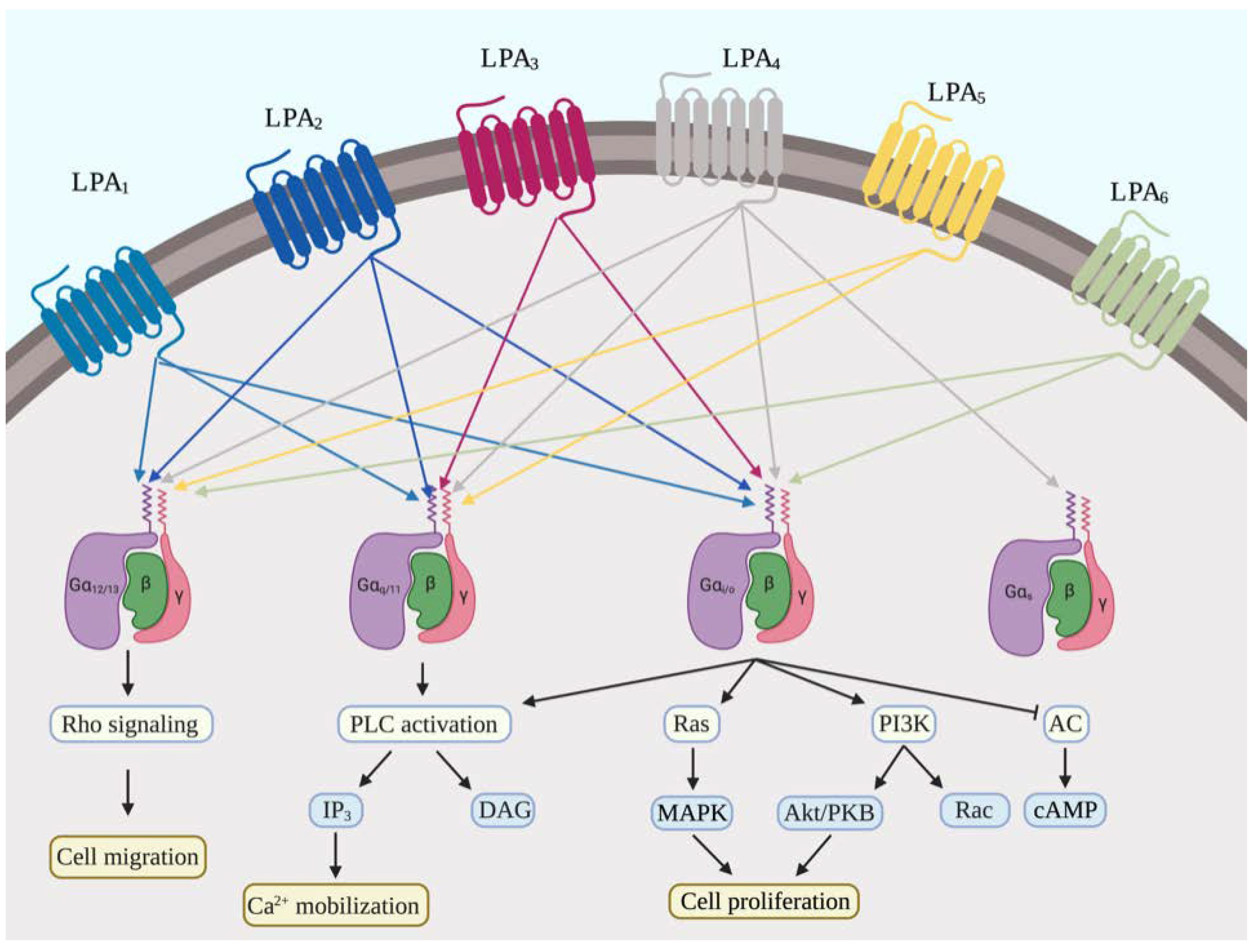
2.2. LPA Receptors
2.3. The Functions of LPA in Stem Cells
3. Regulation of Hematopoiesis
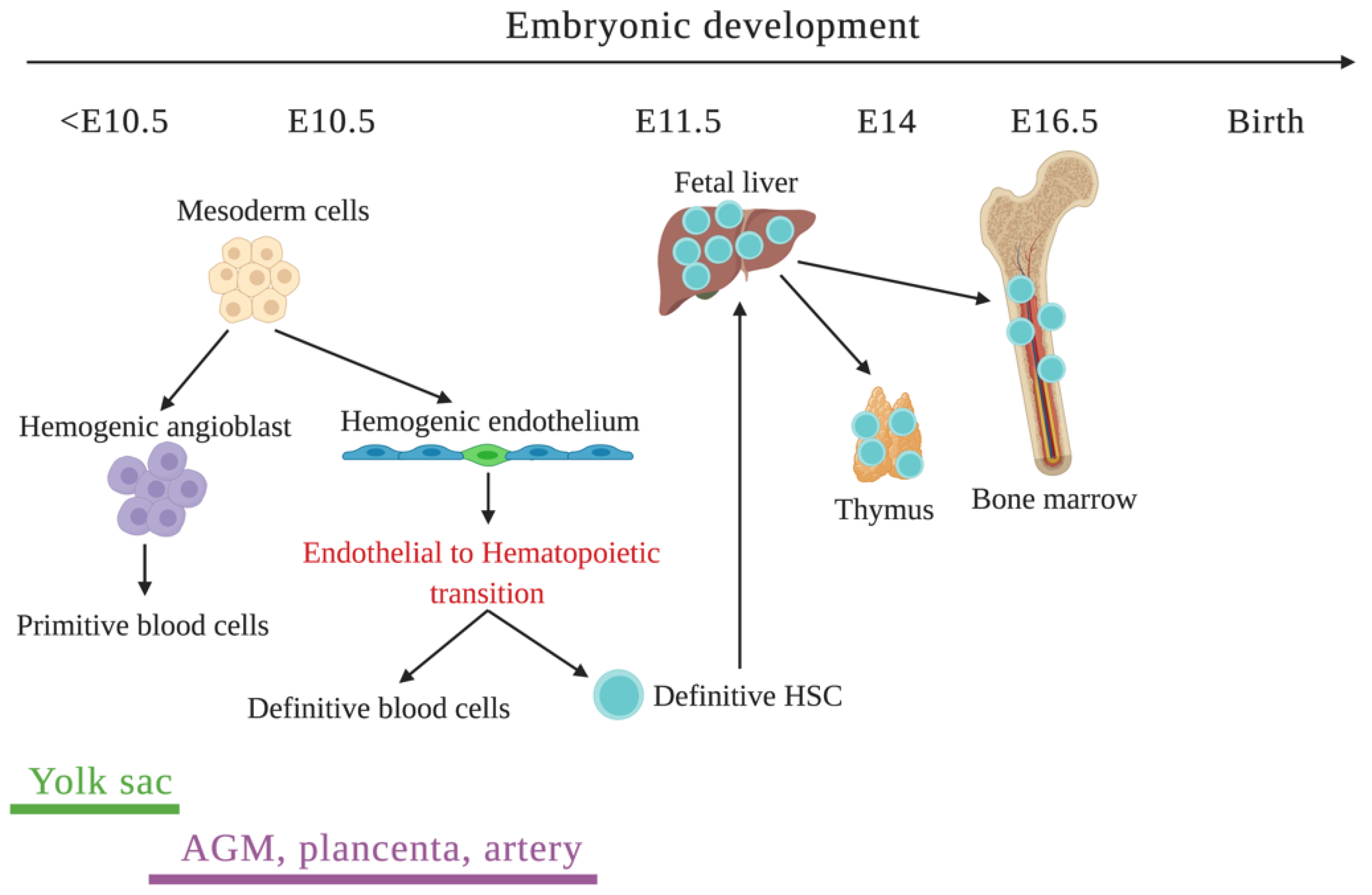
3.1. Initiation of Hematopoiesis
3.2. Microenvironmental Factors
3.2.1. Perivascular Niche
3.2.2. Endosteal Niche
3.3. Transcriptional Factor Networks
3.3.1. EKLF-FLI1 Interactions
3.3.2. GATA Switch
3.3.3. PU.1/GATA-1 Antagonism
4. Microenvironmental and Intracellular Effects of LPA during Hematopoiesis
5. LPA Receptor Agonists and Antagonists as Potential Treatments for Anemia
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef]
- An, S.; Bleu, T.; Hallmark, O.G.; Goetzl, E.J. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem. 1998, 273, 7906–7910. [Google Scholar] [CrossRef]
- Palis, J.; Robertson, S.; Kennedy, M.; Wall, C.; Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Dev. Camb. Engl. 1999, 126, 5073–5084. [Google Scholar]
- Palis, J. Ontogeny of erythropoiesis. Curr. Opin. Hematol. 2008, 15, 155–161. [Google Scholar] [CrossRef]
- Lacaud, G.; Kouskoff, V. Hemangioblast, hemogenic endothelium, and primitive versus definitive hematopoiesis. Exp. Hematol. 2016, 49, 19–24. [Google Scholar] [CrossRef]
- Zovein, A.C.; Hofmann, J.J.; Lynch, M.; French, W.J.; Turlo, K.A.; Yang, Y.; Becker, M.S.; Zanetta, L.; Dejana, E.; Gasson, J.C.; et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 2008, 3, 625–636. [Google Scholar] [CrossRef]
- Jaffredo, T.; Gautier, R.; Eichmann, A.; Dieterlen-Lievre, F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Dev. Camb. Engl. 1998, 125, 4575–4583. [Google Scholar]
- Eilken, H.M.; Nishikawa, S.; Schroeder, T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 2009, 457, 896–900. [Google Scholar] [CrossRef]
- Lancrin, C.; Sroczynska, P.; Stephenson, C.; Allen, T.; Kouskoff, V.; Lacaud, G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 2009, 457, 892–895. [Google Scholar] [CrossRef]
- Fraser, S.T.; Ogawa, M.; Yu, R.T.; Nishikawa, S.; Yoder, M.C.; Nishikawa, S. Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin(+) population. Exp. Hematol. 2002, 30, 1070–1078. [Google Scholar] [CrossRef]
- Ema, M.; Yokomizo, T.; Wakamatsu, A.; Terunuma, T.; Yamamoto, M.; Takahashi, S. Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood 2006, 108, 4018–4024. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Tigyi, G.; Miledi, R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J. Biol. Chem. 1992, 267, 21360–21367. [Google Scholar] [PubMed]
- Contos, J.J.; Ishii, I.; Chun, J. Lysophosphatidic acid receptors. Mol. Pharmacol. 2000, 58, 1188–1196. [Google Scholar] [CrossRef]
- Tigyi, G.J.; Yue, J.; Norman, D.D.; Szabo, E.; Balogh, A.; Balazs, L.; Zhao, G.; Lee, S.C. Regulation of tumor cell - Microenvironment interaction by the autotaxin-lysophosphatidic acid receptor axis. Adv. Biol. Regul. 2019, 71, 183–193. [Google Scholar] [CrossRef]
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591. [Google Scholar] [CrossRef]
- Lidgerwood, G.E.; Pitson, S.M.; Bonder, C.; Pebay, A. Roles of lysophosphatidic acid and sphingosine-1-phosphate in stem cell biology. Prog. Lipid Res. 2018, 72, 42–54. [Google Scholar] [CrossRef]
- Todorova, M.G.; Fuentes, E.; Soria, B.; Nadal, A.; Quesada, I. Lysophosphatidic acid induces Ca2+ mobilization and c-Myc expression in mouse embryonic stem cells via the phospholipase C pathway. Cell. Signal. 2009, 21, 523–528. [Google Scholar] [CrossRef]
- Hsiao, C.; Lampe, M.; Nillasithanukroh, S.; Han, W.; Lian, X.; Palecek, S.P. Human pluripotent stem cell culture density modulates YAP signaling. Biotechnol. J. 2016, 11, 662–675. [Google Scholar] [CrossRef]
- Qin, H.; Hejna, M.; Liu, Y.; Percharde, M.; Wossidlo, M.; Blouin, L.; Durruthy-Durruthy, J.; Wong, P.; Qi, Z.; Yu, J.; et al. YAP Induces Human Naive Pluripotency. Cell Rep. 2016, 14, 2301–2312. [Google Scholar] [CrossRef]
- Evseenko, D.; Latour, B.; Richardson, W.; Corselli, M.; Sahaghian, A.; Cardinal, S.; Zhu, Y.; Chan, R.; Dunn, B.; Crooks, G.M. Lysophosphatidic acid mediates myeloid differentiation within the human bone marrow microenvironment. PLoS ONE 2013, 8, e63718. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Ho, Y.-H.; Chiang, J.-C.; Li, M.-W.; Lin, S.-H.; Chen, W.-M.; Chiang, C.-L.; Lin, Y.-N.; Yang, Y.-J.; Chen, C.-N.; et al. Pharmacological activation of lysophosphatidic acid receptors regulates erythropoiesis. Sci. Rep. 2016, 6, 27050. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Chen, S.S.; Lee, S.J.; Tsao, K.C.; Chu, P.L.; Wen, C.H.; Hwang, S.M.; Yao, C.L.; Lee, H. Lysophosphatidic acid induces erythropoiesis through activating lysophosphatidic acid receptor 3. Stem Cells Dayt. Ohio 2011, 29, 1763–1773. [Google Scholar] [CrossRef]
- Ho, Y.H.; Yao, C.L.; Lin, K.H.; Hou, F.H.; Chen, W.M.; Chiang, C.L.; Lin, Y.N.; Li, M.W.; Lin, S.H.; Yang, Y.J.; et al. Opposing regulation of megakaryopoiesis by LPA receptors 2 and 3 in K562 human erythroleukemia cells. Biochim. Et Biophys. Acta 2015, 1851, 172–183. [Google Scholar] [CrossRef]
- Lin, K.H.; Li, M.W.; Chang, Y.C.; Lin, Y.N.; Ho, Y.H.; Weng, W.C.; Huang, C.J.; Chang, B.E.; Yao, C.L.; Lee, H. Activation of Lysophosphatidic Acid Receptor 3 Inhibits Megakaryopoiesis in Human Hematopoietic Stem Cells and Zebrafish. Stem Cells Dev. 2018, 27, 216–224. [Google Scholar] [CrossRef]
- Pyne, S.; Kong, K.C.; Darroch, P.I. Lysophosphatidic acid and sphingosine 1-phosphate biology: The role of lipid phosphate phosphatases. Semin. Cell Dev. Biol. 2004, 15, 491–501. [Google Scholar] [CrossRef]
- Tokumura, A.; Majima, E.; Kariya, Y.; Tominaga, K.; Kogure, K.; Yasuda, K.; Fukuzawa, K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002, 277, 39436–39442. [Google Scholar] [CrossRef]
- Nakanaga, K.; Hama, K.; Aoki, J. Autotaxin--an LPA producing enzyme with diverse functions. J. Biochem. 2010, 148, 13–24. [Google Scholar] [CrossRef]
- Yang, F.; Chen, G.X. Production of extracellular lysophosphatidic acid in the regulation of adipocyte functions and liver fibrosis. World J. Gastroenterol. 2018, 24, 4132–4151. [Google Scholar] [CrossRef]
- Ortlepp, C.; Steudel, C.; Heiderich, C.; Koch, S.; Jacobi, A.; Ryser, M.; Brenner, S.; Bornhauser, M.; Brors, B.; Hofmann, W.K.; et al. Autotaxin is expressed in FLT3-ITD positive acute myeloid leukemia and hematopoietic stem cells and promotes cell migration and proliferation. Exp. Hematol. 2013, 41, 444–461.e4. [Google Scholar] [CrossRef]
- Ray, R.; Rai, V. Lysophosphatidic acid converts monocytes into macrophages in both mice and humans. Blood 2017, 129, 1177–1183. [Google Scholar] [CrossRef]
- Kime, C.; Sakaki-Yumoto, M.; Goodrich, L.; Hayashi, Y.; Sami, S.; Derynck, R.; Asahi, M.; Panning, B.; Yamanaka, S.; Tomoda, K. Autotaxin-mediated lipid signaling intersects with LIF and BMP signaling to promote the naive pluripotency transcription factor program. Proc. Natl. Acad. Sci. USA 2016, 113, 12478–12483. [Google Scholar] [CrossRef]
- Bradley, R.M.; Marvyn, P.M.; Aristizabal Henao, J.J.; Mardian, E.B.; George, S.; Aucoin, M.G.; Stark, K.D.; Duncan, R.E. Acylglycerophosphate acyltransferase 4 (AGPAT4) is a mitochondrial lysophosphatidic acid acyltransferase that regulates brain phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol levels. Biochim. Biophys. Acta 2015, 1851, 1566–1576. [Google Scholar] [CrossRef]
- Sinderewicz, E.; Grycmacher, K.; Boruszewska, D.; Kowalczyk-Zieba, I.; Staszkiewicz-Chodor, J.; Lukaszuk, K.; Woclawek-Potocka, I. Expression of genes for enzymes synthesizing lysophosphatidic acid, its receptors and follicle developmental factors derived from the cumulus-oocyte complex is dependent on the ovarian follicle type in cows. Anim. Reprod. Sci. 2018, 192, 242–250. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Mohammad, G.; Nawaz, M.I.; Siddiquei, M.M.; Kangave, D.; Opdenakker, G. Expression of lysophosphatidic acid, autotaxin and acylglycerol kinase as biomarkers in diabetic retinopathy. Acta Diabetol. 2013, 50, 363–371. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Salous, A.K.; Brandon, J.; Miriyala, S.; Wheeler, J.; Patil, P.; Sunkara, M.; Morris, A.J.; Escalante-Alcalde, D.; Smyth, S.S. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 837–845. [Google Scholar] [CrossRef]
- DellaPuca, R.; Gallicchio, V.S. The regulation of phospholipase-A2 (PLA-2) by cytokines expressing hematopoietic growth-stimulating properties. Proc. Soc. Exp. Biol. Med. 1996, 212, 174–184. [Google Scholar] [CrossRef]
- Smyth, S.S.; Mueller, P.; Yang, F.; Brandon, J.A.; Morris, A.J. Arguing the case for the autotaxin-lysophosphatidic acid-lipid phosphate phosphatase 3-signaling nexus in the development and complications of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 479–486. [Google Scholar] [CrossRef]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef]
- Hecht, J.H.; Weiner, J.A.; Post, S.R.; Chun, J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 1996, 135, 1071–1083. [Google Scholar] [CrossRef]
- Janssens, R.; Boeynaems, J.M.; Godart, M.; Communi, D. Cloning of a human heptahelical receptor closely related to the P2Y5 receptor. Biochem. Biophys. Res. Commun. 1997, 236, 106–112. [Google Scholar] [CrossRef]
- Kuang, D.; Yao, Y.; Lam, J.; Tsushima, R.G.; Hampson, D.R. Cloning and characterization of a family C orphan G-protein coupled receptor. J. Neurochem. 2005, 93, 383–391. [Google Scholar] [CrossRef]
- Ishii, I.; Contos, J.J.A.; Fukushima, N.; Chun, J. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)NVZG-1/EDG-2, LPA2/EDG-4, and LPA3/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol. Pharmacol. 2000, 58, 895–902. [Google Scholar] [CrossRef]
- Puchałowicz, K.; Tarnowski, M.; Baranowska-Bosiacka, I.; Chlubek, D.; Dziedziejko, V. P2X and P2Y receptors—Role in the pathophysiology of the nervous system. Int. J. Mol. Sci. 2014, 15, 23672–23704. [Google Scholar] [CrossRef]
- Lee, C.W.; Rivera, R.; Dubin, A.E.; Chun, J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J. Biol. Chem. 2007, 282, 4310–4317. [Google Scholar] [CrossRef]
- Lee, C.W.; Rivera, R.; Gardell, S.; Dubin, A.E.; Chun, J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 2006, 281, 23589–23597. [Google Scholar] [CrossRef]
- Pasternack, S.M.; von Kugelgen, I.; Al Aboud, K.; Lee, Y.A.; Ruschendorf, F.; Voss, K.; Hillmer, A.M.; Molderings, G.J.; Franz, T.; Ramirez, A.; et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat. Genet. 2008, 40, 329–334. [Google Scholar] [CrossRef]
- Estivill-Torrus, G.; Llebrez-Zayas, P.; Matas-Rico, E.; Santin, L.; Pedraza, C.; De Diego, I.; Del Arco, I.; Fernandez-Llebrez, P.; Chun, J.; De Fonseca, F.R. Absence of LPA(1) signaling results in defective cortical development. Cereb Cortex 2008, 18, 938–950. [Google Scholar] [CrossRef]
- Ye, X.Q.; Hama, K.; Contos, J.J.A.; Anliker, B.; Inoue, A.; Skinner, M.K.; Suzuki, H.; Amano, T.; Kennedy, G.; Arai, H.; et al. LPA(3)-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005, 435, 104–108. [Google Scholar] [CrossRef]
- Lee, Z.; Cheng, C.T.; Zhang, H.L.; Subler, M.A.; Wu, J.H.; Mukherjee, A.; Windle, J.J.; Chen, C.K.; Fang, X.J. Role of LPA(4)/p2y9/GPR23 in Negative Regulation of Cell Motility. Mol. Biol. Cell 2008, 19, 5435–5445. [Google Scholar] [CrossRef]
- Ohuchi, H.; Hamada, A.; Matsuda, H.; Takagi, A.; Tanaka, M.; Aoki, J.; Arai, H.; Noji, S. Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 3280–3294. [Google Scholar] [CrossRef]
- Araki, M.; Kitayoshi, M.; Dong, Y.; Hirane, M.; Ozaki, S.; Mori, S.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Inhibitory effects of lysophosphatidic acid receptor-5 on cellular functions of sarcoma cells. Growth Factors 2014, 32, 117–122. [Google Scholar] [CrossRef]
- Pebay, A.; Bonder, C.S.; Pitson, S.M. Stem cell regulation by lysophospholipids. Prostag. Oth. Lipid M 2007, 84, 83–97. [Google Scholar] [CrossRef]
- Song, H.Y.; Lee, M.J.; Kim, M.Y.; Kim, K.H.; Lee, I.H.; Shin, S.H.; Lee, J.S.; Kim, J.H. Lysophosphatidic acid mediates migration of human mesenchymal stem cells stimulated by synovial fluid of patients with rheumatoid arthritis. Biochim. Et Biophys. Acta 2010, 1801, 23–30. [Google Scholar] [CrossRef]
- Ryu, J.M.; Han, H.J. Autotaxin-LPA axis regulates hMSC migration by adherent junction disruption and cytoskeletal rearrangement via LPAR1/3-dependent PKC/GSK3beta/beta-catenin and PKC/Rho GTPase pathways. Stem Cells Dayt. Ohio 2015, 33, 819–832. [Google Scholar] [CrossRef]
- Grisanti, L.; Rezza, A.; Clavel, C.; Sennett, R.; Rendl, M. Enpp2/Autotaxin in dermal papilla precursors is dispensable for hair follicle morphogenesis. J. Investig. Dermatol. 2013, 133, 2332–2339. [Google Scholar] [CrossRef]
- Liu, Y.B.; Kharode, Y.; Bodine, P.V.N.; Yaworsky, P.J.; Robinson, J.A.; Billiard, J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J. Cell Biochem. 2010, 109, 794–800. [Google Scholar] [CrossRef]
- Kang, S.; Han, J.; Song, S.Y.; Kim, W.S.; Shin, S.; Kim, J.H.; Ahn, H.; Jeong, J.H.; Hwang, S.J.; Sung, J.H. Lysophosphatidic acid increases the proliferation and migration of adiposederived stem cells via the generation of reactive oxygen species. Mol. Med. Rep. 2015, 12, 5203–5210. [Google Scholar] [CrossRef][Green Version]
- Do, E.K.; Kim, Y.M.; Heo, S.C.; Kwon, Y.W.; Shin, S.H.; Suh, D.S.; Kim, K.H.; Yoon, M.S.; Kim, J.H. Lysophosphatidic acid-induced ADAM12 expression mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth. Int. J. Biochem. Cell Biol. 2012, 44, 2069–2076. [Google Scholar] [CrossRef]
- Matsushita, T.; Amagai, Y.; Soga, T.; Terai, K.; Obinata, M.; Hashimoto, S. A novel oligodendrocyte cell line OLP6 shows the successive stages of oligodendrocyte development: Late progenitor, immature and mature stages. Neuroscience 2005, 136, 115–121. [Google Scholar] [CrossRef]
- Wheeler, N.A.; Lister, J.A.; Fuss, B. The Autotaxin-Lysophosphatidic Acid Axis Modulates Histone Acetylation and Gene Expression during Oligodendrocyte Differentiation. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 11399–11414. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.J.; Nam, J.S.; Sun, Y.; Kim, M.J.; Choi, H.K.; Han, D.H.; Kim, N.H.; Huh, S.O. Lysophosphatidic acid stimulates cAMP accumulation and cAMP response element-binding protein phosphorylation in immortalized hippocampal progenitor cells. Neuroreport 2006, 17, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.L.; Overall, R.W.; Vogler, S.; Sykes, A.M.; Ruhwald, S.; Lasse, D.; Ichwan, M.; Fabel, K.; Kempermann, G. Lysophosphatidic Acid Receptor Is a Functional Marker of Adult Hippocampal Precursor Cells. Stem Cell Rep. 2016, 6, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Dottori, M.; Leung, J.; Turnley, A.M.; Pebay, A. Lysophosphatidic acid inhibits neuronal differentiation of neural stem/progenitor cells derived from human embryonic stem cells. Stem Cells Dayt. Ohio 2008, 26, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Frisca, F.; Crombie, D.E.; Dottori, M.; Goldshmit, Y.; Pebay, A. Rho/ROCK pathway is essential to the expansion, differentiation, and morphological rearrangements of human neural stem/progenitor cells induced by lysophosphatidic acid. J. Lipid Res. 2013, 54, 1192–1206. [Google Scholar] [CrossRef]
- Costa, G.; Kouskoff, V.; Lacaud, G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012, 33, 215–223. [Google Scholar] [CrossRef]
- Lux, C.T.; Yoshimoto, M.; McGrath, K.; Conway, S.J.; Palis, J.; Yoder, M.C. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood 2008, 111, 3435–3438. [Google Scholar] [CrossRef]
- Vogeli, K.M.; Jin, S.-W.; Martin, G.R.; Stainier, D.Y.R. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature 2006, 443, 337. [Google Scholar] [CrossRef]
- Medvinsky, A.L.; Samoylina, N.L.; Muller, A.M.; Dzierzak, E.A. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature 1993, 364, 64–67. [Google Scholar] [CrossRef]
- Ivanovs, A.; Rybtsov, S.; Welch, L.; Anderson, R.A.; Turner, M.L.; Medvinsky, A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J. Exp. Med. 2011, 208, 2417–2427. [Google Scholar] [CrossRef]
- Corbel, C.; Salaun, J.; Belo-Diabangouaya, P.; Dieterlen-Lievre, F. Hematopoietic potential of the pre-fusion allantois. Dev. Biol. 2007, 301, 478–888. [Google Scholar] [CrossRef]
- Zeigler, B.M.; Sugiyama, D.; Chen, M.; Guo, Y.; Downs, K.M.; Speck, N.A. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Dev. Camb. Engl. 2006, 133, 4183–4192. [Google Scholar] [CrossRef]
- Garcia-Porrero, J.A.; Godin, I.E.; Dieterlen-Lievre, F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat. Embryol. 1995, 192, 425–435. [Google Scholar] [CrossRef]
- North, T.; Gu, T.L.; Stacy, T.; Wang, Q.; Howard, L.; Binder, M.; Marin-Padilla, M.; Speck, N.A. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Dev. Camb. Engl. 1999, 126, 2563–2575. [Google Scholar]
- North, T.E.; Stacy, T.; Matheny, C.J.; Speck, N.A.; de Bruijn, M.F.T.R. Runx1 Is Expressed in Adult Mouse Hematopoietic Stem Cells and Differentiating Myeloid and Lymphoid Cells, But Not in Maturing Erythroid Cells. Stem Cells Dayt. Ohio 2004, 22, 158–168. [Google Scholar] [CrossRef]
- Pazianos, G.; Uqoezwa, M.; Reya, T. The elements of stem cell self-renewal: A genetic perspective. BioTechniques 2003, 35, 1240–1247. [Google Scholar] [CrossRef]
- Wilson, A.; Laurenti, E.; Trumpp, A. Balancing dormant and self-renewing hematopoietic stem cells. Curr. Opin. Genet. Dev. 2009, 19, 461–468. [Google Scholar] [CrossRef]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Asada, N.; Takeishi, S.; Frenette, P.S. Complexity of bone marrow hematopoietic stem cell niche. Int. J. Hematol. 2017, 106, 45–54. [Google Scholar] [CrossRef]
- Omatsu, Y.; Sugiyama, T.; Kohara, H.; Kondoh, G.; Fujii, N.; Kohno, K.; Nagasawa, T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 2010, 33, 387–399. [Google Scholar] [CrossRef]
- Zhu, J.; Garrett, R.; Jung, Y.; Zhang, Y.; Kim, N.; Wang, J.; Joe, G.J.; Hexner, E.; Choi, Y.; Taichman, R.S.; et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 2007, 109, 3706–3712. [Google Scholar] [CrossRef]
- Song, K.; Li, L.; Wang, Y.; Liu, T. Hematopoietic stem cells: Multiparameter regulation. Hum. Cell 2016, 29, 53–57. [Google Scholar] [CrossRef]
- da Silva, C.L.; Goncalves, R.; Crapnell, K.B.; Cabral, J.M.; Zanjani, E.D.; Almeida-Porada, G. A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp. Hematol. 2005, 33, 828–835. [Google Scholar] [CrossRef]
- Lui, W.C.; Chan, Y.F.; Chan, L.C.; Ng, R.K. Cytokine combinations on the potential for ex vivo expansion of murine hematopoietic stem cells. Cytokine 2014, 68, 127–132. [Google Scholar] [CrossRef][Green Version]
- Yeoh, J.S.; van Os, R.; Weersing, E.; Ausema, A.; Dontje, B.; Vellenga, E.; de Haan, G. Fibroblast growth factor-1 and -2 preserve long-term repopulating ability of hematopoietic stem cells in serum-free cultures. Stem Cells Dayt. Ohio 2006, 24, 1564–1572. [Google Scholar] [CrossRef]
- Kovtonyuk, L.V.; Manz, M.G.; Takizawa, H. Enhanced thrombopoietin but not G-CSF receptor stimulation induces self-renewing hematopoietic stem cell divisions in vivo. Blood 2016, 127, 3175–3179. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, B.; Wang, S.; Zhang, J.; Liu, Y.; Wang, J.; Fan, Z.; Lv, Y.; Zhang, X.; He, L.; et al. Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation. Sci. Rep. 2015, 5, 12993. [Google Scholar] [CrossRef]
- Khodadi, E.; Shahrabi, S.; Shahjahani, M.; Azandeh, S.; Saki, N. Role of stem cell factor in the placental niche. Cell Tissue Res. 2016, 366, 523–531. [Google Scholar] [CrossRef]
- Winkler, I.G.; Sims, N.A.; Pettit, A.R.; Barbier, V.; Nowlan, B.; Helwani, F.; Poulton, I.J.; van Rooijen, N.; Alexander, K.A.; Raggatt, L.J.; et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010, 116, 4815–4828. [Google Scholar] [CrossRef]
- Kiel, M.J.; Yilmaz, O.H.; Iwashita, T.; Yilmaz, O.H.; Terhorst, C.; Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef]
- Ramalingam, P.; Poulos, M.G.; Butler, J.M. Regulation of the hematopoietic stem cell lifecycle by the endothelial niche. Curr. Opin. Hematol. 2017, 24, 289–299. [Google Scholar] [CrossRef]
- Asada, N.; Kunisaki, Y.; Pierce, H.; Wang, Z.; Fernandez, N.F.; Birbrair, A.; Ma’ayan, A.; Frenette, P.S. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 2017, 19, 214–223. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef]
- Mendez-Ferrer, S.; Lucas, D.; Battista, M.; Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008, 452, 442–447. [Google Scholar] [CrossRef]
- Nakamura-Ishizu, A.; Takubo, K.; Fujioka, M.; Suda, T. Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem. Biophys. Res. Commun. 2014, 454, 353–357. [Google Scholar] [CrossRef]
- Zhao, M.; Perry, J.M.; Marshall, H.; Venkatraman, A.; Qian, P.; He, X.C.; Ahamed, J.; Li, L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 2014, 20, 1321–1326. [Google Scholar] [CrossRef]
- Nilsson, S.K.; Johnston, H.M.; Coverdale, J.A. Spatial localization of transplanted hemopoietic stem cells: Inferences for the localization of stem cell niches. Blood 2001, 97, 2293–2299. [Google Scholar] [CrossRef]
- Silberstein, L.; Goncalves, K.A.; Kharchenko, P.V.; Turcotte, R.; Kfoury, Y.; Mercier, F.; Baryawno, N.; Severe, N.; Bachand, J.; Spencer, J.A.; et al. Proximity-Based Differential Single-Cell Analysis of the Niche to Identify Stem/Progenitor Cell Regulators. Cell Stem Cell 2016, 19, 530–543. [Google Scholar] [CrossRef]
- Asada, N.; Katayama, Y. Regulation of hematopoiesis in endosteal microenvironments. Int. J. Hematol. 2014, 99, 679–684. [Google Scholar] [CrossRef]
- Goncalves, K.A.; Silberstein, L.; Li, S.; Severe, N.; Hu, M.G.; Yang, H.; Scadden, D.T.; Hu, G.F. Angiogenin Promotes Hematopoietic Regeneration by Dichotomously Regulating Quiescence of Stem and Progenitor Cells. Cell 2016, 166, 894–906. [Google Scholar] [CrossRef]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef]
- Asada, N.; Katayama, Y.; Sato, M.; Minagawa, K.; Wakahashi, K.; Kawano, H.; Kawano, Y.; Sada, A.; Ikeda, K.; Matsui, T.; et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell 2013, 12, 737–747. [Google Scholar] [CrossRef]
- Miyamoto, K.; Yoshida, S.; Kawasumi, M.; Hashimoto, K.; Kimura, T.; Sato, Y.; Kobayashi, T.; Miyauchi, Y.; Hoshi, H.; Iwasaki, R.; et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J. Exp. Med. 2011, 208, 2175–2181. [Google Scholar] [CrossRef]
- Chow, A.; Lucas, D.; Hidalgo, A.; Mendez-Ferrer, S.; Hashimoto, D.; Scheiermann, C.; Battista, M.; Leboeuf, M.; Prophete, C.; van Rooijen, N.; et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 2011, 208, 261–271. [Google Scholar] [CrossRef]
- Christopher, M.J.; Rao, M.; Liu, F.; Woloszynek, J.R.; Link, D.C. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J. Exp. Med. 2011, 208, 251–260. [Google Scholar] [CrossRef]
- Nuez, B.; Michalovich, D.; Bygrave, A.; Ploemacher, R.; Grosveld, F. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature 1995, 375, 316–318. [Google Scholar] [CrossRef]
- Perkins, A.C.; Sharpe, A.H.; Orkin, S.H. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature 1995, 375, 318–322. [Google Scholar] [CrossRef]
- Hart, A.; Melet, F.; Grossfeld, P.; Chien, K.; Jones, C.; Tunnacliffe, A.; Favier, R.; Bernstein, A. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity 2000, 13, 167–177. [Google Scholar] [CrossRef]
- Starck, J.; Cohet, N.; Gonnet, C.; Sarrazin, S.; Doubeikovskaia, Z.; Doubeikovski, A.; Verger, A.; Duterque-Coquillaud, M.; Morle, F. Functional cross-antagonism between transcription factors FLI-1 and EKLF. Mol. Cell. Biol. 2003, 23, 1390–1402. [Google Scholar] [CrossRef]
- Bouilloux, F.; Juban, G.; Cohet, N.; Buet, D.; Guyot, B.; Vainchenker, W.; Louache, F.; Morle, F. EKLF restricts megakaryocytic differentiation at the benefit of erythrocytic differentiation. Blood 2008, 112, 576–584. [Google Scholar] [CrossRef]
- Evans, T.; Felsenfeld, G. The erythroid-specific transcription factor Eryf1: A new finger protein. Cell 1989, 58, 877–885. [Google Scholar] [CrossRef]
- Tsai, S.F.; Martin, D.I.; Zon, L.I.; D’Andrea, A.D.; Wong, G.G.; Orkin, S.H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature 1989, 339, 446–451. [Google Scholar] [CrossRef]
- Orkin, S.H. GATA-binding transcription factors in hematopoietic cells. Blood 1992, 80, 575–581. [Google Scholar] [CrossRef]
- Charron, F.; Nemer, M. GATA transcription factors and cardiac development. Semin. Cell Dev. Biol. 1999, 10, 85–91. [Google Scholar] [CrossRef]
- Johnson, K.D.; Kong, G.; Gao, X.; Chang, Y.I.; Hewitt, K.J.; Sanalkumar, R.; Prathibha, R.; Ranheim, E.A.; Dewey, C.N.; Zhang, J.; et al. Cis-regulatory mechanisms governing stem and progenitor cell transitions. Sci. Adv. 2015, 1, e1500503. [Google Scholar] [CrossRef]
- Bresnick, E.H.; Lee, H.Y.; Fujiwara, T.; Johnson, K.D.; Keles, S. GATA switches as developmental drivers. J. Biol. Chem. 2010, 285, 31087–31093. [Google Scholar] [CrossRef]
- Katsumura, K.R.; Ong, I.M.; DeVilbiss, A.W.; Sanalkumar, R.; Bresnick, E.H. GATA Factor-Dependent Positive-Feedback Circuit in Acute Myeloid Leukemia Cells. Cell Rep. 2016, 16, 2428–2441. [Google Scholar] [CrossRef]
- Cortes-Lavaud, X.; Maicas, M.; Vazquez, I.; Vicente, C.; Urquiza, L.; Garcia-Sanchez, M.A.; Odero, M.D. Functional Analysis of the GATA2 Promoter Shows That Mutations of GATA2 Impair Its Own Transcriptional Regulation. Blood 2012, 120, 1233. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, X.; Huo, B.; Wang, Y.; Sun, J.; Meng, L.; Hao, T.; Zhao, Z.J.; Hu, X. GATA2 regulates GATA1 expression through LSD1-mediated histone modification. Am. J. Transl. Res. 2016, 8, 2265–2274. [Google Scholar]
- Grass, J.A.; Boyer, M.E.; Pal, S.; Wu, J.; Weiss, M.J.; Bresnick, E.H. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. USA 2003, 100, 8811–8816. [Google Scholar] [CrossRef]
- Suzuki, M.; Kobayashi-Osaki, M.; Tsutsumi, S.; Pan, X.; Ohmori, S.; Takai, J.; Moriguchi, T.; Ohneda, O.; Ohneda, K.; Shimizu, R.; et al. GATA factor switching from GATA2 to GATA1 contributes to erythroid differentiation. Genes Cells Devoted Mol. Cell. Mech. 2013, 18, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Arinobu, Y.; Mizuno, S.; Chong, Y.; Shigematsu, H.; Iino, T.; Iwasaki, H.; Graf, T.; Mayfield, R.; Chan, S.; Kastner, P.; et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell 2007, 1, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Khandros, E.; Bailey, L.C.; Nichols, K.E.; Vakoc, C.R.; Yao, Y.; Huang, Z.; Crispino, J.D.; Hardison, R.C.; Blobel, G.A.; et al. Graded repression of PU.1/Sfpi1 gene transcription by GATA factors regulates hematopoietic cell fate. Blood 2009, 114, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Nerlov, C.; Graf, T. PU. 1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998, 12, 2403–2412. [Google Scholar] [CrossRef]
- Wontakal, S.N.; Guo, X.; Smith, C.; MacCarthy, T.; Bresnick, E.H.; Bergman, A.; Snyder, M.P.; Weissman, S.M.; Zheng, D.; Skoultchi, A.I. A core erythroid transcriptional network is repressed by a master regulator of myelo-lymphoid differentiation. Proc. Natl. Acad. Sci. USA 2012, 109, 3832–3837. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Chen, Q.; Callon, K.; Xu, X.; Reid, I.R.; Cornish, J. The phospholipids sphingosine-1-phosphate and lysophosphatidic acid prevent apoptosis in osteoblastic cells via a signaling pathway involving G(i) proteins and phosphatidylinositol-3 kinase. Endocrinology 2002, 143, 4755–4763. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, Y.; Wang, X.; Zhao, F.; Sun, M.; Zheng, X.; Dong, H.; Guo, K. Pigment Epithelium-Derived Factor (PEDF) Protects Osteoblastic Cell Line from Glucocorticoid-Induced Apoptosis via PEDF-R. Int. J. Mol. Sci. 2016, 17, 730. [Google Scholar] [CrossRef]
- Karagiosis, S.A.; Karin, N.J. Lysophosphatidic acid induces osteocyte dendrite outgrowth. Biochem. Biophys. Res. Commun. 2007, 357, 194–199. [Google Scholar] [CrossRef]
- David, M.; Machuca-Gayet, I.; Kikuta, J.; Ottewell, P.; Mima, F.; Leblanc, R.; Bonnelye, E.; Ribeiro, J.; Holen, I.; Lopez Vales, R.; et al. Lysophosphatidic acid receptor type 1 (LPA1) plays a functional role in osteoclast differentiation and bone resorption activity. J. Biol. Chem. 2014, 289, 6551–6564. [Google Scholar] [CrossRef]
- Kanehira, M.; Kikuchi, T.; Ohkouchi, S.; Shibahara, T.; Tode, N.; Santoso, A.; Daito, H.; Ohta, H.; Tamada, T.; Nukiwa, T. Targeting lysophosphatidic acid signaling retards culture-associated senescence of human marrow stromal cells. PLoS ONE 2012, 7, e32185. [Google Scholar] [CrossRef]
- Igarashi, H.; Akahoshi, N.; Ohto-Nakanishi, T.; Yasuda, D.; Ishii, S. The lysophosphatidic acid receptor LPA4 regulates hematopoiesis-supporting activity of bone marrow stromal cells. Sci. Rep. 2015, 5, 11410. [Google Scholar] [CrossRef] [PubMed]
- Seitz, G.; Boehmler, A.M.; Kanz, L.; Mohle, R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann. N. Y. Acad. Sci. 2005, 1044, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Whetton, A.D.; Lu, Y.; Pierce, A.; Carney, L.; Spooncer, E. Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving Vav 1. Blood 2003, 102, 2798–2802. [Google Scholar] [CrossRef] [PubMed]
- Kostic, I.; Fidalgo-Carvalho, I.; Aday, S.; Vazao, H.; Carvalheiro, T.; Graos, M.; Duarte, A.; Cardoso, C.; Goncalves, L.; Carvalho, L.; et al. Lysophosphatidic acid enhances survival of human CD34(+) cells in ischemic conditions. Sci. Rep. 2015, 5, 16406. [Google Scholar] [CrossRef]
- Yanai, N.; Matsui, N.; Furusawa, T.; Okubo, T.; Obinata, M. Sphingosine-1-phosphate and lysophosphatidic acid trigger invasion of primitive hematopoietic cells into stromal cell layers. Blood 2000, 96, 139–144. [Google Scholar] [CrossRef]
- Li, H.; Yue, R.; Wei, B.; Gao, G.; Du, J.; Pei, G. Lysophosphatidic acid acts as a nutrient-derived developmental cue to regulate early hematopoiesis. Embo J. 2014, 33, 1383–1396. [Google Scholar] [CrossRef]
- Okabe-Kado, J.; Hagiwara-Watanabe, Y.; Niitsu, N.; Kasukabe, T.; Kaneko, Y. NM23 downregulation and lysophosphatidic acid receptor EDG2/lpa1 upregulation during myeloid differentiation of human leukemia cells. Leuk. Res. 2018, 66, 39–48. [Google Scholar] [CrossRef]
- Dunn, C.J.; Wagstaff, A.J. Epoetin alfa. A review of its clinical efficacy in the management of anaemia associated with renal failure and chronic disease and its use in surgical patients. Drugs Aging 1995, 7, 131–156. [Google Scholar] [CrossRef]
- Epoetin Beta. In Drugs and Lactation Database (LactMed); National Library of Medicine: Bethesda, MD, USA, 2006.
- Palmer, S.C.; Saglimbene, V.; Craig, J.C.; Navaneethan, S.D.; Strippoli, G.F. Darbepoetin for the anaemia of chronic kidney disease. Cochrane Database Syst. Rev. 2014, Cd009297. [Google Scholar] [CrossRef]
- Salamin, O.; Kuuranne, T.; Saugy, M.; Leuenberger, N. Erythropoietin as a performance-enhancing drug: Its mechanistic basis, detection, and potential adverse effects. Mol. Cell. Endocrinol. 2018, 464, 75–87. [Google Scholar] [CrossRef]
- Gupta, N.; Wish, J.B. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2017, 69, 815–826. [Google Scholar] [CrossRef]
- Strowitzki, M.J.; Cummins, E.P.; Taylor, C.T. Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous? Cells 2019, 8, 384. [Google Scholar] [CrossRef]
- Martin, E.R.; Smith, M.T.; Maroni, B.J.; Zuraw, Q.C.; deGoma, E.M. Clinical Trial of Vadadustat in Patients with Anemia Secondary to Stage 3 or 4 Chronic Kidney Disease. Am. J. Nephrol. 2017, 45, 380–388. [Google Scholar] [CrossRef]
- Provenzano, R.; Besarab, A.; Sun, C.H.; Diamond, S.A.; Durham, J.H.; Cangiano, J.L.; Aiello, J.R.; Novak, J.E.; Lee, T.; Leong, R.; et al. Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD. Clin. J. Am. Soc. Nephrol. Cjasn 2016, 11, 982–991. [Google Scholar] [CrossRef]
- Stoddard, N.C.; Chun, J. Promising pharmacological directions in the world of lysophosphatidic Acid signaling. Biomol. Ther. 2015, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]

| Cell Type | LPA-Related Effects | Reference | |
|---|---|---|---|
| Extracellular/ Indirect effects | BM MSCs | Detectable level of ATX expression | [21] |
| BM MSCs | Activation of LPA1 retards cell senescence | [129] | |
| Endosteal osteoblasts | High PPAP2A expression level | [21] | |
| Endosteal osteoblasts | LPA promotes survival and proliferation | [121,126] | |
| MLO-Y4 cell line | LPA triggers dendrite outgrowth | [127] | |
| BM PDGFRα+ cells | LPA4 promotes the production of HSPC proliferation factors | [130] | |
| Intracellular/ Direct regulation | THS119 cell line | LPA-LPA1 axis promotes invasion ability | [134] |
| Hemangioblasts | LPA1 activates hematopoietic differentiation | [135] | |
| Leukemic cell lines | LPA1 is involved in NM23-dependent myeloid differentiation | [136] | |
| Monocytes | Activation of LPA-Akt-mTor-PPARγ signaling converts monocytes into macrophages | [31] | |
| CD34+ HSPCs | LPA promotes the early stage of myeloid differentiation | [21] | |
| MEPs, K562 cell line | Activation of LPA3 promotes erythrocyte differentiation | [23,137] | |
| MEPs | LPA-LPA3 axis inhibits megakaryocyte differentiation | [25] | |
| MEPs, K562 cell line | LPA2 inhibits both erythropoiesis and megakaryopoiesis | [24,137] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-H.; Chiang, J.-C.; Ho, Y.-H.; Yao, C.-L.; Lee, H. Lysophosphatidic Acid and Hematopoiesis: From Microenvironmental Effects to Intracellular Signaling. Int. J. Mol. Sci. 2020, 21, 2015. https://doi.org/10.3390/ijms21062015
Lin K-H, Chiang J-C, Ho Y-H, Yao C-L, Lee H. Lysophosphatidic Acid and Hematopoiesis: From Microenvironmental Effects to Intracellular Signaling. International Journal of Molecular Sciences. 2020; 21(6):2015. https://doi.org/10.3390/ijms21062015
Chicago/Turabian StyleLin, Kuan-Hung, Jui-Chung Chiang, Ya-Hsuan Ho, Chao-Ling Yao, and Hsinyu Lee. 2020. "Lysophosphatidic Acid and Hematopoiesis: From Microenvironmental Effects to Intracellular Signaling" International Journal of Molecular Sciences 21, no. 6: 2015. https://doi.org/10.3390/ijms21062015
APA StyleLin, K.-H., Chiang, J.-C., Ho, Y.-H., Yao, C.-L., & Lee, H. (2020). Lysophosphatidic Acid and Hematopoiesis: From Microenvironmental Effects to Intracellular Signaling. International Journal of Molecular Sciences, 21(6), 2015. https://doi.org/10.3390/ijms21062015






