A Regionalized Genome-Based Mexican Diet Improves Anthropometric and Metabolic Parameters in Subjects at Risk for Obesity-Related Chronic Diseases
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Participants
2.2. Study Design
2.3. Anthropometric Assessment
2.4. Laboratory Tests
2.5. Features of the Regionalized GENOMEX Diet
2.6. Dietary Assessment
2.7. Genetic Analysis
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Genetic Profile of DRAG Polymorphisms
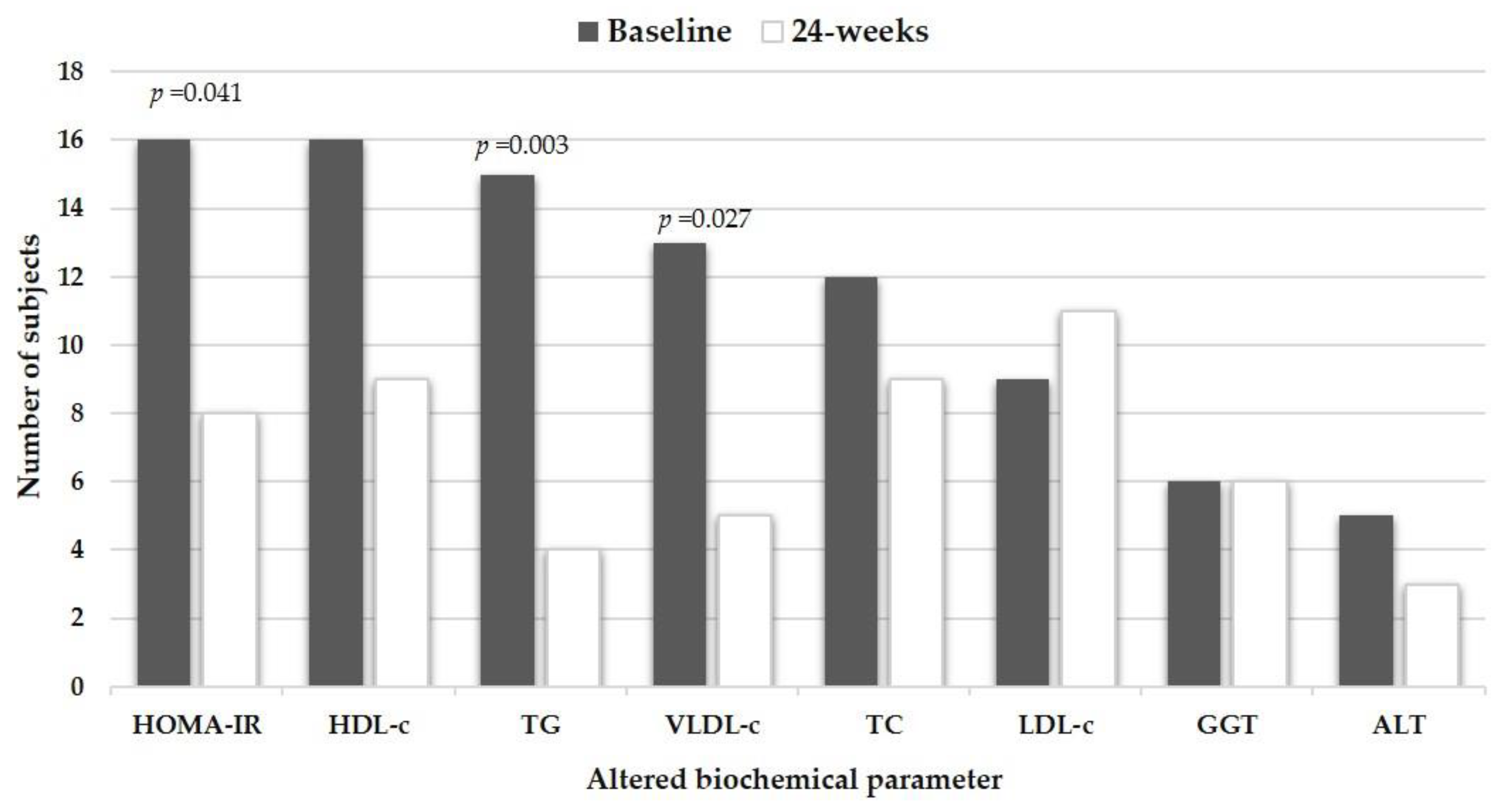
3.3. Metabolic and Anthropometric Response to the Regionalized GENOMEX Diet
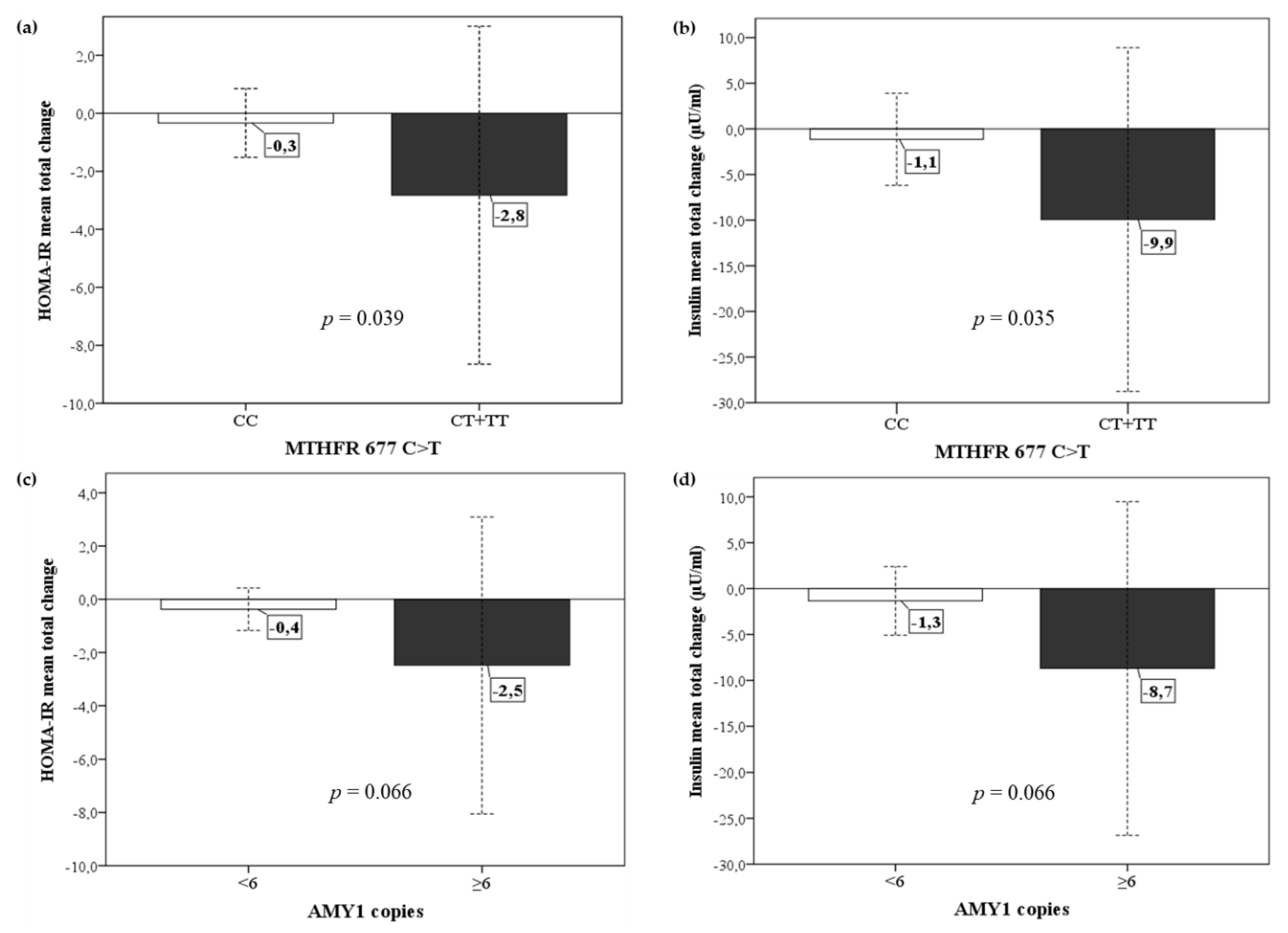
3.4. Metabolic and Anthropometric Response to Diet in Relation to the Genetic Profile of DRAG Polymorphisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.; Perry, G.H.; Di Rienzo, A. Evolutionary Adaptations to Dietary Changes. Annu. Rev. Nutr. 2010, 30, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Gu, Z. Recent advances in understanding the role of nutrition in human genome evolution. Adv. Nutr. 2011, 2, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Sazzini, M.; Gnecchi Ruscone, G.A.; Giuliani, C.; Sarno, S.; Quagliariello, A.; De Fanti, S.; Boattini, A.; Gentilini, D.; Fiorito, G.; Catanoso, M.; et al. Complex interplay between neutral and adaptive evolution shaped differential genomic background and disease susceptibility along the Italian peninsula. Sci. Rep. 2016, 6, 32513. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Roman, S.; Ojeda-Granados, C.; Panduro, A. Genética y evolución de la alimentación de la población en México. Rev. Endocrinol. Nutr. 2013, 21, 42–51. [Google Scholar]
- Sepulveda-Villegas, M.; Roman, S.; Rivera-Iñiguez, I.; Ojeda-Granados, C.; Gonzalez-Aldaco, K.; Torres-Reyes, L.A.; Jose-Abrego, A.; Panduro, A. High prevalence of nonalcoholic steatohepatitis and abnormal liver stiffness in a young and obese Mexican population. PLoS ONE 2019, 14, e0208926. [Google Scholar] [CrossRef]
- Ramos-López, O.; Román, S.; Ojeda-Granados, C.; Sepúlveda-Villegas, M.; Martínez-López, E.; Torres-Valadez, R.; Trujillo-Trujillo, E.; Panduro, A. Patrón de ingesta alimentaria y actividad física en pacientes hepatópatas en el Occidente de México. Rev. Endocrinol. Nutr. 2013, 21, 7–17. [Google Scholar]
- Ojeda-Granados, C.; Panduro, A.; Gonzalez-Aldaco, K.; Sepulveda-Villegas, M.; Rivera-Iñiguez, I.; Roman, S. Tailoring Nutritional Advice for Mexicans Based on Prevalence Profiles of Diet-Related Adaptive Gene Polymorphisms. J. Pers. Med. 2017, 7, 16. [Google Scholar] [CrossRef]
- Isordia-Salas, I.; Barinagarrementería-Aldatz, F.; Leaños-Miranda, A.; Borrayo-Sánchez, G.; Vela-Ojeda, J.; García-Chávez, J.; Ibarra-González, I.; Majluf-Cruz, A. The C677T polymorphism of the methylenetetrahydrofolate reductase gene is associated with idiopathic ischemic stroke in the young Mexican-Mestizo population. Cerebrovasc. Dis. 2010, 29, 454–459. [Google Scholar] [CrossRef]
- Gallegos-Arreola, M.P.; García-Ortiz, J.E.; Figuera, L.E.; Puebla-Pérez, A.M.; Morgan-Villela, G.; Zúñiga-González, G.M. Association of the 677C →T polymorphism in the MTHFR gene with colorectal cancer in Mexican patients. Cancer Genom. Proteom. 2009, 6, 183–188. [Google Scholar]
- Aguilar-Salinas, C.A.; Canizales-Quinteros, S.; Rojas-Martínez, R.; Mehta, R.; Rodriguez-Guillén, R.; Ordoñez-Sanchez, M.L.; Riba, L.; Tusié-Luna, M.T. The non-synonymous Arg230Cys variant (R230C) of the ATP-binding cassette transporter A1 is associated with low HDL cholesterol concentrations in Mexican adults: A population based nation wide study. Atherosclerosis 2011, 216, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, E.; Curiel-Lopez, F.; Hernandez-Nazara, A.; Moreno-Luna, L.E.; Ramos-Marquez, M.E.; Roman, S.; Panduro, A. Influence of ApoE and FABP2 polymorphisms and environmental factors in the susceptibility to gallstone disease. Ann. Hepatol. 2015, 14, 515–523. [Google Scholar] [CrossRef]
- Cahua-Pablo, G.; Cruz, M.; Moral-Hernández, O.D.; Leyva-Vázquez, M.A.; Antúnez-Ortiz, D.L.; Cahua-Pablo, J.A.; Alarcón-Romero, L.D.C.; Ortuño-Pineda, C.; Moreno-Godínez, M.E.; Hernández-Sotelo, D.; et al. Elevated Levels of LDL-C are Associated With ApoE4 but Not With the rs688 Polymorphism in the LDLR Gene. Clin. Appl. Thromb. Hemost. 2016, 22, 465–470. [Google Scholar] [CrossRef]
- Ojeda-Granados, C.; Panduro, A.; Rebello Pinho, J.R.; Ramos-Lopez, O.; Gleyzer, K.; Malta, F.M.; Gonzalez-Aldaco, K.; Roman, S. Association of Lactase Persistence Genotypes with High Intake of Dairy Saturated Fat and High Prevalence of Lactase Non-Persistence among the Mexican Population. Lifestyle Genom. 2016, 9, 83–94. [Google Scholar] [CrossRef]
- Mejía-Benítez, M.A.; Bonnefond, A.; Yengo, L.; Huyvaert, M.; Dechaume, A.; Peralta-Romero, J.; Klünder-Klünder, M.; García Mena, J.; El-Sayed Moustafa, J.S.; Falchi, M.; et al. Beneficial effect of a high number of copies of salivary amylase AMY1 gene on obesity risk in Mexican children. Diabetologia 2015, 58, 290–294. [Google Scholar] [CrossRef]
- Santiago-Torres, M.; De Dieu Tapsoba, J.; Kratz, M.; Lampe, J.W.; Breymeyer, K.L.; Levy, L.; Song, X.; Villaseñor, A.; Wang, C.-Y.; Fejerman, L.; et al. Genetic ancestry in relation to the metabolic response to a US versus traditional Mexican diet: A randomized crossover feeding trial among women of Mexican descent. Eur. J. Clin. Nutr. 2017, 71, 395–401. [Google Scholar] [CrossRef]
- Aguilar, C.A.; Talavera, G.; Ordovas, J.M.; Barriguete, J.A.; Guillén, L.E.; Leco, M.E.; Pedro-Botet, J.; Gonzalez-Barranco, J.; Gómez-Pérez, F.J.; Rull, J.A. The apolipoprotein E4 allele is not associated with an abnormal lipid profile in a Native American population following its traditional lifestyle. Atherosclerosis 1999, 142, 409–414. [Google Scholar] [CrossRef]
- Roman, S.; Ojeda-Granados, C.; Ramos-Lopez, O.; Panduro, A. Genome-based nutrition: An intervention strategy for the prevention and treatment of obesity and nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 3449–3461. [Google Scholar] [CrossRef]
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; de Luis, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. Lifestyle Genom. 2016, 9, 12–27. [Google Scholar] [CrossRef]
- San-Cristobal, R.; Navas-Carretero, S.; Livingstone, K.M.; Celis-Morales, C.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Lambrinou, C.P.; Moschonis, G.; Marsaux, C.F.M.; et al. Mediterranean Diet Adherence and Genetic Background Roles within a Web-Based Nutritional Intervention: The Food4Me Study. Nutrients 2017, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Galilea-Zabalza, I.; Buil-Cosiales, P.; Salas-Salvadó, J.; Toledo, E.; Ortega-Azorín, C.; Díez-Espino, J.; Vázquez-Ruiz, Z.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS ONE 2018, 13, e0198974. [Google Scholar] [CrossRef]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Tovar, A.R.; Aguilar-Salinas, C.A.; Medina-Vera, I.; Gil-Zenteno, L.; Hernández-Viveros, I.; López-Romero, P.; Ordaz-Nava, G.; Canizales-Quinteros, S.; Guillen Pineda, L.E.; et al. A dietary pattern including nopal, chia seed, soy protein, and oat reduces serum triglycerides and glucose intolerance in patients with metabolic syndrome. J. Nutr. 2012, 142, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Torres, M.; Kratz, M.; Lampe, J.W.; Tapsoba, J.D.D.; Breymeyer, K.L.; Levy, L.; Villaseñor, A.; Wang, C.-Y.; Song, X.; Neuhouser, M.L. Metabolic responses to a traditional Mexican diet compared with a commonly consumed US diet in women of Mexican descent: A randomized crossover feeding trial1,2. Am. J. Clin. Nutr. 2016, 103, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Avila-Nava, A.; Noriega, L.G.; Tovar, A.R.; Granados, O.; Perez-Cruz, C.; Pedraza-Chaverri, J.; Torres, N. Food combination based on a pre-hispanic Mexican diet decreases metabolic and cognitive abnormalities and gut microbiota dysbiosis caused by a sucrose-enriched high-fat diet in rats. Mol. Nutr. Food Res. 2017, 61, 1501023. [Google Scholar] [CrossRef]
- Bjornstad, P.; Eckel, R.H. Pathogenesis of Lipid Disorders in Insulin Resistance: A Brief Review. Curr. Diabetes Rep. 2018, 18, 127. [Google Scholar] [CrossRef]
- Ortiz Solís, G.R.; Hernández y Hernández, H. Resumen integrado de la NOM-037-SSA2-2012 y Guía de Tratamiento Farmacológico de las Dislipidemias. Rev. Mex. Cardiol. 2013, 24, 3–22. [Google Scholar]
- NHANES Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Anthropometry Procedures Manual; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2013.
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Z.; Shen, W.; Heymsfield, S.B.; Heshka, S. Percentage body fat ranges associated with metabolic syndrome risk: Results based on the third National Health and Nutrition Examination Survey (1988–1994). Am. J. Clin. Nutr. 2003, 78, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.J.; Morrissette, H.; Gagné, J.-M.; Bergeron, J.; Gagné, C.; Couture, P. Validation of the Friedewald formula for the determination of low-density lipoprotein cholesterol compared with beta-quantification in a large population. Clin. Biochem. 2004, 37, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Yamada, C.; Mitsuhashi, T.; Hiratsuka, N.; Inabe, F.; Araida, N.; Takahashi, E. Optimal reference interval for homeostasis model assessment of insulin resistance in a Japanese population. J. Diabetes Investig. 2011, 2, 373–376. [Google Scholar] [CrossRef]
- Roman, S.; Rivera-Iñiguez, I.; Ojeda-Granados, C.; Sepulveda-Villegas, M.; Panduro, A. Chapter 1—Genome-Based Nutrition in Chronic Liver Disease. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–14. [Google Scholar]
- Pérez Lizaur, A.B.; Palacios González, B.; Castro Becerra, A.L.; Flores Galicia, I. Sistema Mexicano de Alimentos Equivalentes, 4th ed.; Cuadernos de Nutrición (Fomento de Nutrición y Salud, A.C.): CDMX, Mexico, 2011; ISBN 978-607-00-7928-3. [Google Scholar]
- Medicine, I. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-08525-0. [Google Scholar]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Romero-Hidalgo, S.; Villarreal-Molina, T.; González-Barrios, J.A.; Canizales-Quinteros, S.; Rodríguez-Arellano, M.E.; Yañez-Velazco, L.B.; Bernal-Alcantara, D.A.; Villa, A.R.; Antuna-Puente, B.; Acuña-Alonzo, V.; et al. Carbohydrate Intake Modulates the Effect of the ABCA1-R230C Variant on HDL Cholesterol Concentrations in Premenopausal Women. J. Nutr. 2012, 142, 278–283. [Google Scholar] [CrossRef]
- Jensen, M.D. Role of Body Fat Distribution and the Metabolic Complications of Obesity. J. Clin. Endocrinol. Metab. 2008, 93, S57–S63. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Behavioural Weight Management Review Group Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Ghanavati, M.; Lamuchi-Deli, N.; Payami, S.A.; Alavi-Rad, S.; Boustaninejad, M.; Afrisham, R.; Abbasnezhad, A.; Alipour, M. Rapid Weight Loss vs. Slow Weight Loss: Which is More Effective on Body Composition and Metabolic Risk Factors? Int. J. Endocrinol. Metab. 2017, 15, e13249. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of Nonalcoholic Hepatic Steatosis, Hepatic Insulin Resistance, and Hyperglycemia by Moderate Weight Reduction in Patients With Type 2 Diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Manzanero-Medina, G.I.; Pérez-Herrera, A.; Lustre-Sánchez, H.; Vásquez-Dávila, M.A.; Santos-Sánchez, N.F.; Sánchez-Medina, M.A. Ethnobotanical and nutritional study of quelites sold in two traditional markets of Oaxaca, Mexico. BioRxiv 2018. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Tovar, J.; Osorio-Díaz, P.; Paredes-López, O.; Bello-Pérez, L.A. In vitro starch digestibility and predicted glycemic index of corn tortilla, black beans, and tortilla-bean mixture: Effect of cold storage. J. Agric. Food Chem. 2005, 53, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Valdivia-López, M.Á.; Tecante, A. Chia (Salvia hispanica): A Review of Native Mexican Seed and its Nutritional and Functional Properties. Adv. Food Nutr. Res. 2015, 75, 53–75. [Google Scholar]
- Orona-Tamayo, D.; Valverde, M.E.; Paredes-López, O. Bioactive peptides from selected latin american food crops—A nutraceutical and molecular approach. Crit. Rev. Food Sci. Nutr. 2019, 59, 1949–1975. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Samblas, M.; Milagro, F.I.; Zulet, M.A.; Mansego, M.L.; Riezu-Boj, J.I.; Martinez, J.A. Association of low dietary folate intake with lower CAMKK2 gene methylation, adiposity, and insulin resistance in obese subjects. Nutr. Res. 2018, 50, 53–62. [Google Scholar] [CrossRef]
- Colson, N.J.; Naug, H.L.; Nikbakht, E.; Zhang, P.; McCormack, J. The impact of MTHFR 677 C/T genotypes on folate status markers: A meta-analysis of folic acid intervention studies. Eur. J. Nutr. 2017, 56, 247–260. [Google Scholar] [CrossRef]
- Lisboa, J.V.d.C.; Ribeiro, M.R.; Luna, R.C.P.; Lima, R.P.A.; Nascimento, R.A.F.; Monteiro, M.G.C.A.; Lima, K.Q.d.F.; Fechine, C.P.N.d.S.; Oliveira, N.F.P.d.; Persuhn, D.C.; et al. Food Intervention with Folate Reduces TNF-α and Interleukin Levels in Overweight and Obese Women with the MTHFR C677T Polymorphism: A Randomized Trial. Nutrients 2020, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.L.; Breslin, P.A.S. High Endogenous Salivary Amylase Activity Is Associated with Improved Glycemic Homeostasis following Starch Ingestion in Adults123. J. Nutr. 2012, 142, 853–858. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.; Valsesia, A.; Kulkarni, S.; Marquis, J.; Leone, P.; Mironova, P.; Walter, O.; Saris, W.; Descombes, P.; Hager, J.; et al. Understanding Determinants of Carbohydrate Metabolism and Their Contribution to Metabolic Health; The Impact of AMY1 CNV (P21-015-19). Curr. Dev. Nutr. 2019, 3, nzz041.P21-015-19. [Google Scholar] [CrossRef]
- Gasteyger, C.; Larsen, T.M.; Vercruysse, F.; Astrup, A. Effect of a dietary-induced weight loss on liver enzymes in obese subjects. Am. J. Clin. Nutr. 2008, 87, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
| GENOMEX Diet | Study Subjects (Baseline) | p | Reference Values [39] | |
|---|---|---|---|---|
| Macronutrients | ||||
| Total energy (kcal) | 1453.6 ± 113.0 | 2332.4 ± 853.0 | <0.001 | − |
| Protein (%) | 20.1 ± 2.5 | 17.6 ± 4.2 | <0.001 | 15–20 |
| Total fat (%) | 31.6 ± 5.1 | 31.3 ± 7.8 | 0.808 | 25–30 |
| SFAs (%) | 5.6 ± 3.9 | 9.0 ± 3.9 | <0.001 | <7 |
| MUFAs (%) | 11.5 ± 3.6 | 10.6 ± 4.0 | 0.208 | 10–15 |
| PUFAs (%) | 8.3 ± 2.5 | 5.1 ± 2.5 | <0.001 | 7–10 |
| Cholesterol (mg) | 155.7 ± 105.3 | 300.4 ± 186.6 | <0.001 | <200 |
| Carbohydrates (%) | 52.7 ± 4.7 | 53.4 ± 9.0 | 0.642 | 50–55 |
| Fiber (g/d) | 32.0 ± 6.9 | 25.1 ± 11.7 | 0.001 | 25–38 |
| Micronutrients | ||||
| Folates (µg/d of DFE) | 301.0 ± 130.5 | 246.1 ± 164.6 | 0.022 | 300–600 |
| Vitamin A (µg/d) | 1342.9 ± 961.1 | 1198.5 ± 991.6 | 0.396 | 900 |
| Vitamin C (mg/d) | 269.3 ± 135.2 | 155.1 ± 117.5 | <0.001 | 90 |
| Vitamin E (mg/d) | 6.1 ± 2.4 | 16.6 ± 73.5 | 0.392 | 15 |
| Thiamin (mg/d) | 1.3 ± 0.3 | 1.6 ± 0.6 | 0.007 | 1.1–1.2 |
| Riboflavin (mg/d) | 1.2 ± 0.3 | 1.6 ± 0.8 | 0.009 | 1.1–1.3 |
| Niacin (mg/d) | 14.2 ± 5.4 | 20.6 ± 8.8 | <0.001 | 16 |
| Pyridoxine (mg/d) | 1.5 ± 0.4 | 1.7 ± 0.7 | 0.009 | 1.7 |
| Cobalamin (µg/d) | 2.1 ± 5.3 | 7.2 ± 20.5 | 0.138 | 2.4 |
| Pantothenic acid (mg/d) | 8.4 ± 21.6 | 6.1 ± 15.3 | 0.522 | 5 |
| Calcium (mg/d) | 1121.1 ± 428.9 | 1180.8 ± 588.3 | 0.454 | 1000 |
| Iron (mg/d) | 14.8 ± 2.9 | 17.6 ± 6.7 | 0.018 | 8–18 |
| Sodium (mg/d) | 1111.8 ± 481.3 | 1911.3 ± 1107.6 | <0.001 | 1500 |
| Potassium (mg/d) | 2888.2 ± 614.3 | 3001.9 ± 1078.2 | 0.534 | 4700 |
| Selenium (µg/d) | 45.0 ± 18.4 | 52.8 ± 22.1 | 0.020 | 55 |
| Phosphorus (mg/d) | 721.7 ± 216.2 | 909.9 ± 419.0 | 0.011 | 700 |
| Magnesium (mg/d) | 367.1 ± 123.4 | 339.3 ± 175.2 | 0.359 | 310–420 |
| Zinc (mg/d) | 5.3 ± 1.7 | 10.8 ± 6.8 | <0.001 | 8–11 |
| LCT-13910 C>T | MTHFR 677 C>T | ABCA1 R230C | APOE ε2, ε3, ε4 | AMY1 Copies | |||||
|---|---|---|---|---|---|---|---|---|---|
| CC | 28 (75.7) | CC | 19 (51.4) | RR | 28 (75.7) | E2/E2 | 0 (0.0) | 6.27 ± 2.9 | |
| E2/E3 | 2 (5.4) | ||||||||
| CT | 8 (21.6) | CT | 13 (35.1) | RC | 9 (24.3) | E2/E4 | 0 (0.0) | ||
| E3/E3 | 28 (75.7) | ||||||||
| TT | 1 (2.7) | TT | 5 (13.5) | CC | 0 (0.0) | E3/E4 | 7 (18.9) | ||
| E4/E4 | 0 (0.0) | ||||||||
| C | 64 (86.5) | C | 51 (68.9) | R | 65 (87.8) | ε2 | 2 (2.7) | <6 | 18 (48.6) |
| T | 10 (13.5) | T | 23 (31.1) | C | 9 (12.2) | ε3 | 65 (87.8) | ≥6 | 19 (51.4) |
| ε4 | 7 (9.5) | ||||||||
| HWE | 0.371 | 0.137 | 0.352 | 0.588 | --- | ||||
| Baseline | 14 Weeks | 24 Weeks | Total Change | p * | p ** | |
|---|---|---|---|---|---|---|
| Anthropometrics | ||||||
| Weight (kg) | 80.4 ± 18.5 | 74.5 ± 16.3 | 75.0 ± 16.6 | 5.3 ± 5.3 | < 0.001 | < 0.001 |
| BMI (kg/m2) | 30.0 ± 5.6 | 27.9 ± 5.1 | 28.0 ± 5.1 | 2.0 ± 1.9 | < 0.001 | < 0.001 |
| WC (cm) | 94.8 ± 14.7 | 88.5 ± 13.6 | 88.7 ± 14.1 | 5.9 ± 5.5 | < 0.001 | < 0.001 |
| Body water (kg) | 34.8 ± 8.4 | 35.2 ± 7.0 | 35.0 ± 6.8 | −0.2 ± 3.8 | 0.794 | 0.780 |
| Muscle mass (kg) | 12.9 ± 2.6 | 12.9 ± 2.5 | 12.7 ± 2.6 | 0.3 ± 0.8 | 0.447 | 0.091 |
| Fat mass (kg) | 29.2 ± 11.4 | 23.9 ± 9.6 | 24.6 ± 10.1 | 4.6 ± 4.3 | < 0.001 | < 0.001 |
| Body fat (%) | 35.6 ± 7.0 | 31.3 ± 7.1 | 31.9 ± 7.9 | 3.7 ± 3.3 | < 0.001 | < 0.001 |
| EBW (kg) | 15.7 ± 10.4 | 9.8 ± 9.5 | 10.6 ± 10.3 | 5.3 ± 7.1 | < 0.001 | < 0.001 |
| EFM (kg) | 15.8 ± 10.4 | 10.1 ± 9.1 | 10.5 ± 10.2 | 5.3 ± 7.0 | < 0.001 | < 0.001 |
| Normal weight ղ (%) | 0 (0.0) | 10 (30.3) | 12 (36.4) | −12 (36.4) | − | < 0.001 |
| Overweight ղ (%) | 20 (60.6) | 13 (39.4) | 10 (30.3) | 10 (30.3) | − | 0.013 |
| Obesity ղ (%) | 13 (39.4) | 8 (24.2) | 11 (33.3) | 2 (6.06) | − | 0.609 |
| Biochemicals | ||||||
| Glucose (mg/dL) | 89.0 ± 10.5 | 86.1 ± 11.4 | 84.3 ± 6.6 | 4.7 ± 8.7 | 0.326 | 0.004 |
| Insulin (µU/mL) | 15.0 ± 15.8 | 8.1 ± 4.0 | 8.9 ± 4.9 | 5.5 ± 14.2 | < 0.001 | 0.002 |
| HOMA-IR | 3.6 ± 4.8 | 1.8 ± 0.8 | 1.9 ± 1.0 | 1.6 ± 4.3 | 0.001 | 0.002 |
| TC (mg/dL) | 185.2 ± 39.0 | 184.6 ± 38.0 | 187.5 ± 34.1 | −3.4 ± 30.0 | 0.830 | 0.516 |
| Triglycerides (mg/dL) | 151.5 ± 88.3 | 115.9 ± 47.4 | 108.5 ± 44.7 | 43.0 ± 60.6 | 0.003 | < 0.001 |
| HDL-c (mg/dL) | 41.2 ± 9.0 | 41.6 ± 6.8 | 43.2 ± 7.3 | −0.8 ± 5.8 | 0.790 | 0.503 |
| LDL-c (mg/dL) | 112.0 ± 37.8 | 117.8 ± 32.2 | 124.8 ± 29.6 | −14.3 ± 32.7 | 0.091 | 0.035 |
| VLDL-c (mg/dL) | 31.8 ± 21.5 | 23.5 ± 9.4 | 23.3 ± 11.3 | 9.0 ± 19.7 | 0.068 | 0.004 |
| ALT (IU/L) | 25.3 ± 15.9 | 21.8 ± 14.4 | 22.4 ± 14.7 | 2.6 ± 14.0 | 0.203 | 0.327 |
| AST (IU/L) | 21.4 ± 10.5 | 20.8 ± 16.2 | 20.1 ± 11.4 | 1.2 ± 6.8 | 0.033 | 0.295 |
| GGT (IU/L) | 27.5 ± 22.5 | 23.2 ± 16.6 | 25.2 ± 21.9 | 2.3 ± 10.7 | 0.060 | 0.110 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda-Granados, C.; Panduro, A.; Rivera-Iñiguez, I.; Sepúlveda-Villegas, M.; Roman, S. A Regionalized Genome-Based Mexican Diet Improves Anthropometric and Metabolic Parameters in Subjects at Risk for Obesity-Related Chronic Diseases. Nutrients 2020, 12, 645. https://doi.org/10.3390/nu12030645
Ojeda-Granados C, Panduro A, Rivera-Iñiguez I, Sepúlveda-Villegas M, Roman S. A Regionalized Genome-Based Mexican Diet Improves Anthropometric and Metabolic Parameters in Subjects at Risk for Obesity-Related Chronic Diseases. Nutrients. 2020; 12(3):645. https://doi.org/10.3390/nu12030645
Chicago/Turabian StyleOjeda-Granados, Claudia, Arturo Panduro, Ingrid Rivera-Iñiguez, Maricruz Sepúlveda-Villegas, and Sonia Roman. 2020. "A Regionalized Genome-Based Mexican Diet Improves Anthropometric and Metabolic Parameters in Subjects at Risk for Obesity-Related Chronic Diseases" Nutrients 12, no. 3: 645. https://doi.org/10.3390/nu12030645
APA StyleOjeda-Granados, C., Panduro, A., Rivera-Iñiguez, I., Sepúlveda-Villegas, M., & Roman, S. (2020). A Regionalized Genome-Based Mexican Diet Improves Anthropometric and Metabolic Parameters in Subjects at Risk for Obesity-Related Chronic Diseases. Nutrients, 12(3), 645. https://doi.org/10.3390/nu12030645






