Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
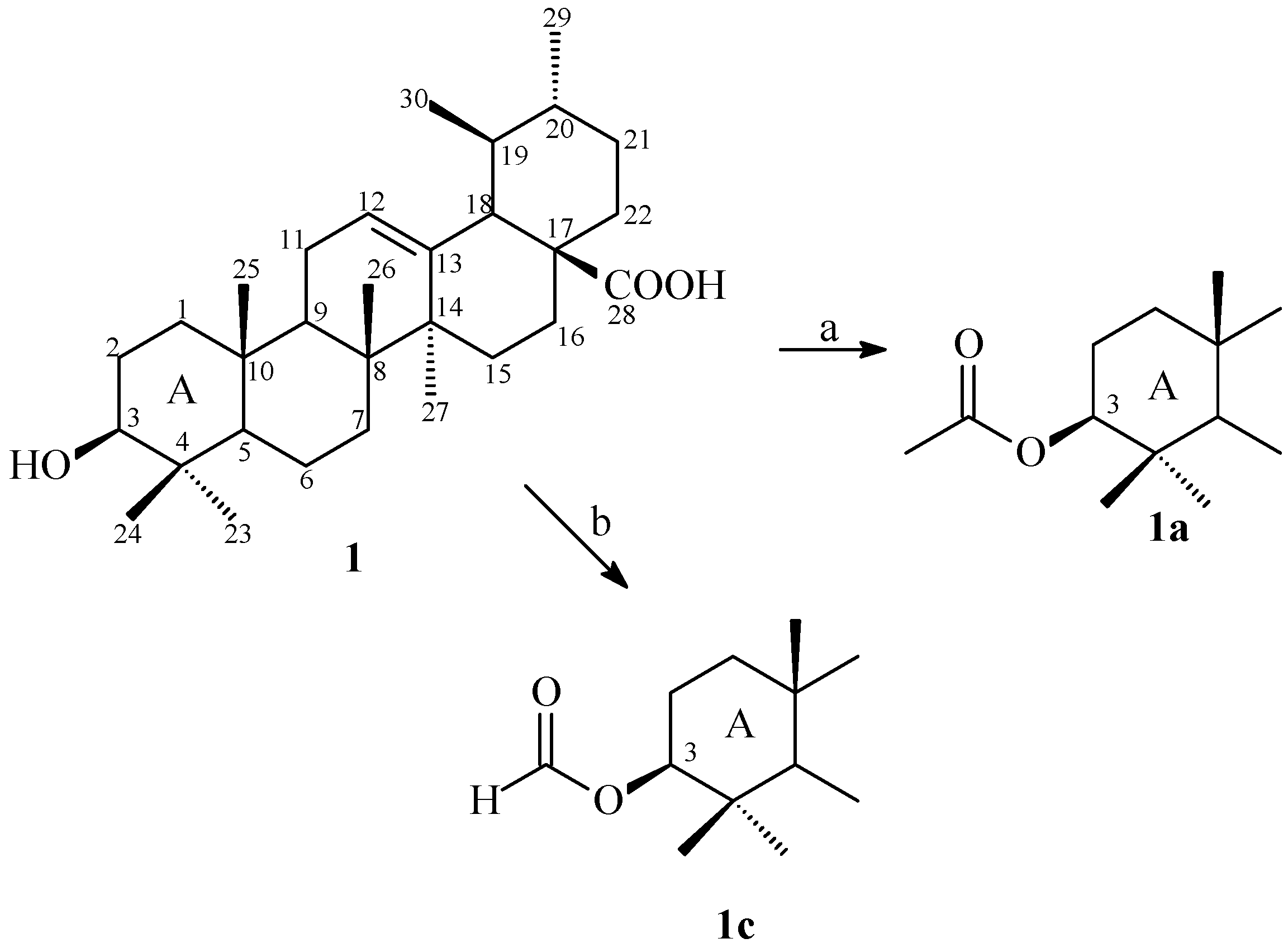
2.2. Antimicrobial Activity and Evaluation of the Modulatory Activity by Direct Contact
| Bacterial strains | MIC (µg/mL) | ||
|---|---|---|---|
| 1 | 1a | 1b | |
| Staphylococcus aureus (ATCC 12692) | ≥1024 | 256 | 512 |
| Staphylococcus aureus (ATCC 12624) | ≥1024 | ≥1024 | ≥1024 |
| Staphylococcus aureus (ATCC 6538) | 32 | 128 | 256 |
| Bacillus cereus (ATCC 33018) | ≥1024 | 512 | 512 |
| Escherichia coli (ATCC 25922) | 64 | 32 | 512 |
| Escherichia coli (ATCC 27) | 512 | 256 | 512 |
| Pseudomonas aeruginosa (ATCC 15442) | 512 | 512 | 512 |
| Aeromonas caveae (ATCC 15468) | ≥1024 | 256 | 256 |
| Klebsiella pneumoniae (ATCC 10031) | 64 | 64 | 128 |
| Shigella flexneri (ATCC 12022) | 64 | 32 | 128 |
| Vibrio colareae (ATCC 15748) | ≥1024 | 512 | ≥1024 |
| Listeria monocytogenes (ATCC 19117) | ≥1024 | 256 | 256 |
| Bacterial strains | Combination tested Antibiotic + Substance (µg/mL) | MIC (µg/mL) | |||
|---|---|---|---|---|---|
| Neomycin | Amikacin | Kanamycin | Gentamicin | ||
| S. aureus 12692 | * | 128 | 64 | 64 | 128 |
| 1a (32) | 16 | 4 | 8 | 32 | |
| 1b (64) | 64 | 32 | 16 | 128 | |
| S. aureus 6538 | * | 128 | 128 | 64 | 64 |
| 1 (4) | 18 | 32 | 16 | 4 | |
| 1a (16) | 16 | 32 | 16 | 16 | |
| 1b (32) | 32 | 64 | 32 | 16 | |
| B. cereus 33018 | * | 128 | 128 | 64 | 64 |
| 1a (64) | 64 | 32 | 8 | 16 | |
| 1b (64) | 64 | 64 | 32 | 64 | |
| E. coli 25922 | * | 128 | 64 | 128 | 64 |
| 1 (8) | 32 | 64 | 64 | 64 | |
| 1a (4) | 32 | 32 | 16 | 16 | |
| 1b (64) | 64 | 32 | 64 | 32 | |
| E. coli 27 | * | 64 | 128 | 128 | 64 |
| 1 (8) | 4 | 32 | 16 | 32 | |
| 1a (32) | 16 | 32 | 32 | 8 | |
| 1b (64) | 32 | 16 | 8 | 8 | |
| P. aeruginosa 15442 | * | 128 | 128 | 64 | 32 |
| 1 (64) | 64 | 32 | 64 | 16 | |
| 1a (64) | 64 | 32 | 16 | 4 | |
| 1b (64) | 32 | 32 | 16 | 8 | |
| A. caveae 15468 | * | 64 | 128 | 128 | 128 |
| 1a (32) | 8 | 16 | 32 | 32 | |
| 1b (64) | 16 | 64 | 32 | 32 | |
| K. pneumonia 10031 | * | 256 | 64 | 64 | 128 |
| 1 (8) | 128 | 32 | 64 | 16 | |
| 1a (8) | 64 | 32 | 16 | 64 | |
| 1b (16) | 128 | 64 | 32 | 64 | |
| S. flexneri 12022 | * | 512 | 128 | 64 | 64 |
| 1 (8) | 64 | 32 | 16 | 16 | |
| 1a (4) | 256 | 64 | 16 | 8 | |
| 1b (16) | 128 | 32 | 16 | 32 | |
| L. monocytogenes 19117 | * | 128 | 128 | 64 | 64 |
| 1a (32) | 16 | 32 | 32 | 8 | |
| 1b (32) | 32 | 64 | 64 | 16 | |
| V. colareae 15748 | * | 64 | 32 | 128 | 128 |
| 1a (64) | 16 | 4 | 16 | 32 | |
2.3. Antioxidant Activity
| Samples | IC50 (mg/mL) |
|---|---|
| 1 | 5.97 × 10−2 ± 1 × 10−3 |
| 1a | 0.73 ± 9.3 × 10−2 |
| 1b | not active |
| Trolox | 2.6 × 10−3 ± 2.3 × 10−4 |
| Vitamin C | 4.3 × 10−2 ± 1.9 × 10−2 |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Synthesis 3β-Acetoxy-urs-12-en-28-oic Acid (1a)
3.5. Synthesis 3β-Formiloxy-urs-12-en-28-oic Acid (1b)
3.6. Antibacterial Activity and Minimal Inhibitory Concentration
3.7. Evaluation of the Modulatory Activity by Direct Contact
3.8. Antioxidant Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kursvietiene, L.; Masteikova, R.; Kopustinskiene, D.M.; Savickas, A.; Bernatoniene, J. Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. J. Nat. Prod. 2011, 74, 1640–1644. [Google Scholar] [CrossRef]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef]
- Shanmungam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Van Baren, C.; Anao, I.; Lira, P.L.; Debenedetti, S.; Houghton, P.; Croft, S.; Martino, V. Triterpenic acids and flavonoids from Satureja parvifolia. Evaluation of their antiprotozoal activity. Z. Naturforsch. C 2006, 61, 189–192. [Google Scholar]
- Ali, M.S.; Ibrahim, S.A.; Jalil, S.; Choudhary, M.I. Ursolic acid: A potent inhibitor of superoxides produced in the cellular system. Phytother. Res. 2007, 21, 558–561. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Fiorentino, A.; Monaco, P.; Pacifico, S. Radical-scavenging activities of new hydroxylated ursane triterpenes from cv. Annurca Apples. Chem. Biodiv. 2005, 2, 953–958. [Google Scholar] [CrossRef]
- Acebey-Castellon, I.L.; Voutquenne-Nazabadioko, L.; Mai, H.D.T.; Roseau, N.; Bouthagane, N.; Muhammad, D.; Debar, E.M.; Gangloff, S.C.; Litaudon, M.; Sevenet, T.; et al. Triterpenoid saponins from Symplocos lancifolia. J. Nat. Prod. 2011, 74, 163–168. [Google Scholar] [CrossRef]
- Rao, V.S.; de Melo, C.L.; Queiroz, M.G.R.; Lemos, T.L.G.; Menezes, D.B.; Melo, T.S.; Santos, F.A. Ursolic Acid, a pentacyclic triterpene from Sambucus australis, prevents abdominal adiposity in mice fed a high-fat diet. J. Med. Food 2011, 14, 1375–1382. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Mahapatra, A.; Jamil, K.; Reddy, P.S. Antimicrobial activity of some pentacyclic triterpenes and their synthesized 3-O-lipophilic chains. Biol. Pharm. Bull. 2004, 27, 1576–1579. [Google Scholar] [CrossRef]
- Cunha, W.R.; de Matos, G.X.; Souza, M.G.M.; Tozatti, M.G.; Silva, M.L.A.; Martins, C.H.G.; da Silva, R.; da Silva Filho, A.A. Evaluation of the antibacterial activity of the methylene chloride extract of Miconia ligustroides, Isolated triterpene acids, and ursolic acid derivatives. Pharm. Biol. 2010, 48, 166–169. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Silva, M.L.; David, J.P.; Silva, L.C.R.C.; Santos, R.A.F.; David, J.M.; Lima, L.S.; Reis, P.S.; Fontana, R. Bioactive oleanane, lupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef]
- Bano, T.; Kumar, N.; Dudhe, R. Free radical scavenging properties of pyrimidine derivatives. Org. Med. Chem. Lett. 2012, 34, 1–6. [Google Scholar]
- Mahato, S.B.; Kundu, A.P. 13C NMR spectra of pentacyclic triterpenoids—A compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Gnoatto, S.C.B.; Dassonville-Klimpt, A.; Nascimento, S.D.; Galéra, P.; Boumediene, K.; Gosmann, G.; Sonnet, P.; Moslemi, S. Evaluation of ursolic acid isolated from Ilex paraguariensis and derivatives on aromatase inhibition. Eur. J. Med. Chem. 2008, 43, 1865–1877. [Google Scholar] [CrossRef]
- Tkachev, A.V.; Denisov, A.Y.; Gatilov, Y.V.; Bagryanskaya, I.Y.; Shevtsov, S.A.; Rybalova, T.V. Stereochemistry of hydrogen peroxide—Acetic acid oxidation of ursolic acid and related compounds. Tetrahedron 1994, 50, 11459–11488. [Google Scholar] [CrossRef]
- Lemos, T.L.G.; Mcchesney, J.D. Utilization of common natural products as synthons: Preparation of progesterone from lithocholic acid. J. Nat. Prod. 1990, 53, 152–156. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard—Sixth Edition; CLSI document M7-A6; NCCLS: Wayne, PA, USA, 2003. [Google Scholar]
- Hadacek, F.; Greger, H. Testing of antifungal natural products: Methodologies, comparability of results and assay choice. Phytochem. Anal. 2000, 11, 137–147. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing: Twelfth Informational Supplement; CLSI document M100-S12; NCCLS: Wayne, PA, USA, 2002; Volume 22, Number 1. [Google Scholar]
- Viljoen, A.; Vuuren, A.V.; Ernst, E.; Klepser, M.; Demirci, B.; Baser, H.; Vanwyk, B.E. Osmitopsis asteriscoides (Asteraceae) the antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol. 2003, 88, 137–143. [Google Scholar] [CrossRef]
- Salvat, A.; Antonnacci, L.; Fortunato, R.H.; Suarez, E.Y.; Godoy, H.M. Screening of some plants from Northern Argentina for their antimicrobial activity. Lett. Appl. Microbiol. 2001, 32, 293–297. [Google Scholar] [CrossRef]
- Sagdiç, O. Sensitivity of four pathogens pathogenic bacteria to Turkish thyme and oregano hydrosols, Lebensm. Wiss. Technol. 2003, 36, 467–473. [Google Scholar] [CrossRef]
- Bandeira, P.N.; Fonseca, A.M.; Costa, S.M.O.; Lins, M.U.D.S.; Pessoa, O.D.L.; Monte, F.J.Q.; Nogueira, N.A.P.; Lemos, T.L.G. Antimicrobial and antioxidant activities of the essential oil of resin of Protium heptaphyllum. Nat. Prod. Commun. 2006, 1, 117–120. [Google Scholar]
- Sample Availability: Samples 1, 1a and 1b are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Do Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, Â.M.C.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules 2014, 19, 1317-1327. https://doi.org/10.3390/molecules19011317
Do Nascimento PGG, Lemos TLG, Bizerra AMC, Arriaga ÂMC, Ferreira DA, Santiago GMP, Braz-Filho R, Costa JGM. Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules. 2014; 19(1):1317-1327. https://doi.org/10.3390/molecules19011317
Chicago/Turabian StyleDo Nascimento, Patrícia G.G., Telma L.G. Lemos, Ayla M.C. Bizerra, Ângela M.C. Arriaga, Daniele A. Ferreira, Gilvandete M.P. Santiago, Raimundo Braz-Filho, and José Galberto M. Costa. 2014. "Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives" Molecules 19, no. 1: 1317-1327. https://doi.org/10.3390/molecules19011317
APA StyleDo Nascimento, P. G. G., Lemos, T. L. G., Bizerra, A. M. C., Arriaga, Â. M. C., Ferreira, D. A., Santiago, G. M. P., Braz-Filho, R., & Costa, J. G. M. (2014). Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules, 19(1), 1317-1327. https://doi.org/10.3390/molecules19011317




