Fluorescence Sensing Platforms for Epinephrine Detection Based on Low Temperature Cofired Ceramics
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Apparatus and Procedures
2.2.1. Inorganic Dye Preparation
2.2.2. Electrochemical Polymerization
2.2.3. Modification of an Outside Electrode
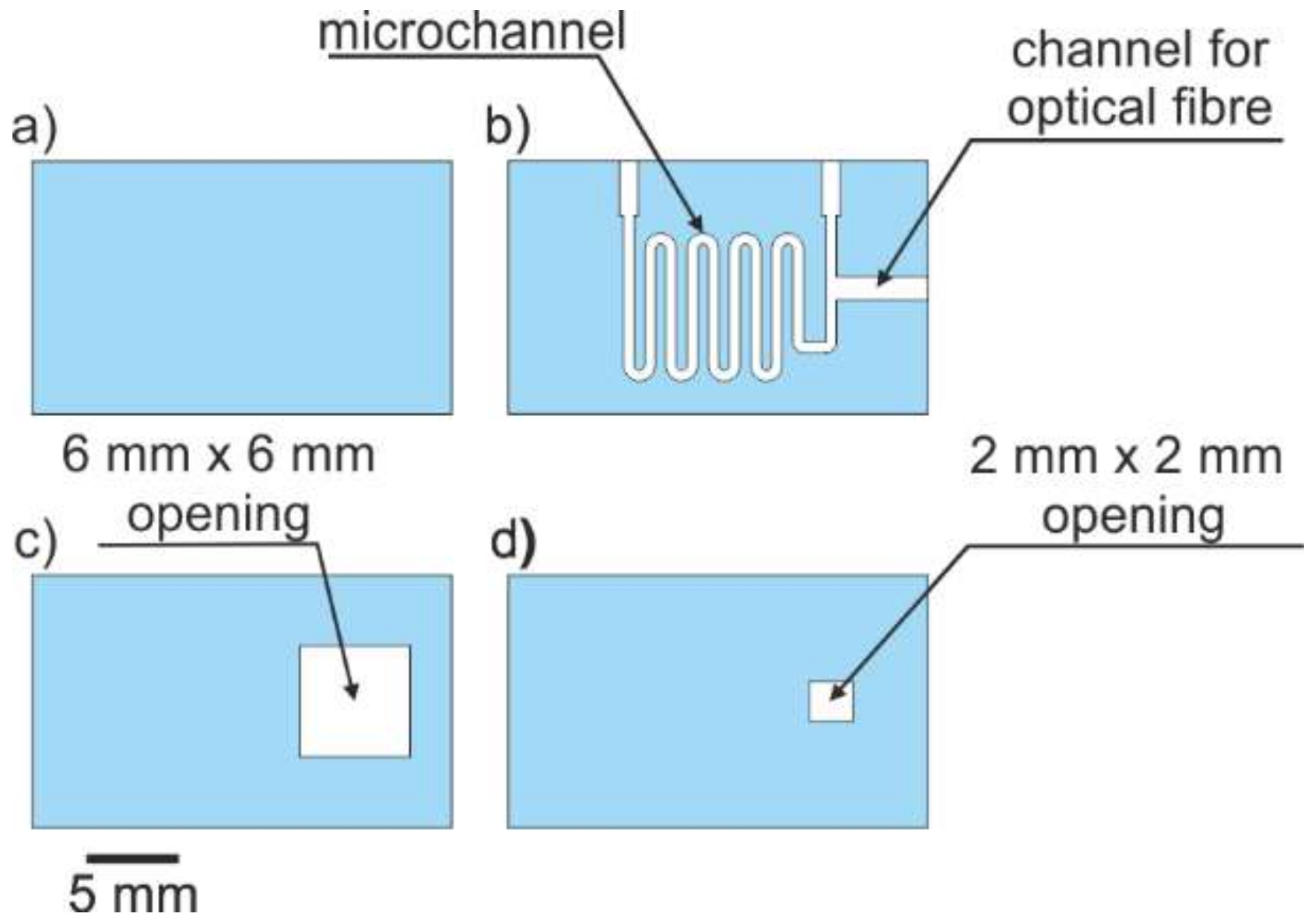
2.2.4. Microsystem Technology
2.2.5. An Optical Epinephrine Determination
2.2.6. Influence of Interfering Substances
3. Results and Discussion
3.1. EP–Fe2+ Complex Creation
3.2. Pt–LTCC Electrode Modification
3.3. Detection Principles and Optical EP Determination
3.4. Parameters for Sensor Characteristics
3.4.1. Effect of the Flow Rate
3.4.2. Effect of the Inorganic Dye Ratio on Fluorescence Intensity
3.5. Selectivity
3.6. Reproducibility and Stability of the Sensor
3.7. Real Sample Analysis of Epinephrine
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergquist, J.; Sciubisz, A.; Kaczor, A.; Silberring, J. Catecholamines and methods for their identification and quantitation in biological tissues and fluids. J. Neurosci. Methods 2002, 113, 1–13. [Google Scholar] [CrossRef]
- Robinson, D.L.; Hermans, A.; Seipel, A.T.; Wightman, R.M. Monitoring rapid chemical communication in the brain. Chem. Rev. 2008, 108, 2554–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tselea, T.; Adekunlea, A.; Fayemia, O.; Ebenso, E. Electrochemical detection of Epinephrine using Polyaniline nanocomposite films doped with TiO2 and RuO2 Nanoparticles on Multi-walled Carbon Nanotube. Electrochim. Acta 2017, 243, 331–348. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koninckx, M.; Buysse, C.; de Hoog, M. Management of status asthmaticus in children. Pediatric Respir. Rev. 2013, 14, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Stroumpoulis, K.; Xanthos, T.; Rokas, G.; Kitsou, V.; Papadimitriou, D.; Serpetinis, I.; Perrea, D.; Papadimitriou, L.; Kouskouni, E. Vasopressin and epinephrine in the treatment of cardiac arrest: An experimental study. Crit. Care 2008, 12, R40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, B.M.; Cristovao, A.C.; Goncalves, D.; Fortuna, A.; Bernardino, L.; Falcao, A.; Alves, G. Determination of catecholamines and endogenous related compounds in rat brain tissue exploring their native fluorescence and liquid chromatography. J. Chromatogr. B. 2017, 1049, 51–59. [Google Scholar]
- Nagaraja, P.; Vasantha, R.A.; Sunitha, K.R. A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 2001, 55, 1039–1046. [Google Scholar] [CrossRef]
- Kartsova, L.A.; Sidorova, A.A.; Kazakov, V.A.; Bessonova, E.A.; Yashin, A.Y. Determination of catecholamines by capillary electrophoresis and reversed-phase high-performance liquid chromatography. J. Anal. Chem. 2004, 59, 737–741. [Google Scholar] [CrossRef]
- Menon, S.; Jesny, S.; Sivasankaran, U. Girish Kumar K. Fluorometric Determination of Epinephrine: A Green Approach. Anal. Sci. 2016, 32, 999–1001. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, D.; Yang, J.Y.; Trehy, M. Determination of the enantiomeric purity of epinephrine by HPLC with circular dichroism detection. J. Liquid Chromatogr. Related Technol. 2017, 40, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ren, H.X.; Zhao, H.; Li, Z.X. Ultrasensitive visual and colorimetric determination of dopamine based on the prevention of etching of silver nanoprisms by chloride. Microchim. Acta 2017, 184, 415–421. [Google Scholar] [CrossRef]
- Mukdasai, S.; Langsi, V.; Pravda, M.; Srijaranai, S.; Glennon, J.D. A highly sensitive electrochemical determination of norepinephrine using L-cysteine self-assembled monolayers over gold nanoparticles/multi-walled carbon nanotubes electrode in the presence of sodium dodecyl sulfate. Sens. Actuators B 2016, 236, 126–135. [Google Scholar] [CrossRef]
- Lu, L.P.; Wang, S.-Q.; Lin, X.-Q. Fabrication of layer-by-layer deposited multilayer films containing DNA and gold nanoparticle for norepinephrine biosensor. Anal. Chim. Acta 2004, 519, 161–166. [Google Scholar] [CrossRef]
- Kang, G.; Lin, X. RNA modified electrodes for simultaneous determination of dopamine and uric acid in the presence of high amounts of ascorbic acid. Electroanal 2006, 18, 2458–2466. [Google Scholar] [CrossRef]
- Mohsin, M.A.; Liu, B.D.; Zhang, X.L.; Yang, W.J.; Liu, L.S.; Jiang, X. Cellular-membrane inspired surface modification of well aligned ZnO nanorods for chemosensing of epinephrine. RSC Adv. 2017, 7, 3012–3020. [Google Scholar] [CrossRef] [Green Version]
- Mazloum-Ardakani, M.; Beitollahi, H.; Amini, M.K.; Mirjalili, B.-F.; Mirkhalaf, F. Simultaneous determination of epinephrine and uric acid at a gold electrode modified by a 2-(2,3-dihydroxy phenyl)-1, 3-dithiane self-assembled monolayer. J. Electroanal. Chem. 2011, 651, 243–249. [Google Scholar] [CrossRef]
- Zohreh, M.; Ghoreishi, S.M.; Behpour, M.; Mohammadhassan, M. Applied electrochemical biosensor based on covalently self-assembled monolayer at gold surface for determination of epinephrine in the presence of ascorbic acid. Arab. J. Chem. 2017, 10, S657–S664. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, B.N.; Swamy, B.E.K.; Gururaj, K.J.; Cheng, C. Simultaneous determination of epinephrine, ascorbic acid and folic acid using TX-100 modified carbon paste electrode: A cyclic voltammetric study. J. Mol. Liq. 2017, 231, 379–385. [Google Scholar] [CrossRef]
- Rajabi, H.; Noroozifar, M.; Khorasani-Motlagh, M. An Electrochemical Sensor Based on Ionic Liquid-Multiwall Carbon Nanotubes/Graphite Paste Electrode for Simultaneous Determination of Epinephrine and Xanthine in Pharmaceutical and Biological Samples. Anal. Bioanal. Electrochem. 2016, 8, 522–534. [Google Scholar]
- Akyilmaz, E.; Canbay, E.; Dinckaya, E.; Guvenc, C.; Yas I¸ Bayram, E. Simultaneous Determination of Epinephrine and Dopamine by Using Candida tropicalis Yeast Cells Immobilized in a Carbon Paste Electrode Modified with Single Wall Carbon Nanotube. Electroanalysis 2017, 29, 1976–1984. [Google Scholar] [CrossRef]
- Alpat, Ş.; Özdemir, K.; Kılınç Alpat, S. Voltammetric Determination of Epinephrine in Pharmaceutical Sample with a Tyrosinase Nanobiosensor. J. Sens. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sabri, N.; Aljunid, S.A.; Salim, M.S.; Ahmad, R.B.; Kamaruddin, R. Toward optical sensors: Review and applications. J. Phys. Conf. Ser. 2013, 423, 012064. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.N.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef]
- Rasheed, T.; Li, C.; Zhang, Y.; Nabeel, F.; Peng, J.; Qi, J.; Gong, L.; Yu, C. Rhodamine-based multianalyte colorimetric probe with potentialities as on-site assay kit and in biological systems. Sens. Actuators B 2018, 258, 115–124. [Google Scholar] [CrossRef]
- Rasheed, T.; Li, C.; Nabeel, F.; Qi, M.; Zhang, Y.; Yu, C. Real-time probing of mercury using an efficient “turn-on” strategy with potential as in-field mapping kit and in live cell imaging. New J. Chem. 2018, 42, 1094010946. [Google Scholar] [CrossRef]
- Weng, S.; Liang, D.; Qiu, H.; Liu, Z.; Lin, Z.; Zheng, Z.; Liu, A.; Chen, W.; Lin, X. An Unique Turn-off Fluorescent Strategy for Sensing Dopamine Based on Formed Polydopamine (pDA) Using Graphene Quantum Dots (GQDs) as Fluorescent Probe. Sens. Actuators B Chem. 2015, 221, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Solich, P.; Polydorou, C.K.; Koupparis, M.A.; Efstathiou, C.E. Automated flow-injection spectrophotometric determination of catecholamines (epinephrine and isoproterenol) in pharmaceutical formulations based on ferrous complex formation. J. Pharm. Biomed. Anal. 2000, 22, 781–789. [Google Scholar] [CrossRef]
- Golonka, L. Technology and application of Low Temperature Cofired Ceramic (LTCC) based sensors and microsystems. Bull. Polish Acad. Sci. Tech. Sci. 2006, 54, 221–231. [Google Scholar]
- Ibáñez-Garcia, N.; Martinez-Cisneros, C.S.; Valdés, F.; Alonso, J. Green-tape ceramics. New technological approach for integrating electronics and fluidics in microsystems. Trends Anal. Chem. 2008, 27, 24–33. [Google Scholar] [CrossRef]
- Groß, G.A.; Thelemann, T.; Schneider, S.; Boskovic, D.; Köhler, J.M. Fabrication and fluidic characterization of static micromixers made of low temperature co-fired ceramic (LTCC). Chem. Eng. Sci. 2008, 63, 2773–2784. [Google Scholar] [CrossRef]
- Pander, P.; Data, P.; Turczyn, R.; Lapkowski, M.; Swist, A.; Soloducho, J.; Monkman, A.P. Synthesis and characterization of chalcogenophene-based monomers with pyridine acceptor unit. Electrochim. Acta 2016, 210, 773–782. [Google Scholar] [CrossRef]
- Malecha, K. The implementation of fluorescence-based detection in LTCC (low-temperature-co-fired-ceramics) microfluidic modules. Int. J. Appl. Ceram. Technol. 2016, 13, 69–77. [Google Scholar] [CrossRef]
- Malecha, K. Low temperature co-fired ceramics based modular fluidic system for efficient optical measurements. Int. J. Appl. Ceram. Technol. 2017, 14, 630–635. [Google Scholar] [CrossRef]
- Baluta, S.; Cabaj, J.; Malecha, K. Neurotransmitters detection using a fluorescence-based sensor with graphene quantum dots. Opt. Appl. 2017, 47, 225–231. [Google Scholar]
- Melin, V.; Henríquez, A.; Freer, J. Contreras D. Reactivity of catecholamine-driven Fenton reaction and its relationships with iron (III) speciation. Redox Rep. 2015, 20, 89–96. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of L-adrenaline: A structure–activity insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef]
- Korać, J.; Stanković, D.M.; Stanić, M.; Bajuk-Bogdanović, D.; Žižić, M.; Pristov, J.B.; Grgurić-Šipka, S.; Popović-Bijelić, A.; Spasojević, I. Coordinate and redox interactions of epinephrine with ferric and ferrous iron at physiological pH. Sci. Rep. 2018, 8, 3530. [Google Scholar] [CrossRef]
- Cabaj, J.; Jędrychowska, A.; Zając, D.; Krawiec, S.; Sołoducho, J. Phenolic Compounds Determination Using Laccase-based Electrode Modified with Conducting Polymer Support. Int. J. Electrochem. Sci. 2016, 11, 609–620. [Google Scholar]
- Baluta, S.; Malecha, K.; Zając, D.; Sołoducho, J.; Cabaj, J. Dopamine sensing with fluorescence strategy based on low temperature co-fired ceramic technology modified with conducting polymers. Sens. Actuators B Chem. 2017, 252, 803–812. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Li, G.L.; Zhou, N.N.; Wang, R.X.; Chi, Y.W.; Chen, G.N. Graphene quantum dot as a green and facile sensor for free chlorine in drinking water. Anal. Chem. 2012, 84, 8378–8382. [Google Scholar] [CrossRef] [PubMed]
- Faber, K. Biotransformations in Organic Chemistry, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Thanh, T.D.; Balamurugan, J.; Tuan, N.T.; Jeong, H.; Lee, S.H.; Kim, N.H.; Lee, J.H. Enhanced electrocatalytic performance of an ultrafine AuPt nanoalloy framework embedded in graphene towards epinephrine sensing. Biosens. Bioelectron. 2016, 89, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Khafaji, M. Application of pyrolytic graphite modified with nano-diamond/graphite film for simultaneous voltammetric determination of epinephrine and uric acid in the presence of ascorbic acid. Electrochim. Acta 2010, 55, 9090–9096. [Google Scholar] [CrossRef]















| EP Concentration in Real Sample (μM) | EP Concentration Detected Using Proposed Method (μM) | Recovery (%) |
|---|---|---|
| 20.00 | 17.80 | 89 |
| 120.00 | 116.95 | 97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baluta, S.; Malecha, K.; Świst, A.; Cabaj, J. Fluorescence Sensing Platforms for Epinephrine Detection Based on Low Temperature Cofired Ceramics. Sensors 2020, 20, 1429. https://doi.org/10.3390/s20051429
Baluta S, Malecha K, Świst A, Cabaj J. Fluorescence Sensing Platforms for Epinephrine Detection Based on Low Temperature Cofired Ceramics. Sensors. 2020; 20(5):1429. https://doi.org/10.3390/s20051429
Chicago/Turabian StyleBaluta, Sylwia, Karol Malecha, Agnieszka Świst, and Joanna Cabaj. 2020. "Fluorescence Sensing Platforms for Epinephrine Detection Based on Low Temperature Cofired Ceramics" Sensors 20, no. 5: 1429. https://doi.org/10.3390/s20051429





