Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus mutans Grown over Dental Resins: An In Vitro Study
Abstract
:1. Introduction
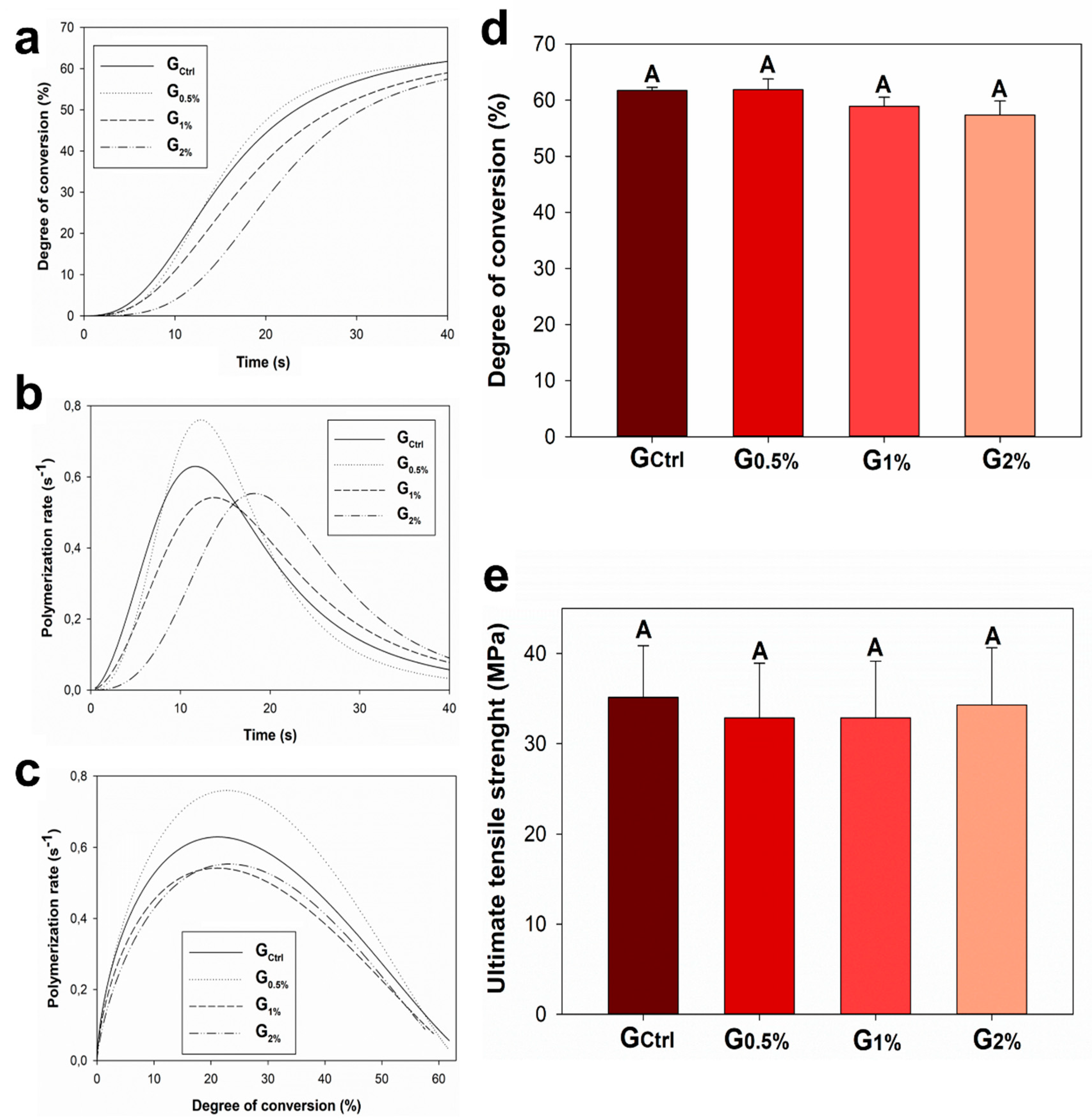
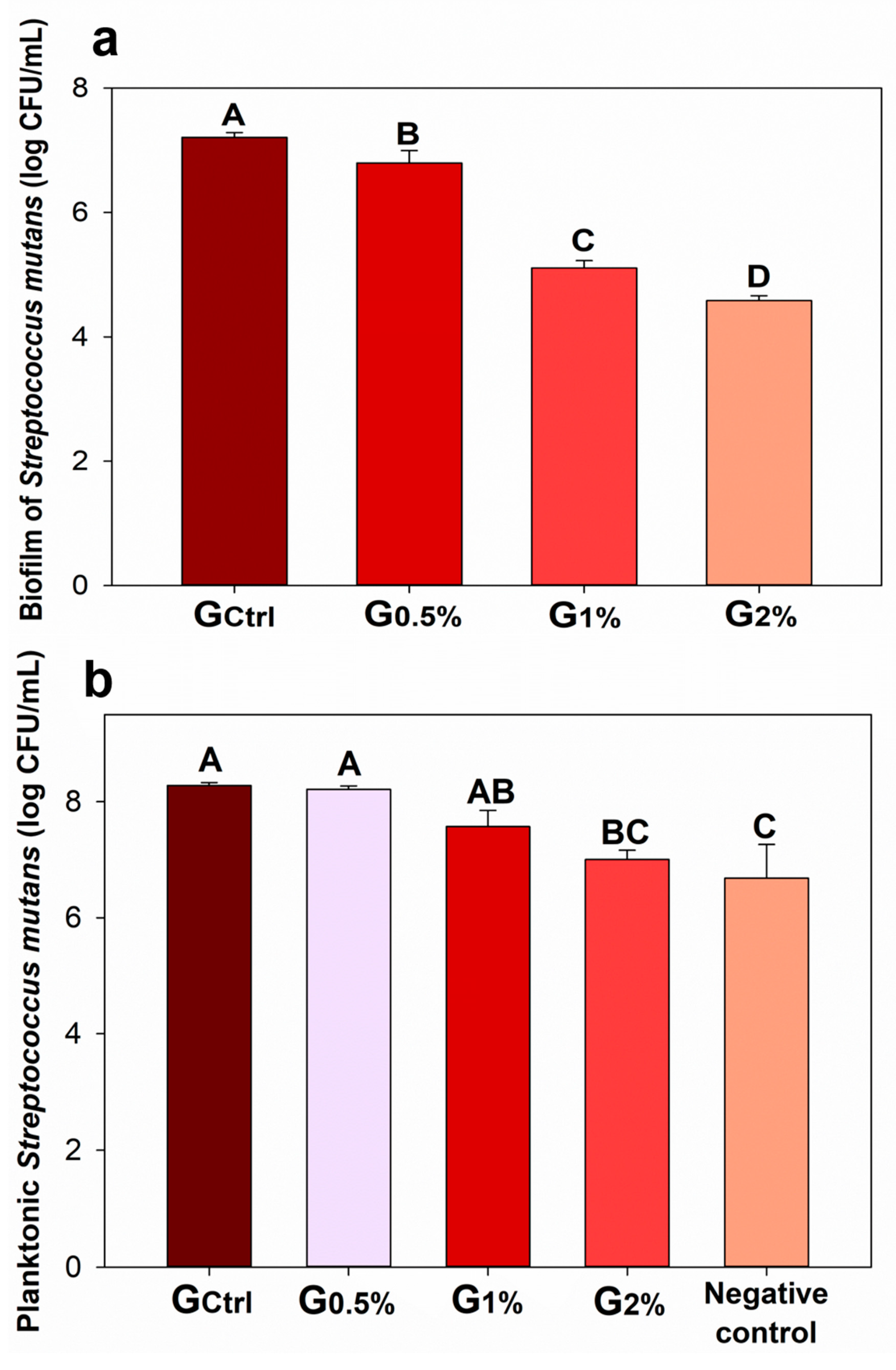
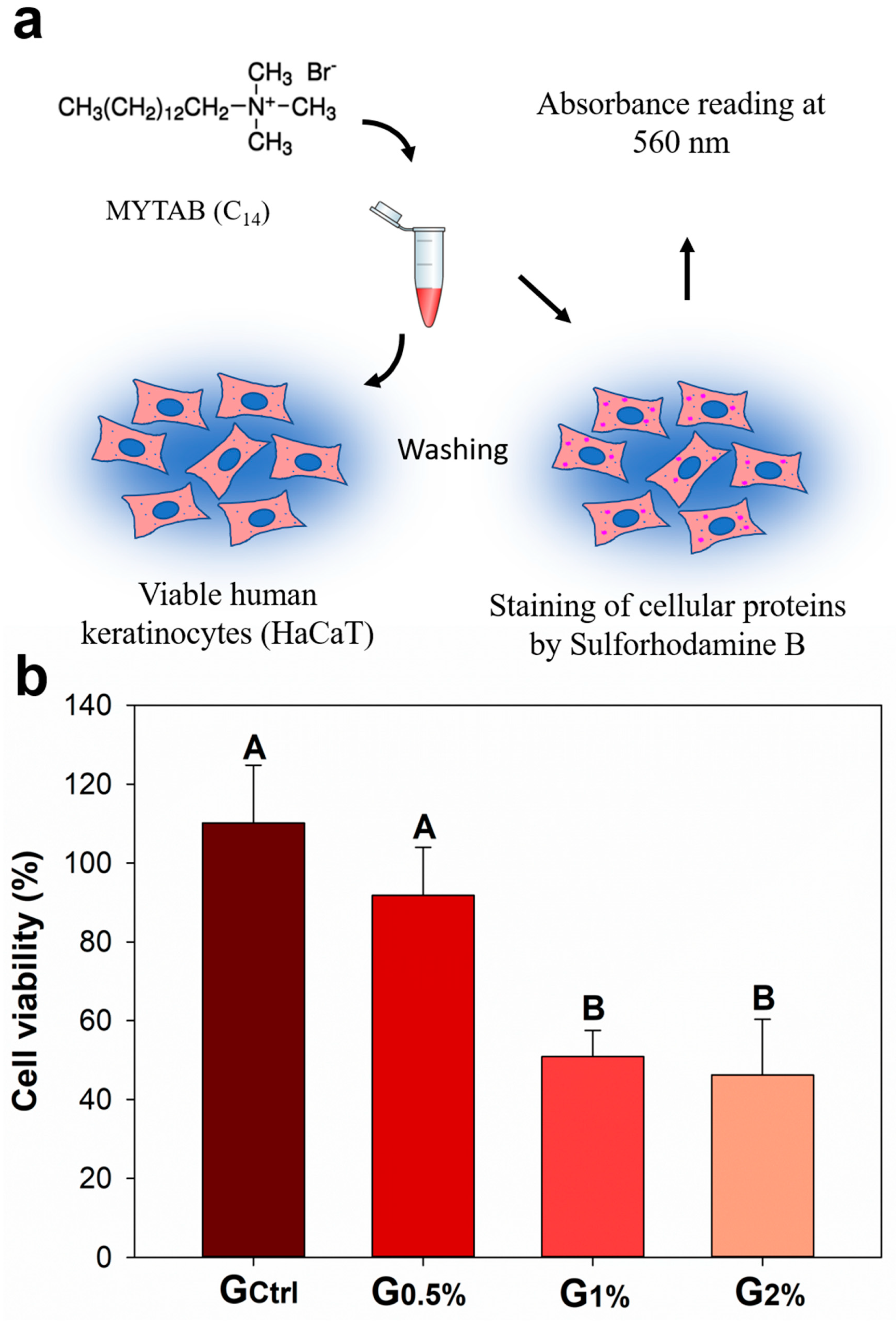
2. Results
3. Discussion
4. Materials and Methods
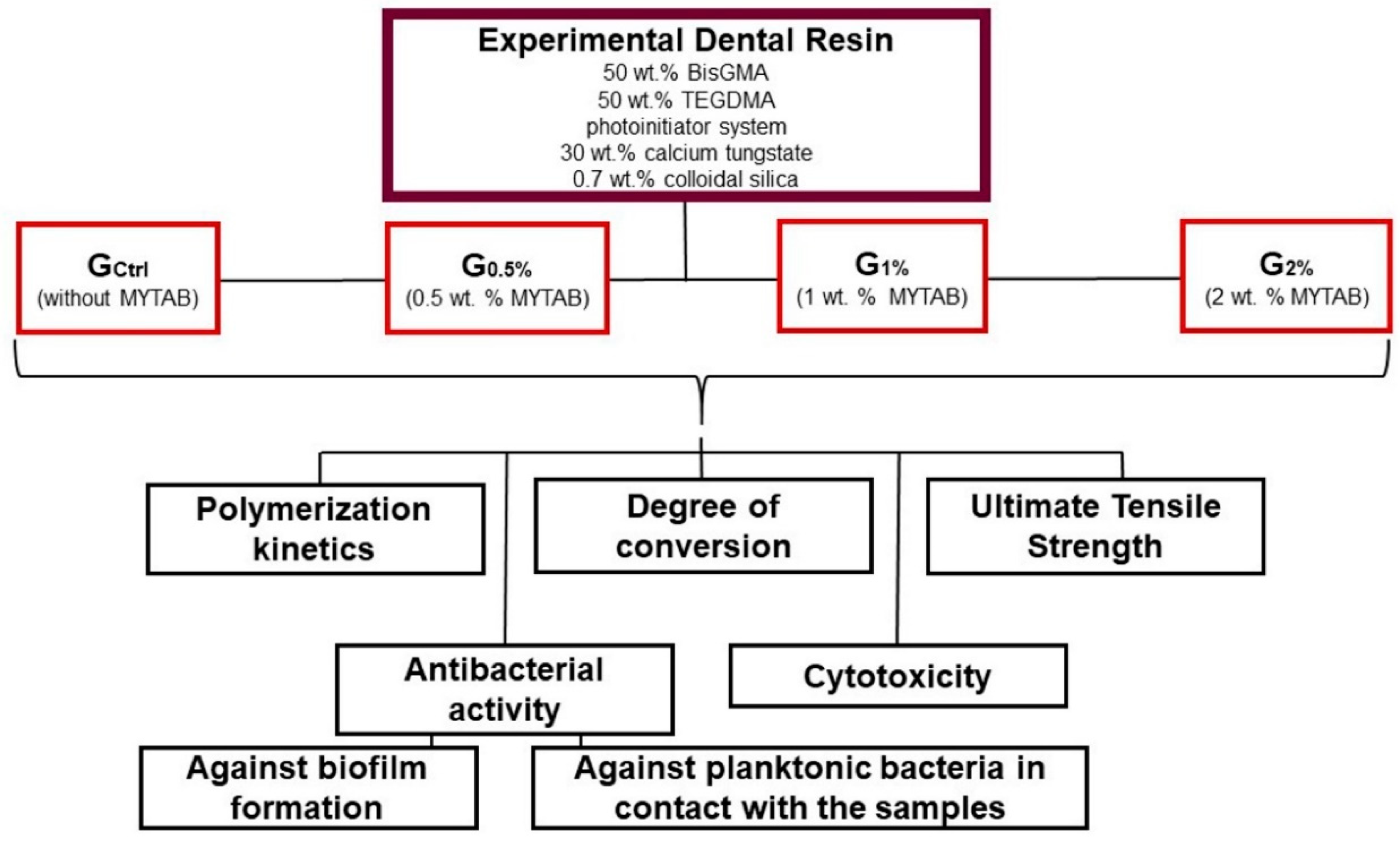
4.1. Study Design and Formulation of Dental Resins
4.2. Polymerization Kinetics and Degree of Conversion
4.3. Ultimate Tensile Strength (UTS)
4.4. Antibacterial Activity
4.5. Cytotoxicity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, N.J. Biofilm over teeth and restorations: What do we need to know? Dent. Mater. 2017, 33, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Srivastava, K.R.; Ajmal, G.; Thakur, V.K.; Gupta, V.K.; Upadhyay, S.N.; Mishra, P.K. Differential Susceptibility of Catheter Biomaterials to Biofilm-Associated Infections and Their Remedy by Drug-Encapsulated Eudragit RL100 Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, D.; Vaz, A.T.; Grainha, T.; Rodrigues, C.F.; Pereira, M.O. Design of an Antifungal Surface Embedding Liposomal Amphotericin B Through a Mussel Adhesive-Inspired Coating Strategy. Front. Chem. 2019, 7, 431. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- He, Y.; Wan, X.; Xiao, K.; Lin, W.; Li, J.; Li, Z.; Luo, F.; Tan, H.; Li, J.; Fu, Q. Anti-biofilm surfaces from mixed dopamine-modified polymer brushes: Synergistic role of cationic and zwitterionic chains to resist staphyloccocus aureus. Biomater. Sci. 2019, 7, 5369–5382. [Google Scholar] [CrossRef]
- Jung, J.; Li, L.; Yeh, C.K.; Ren, X.; Sun, Y. Amphiphilic quaternary ammonium chitosan/sodium alginate multilayer coatings kill fungal cells and inhibit fungal biofilm on dental biomaterials. Mater. Sci. Eng. C 2019, 104, 109961. [Google Scholar] [CrossRef]
- Melo, M.A.; Orrego, S.; Weir, M.D.; Xu, H.H.; Arola, D.D. Designing Multiagent Dental Materials for Enhanced Resistance to Biofilm Damage at the Bonded Interface. ACS Appl. Mater. Interfaces 2016, 8, 11779–11787. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Genari, B.; Leitune, V.C.B.; Arthur, R.A.; Luxan, S.A.; Samuel, S.M.W.; Collares, F.M.; Sauro, S. Polymerisation, antibacterial and bioactivity properties of experimental orthodontic adhesives containing triclosan-loaded halloysite nanotubes. J. Dent. 2018, 69, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Braga, R.R.; Fronza, B.M. The use of bioactive particles and biomimetic analogues for increasing the longevity of resin-dentin interfaces: A literature review. Dent. Mater. J. 2019. [Google Scholar] [CrossRef] [Green Version]
- Andre, C.B.; Chan, D.C.; Giannini, M. Antibacterial-containing dental adhesives’ effects on oral pathogens and on Streptococcus mutans biofilm: Current perspectives. Am. J. Dent. 2018, 31, 37B–41B. [Google Scholar] [PubMed]
- Chi, M.; Qi, M.; A, L.; Wang, P.; Weir, M.D.; Melo, M.A.; Sun, X.; Dong, B.; Li, C.; Wu, J.; et al. Novel Bioactive and Therapeutic Dental Polymeric Materials to Inhibit Periodontal Pathogens and Biofilms. Int. J. Mol. Sci. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, I.M.; Souza, V.S.; Hellriegel, C.; Scholten, J.D.; Collares, F.M. Ionic Liquid-Stabilized Titania Quantum Dots Applied in Adhesive Resin. J. Dent. Res. 2019, 98, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Frencken, J.E.; Bjorndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Domejean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Carious Tissue Removal. Adv. Dent. Res. 2016, 28, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, C.D.; Opdam, N.J.; Hickel, R.; Brunton, P.A.; Gurgan, S.; Kakaboura, A.; Shearer, A.C.; Vanherle, G.; Wilson, N.H. Guidance on posterior resin composites: Academy of Operative Dentistry—European Section. J. Dent. 2014, 42, 377–383. [Google Scholar] [CrossRef]
- Tyas, M.J.; Anusavice, K.J.; Frencken, J.E.; Mount, G.J. Minimal intervention dentistry—A review. FDI Commission Project 1–97. Int. Dent. J. 2000, 50, 1–12. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Ahovuo-Saloranta, A.; Forss, H.; Walsh, T.; Nordblad, A.; Makela, M.; Worthington, H.V. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst. Rev. 2017, 7, CD001830. [Google Scholar] [CrossRef] [Green Version]
- De Munck, J.; Van Meerbeek, B.; Yoshida, Y.; Inoue, S.; Vargas, M.; Suzuki, K.; Lambrechts, P.; Vanherle, G. Four-year water degradation of total-etch adhesives bonded to dentin. J. Dent. Res. 2003, 82, 136–140. [Google Scholar] [CrossRef]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, e41–e53. [Google Scholar] [CrossRef]
- Kusuma Yulianto, H.D.; Rinastiti, M.; Cune, M.S.; de Haan-Visser, W.; Atema-Smit, J.; Busscher, H.J.; van der Mei, H.C. Biofilm composition and composite degradation during intra-oral wear. Dent. Mater. 2019, 35, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.J.; van de Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.; van Dijken, J.W. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ma, S.; Chen, J.H.; Xu, H.H. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent. Mater. 2014, 30, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Shanshoury, A.E.-R.R.; El-Newehy, M.H. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J. Polym. Sci. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polymer 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Quan, A.; McGeachie, A.B.; Keating, D.J.; van Dam, E.M.; Rusak, J.; Chau, N.; Malladi, C.S.; Chen, C.; McCluskey, A.; Cousin, M.A.; et al. Myristyl trimethyl ammonium bromide and octadecyl trimethyl ammonium bromide are surface-active small molecule dynamin inhibitors that block endocytosis mediated by dynamin I or dynamin II. Mol. Pharmacol. 2007, 72, 1425–1439. [Google Scholar] [CrossRef]
- Monteiro, J.C.; Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; de Souza Balbinot, G.; Samuel, S.M.W.; Makeeva, I.; Collares, F.M.; Sauro, S. Halloysite nanotubes loaded with alkyl trimethyl ammonium bromide as antibacterial agent for root canal sealers. Dent. Mater. 2019, 35, 789–796. [Google Scholar] [CrossRef]
- Pallesen, U.; van Dijken, J.W. A randomized controlled 27 years follow up of three resin composites in Class II restorations. J. Dent. 2015, 43, 1547–1558. [Google Scholar] [CrossRef]
- Munoz-Sandoval, C.; Gambetta-Tessini, K.; Giacaman, R.A. Microcavitated (ICDAS 3) carious lesion arrest with resin or glass ionomer sealants in first permanent molars: A randomized controlled trial. J. Dent. 2019, 88, 103163. [Google Scholar] [CrossRef]
- Chisini, L.A.; Collares, K.; Cademartori, M.G.; de Oliveira, L.J.C.; Conde, M.C.M.; Demarco, F.F. Restorations in primary teeth: A systematic review on survival and reasons for failures. Int. J. Paediatr. Dent. 2018, 28, 123–139. [Google Scholar] [CrossRef]
- Collares, F.M.; Ogliari, F.A.; Zanchi, C.H.; Petzhold, C.L.; Piva, E.; Samuel, S.M. Influence of 2-hydroxyethyl methacrylate concentration on polymer network of adhesive resin. J. Adhes. Dent. 2011, 13, 125–129. [Google Scholar] [PubMed]
- Barszczewska-Rybarek, I.M. Characterization of urethane-dimethacrylate derivatives as alternative monomers for the restorative composite matrix. Dent. Mater. 2014, 30, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Martini Garcia, I.; Jung Ferreira, C.; de Souza, V.S.; Castelo Branco Leitune, V.; Samuel, S.M.W.; de Souza Balbinot, G.; de Souza da Motta, A.; Visioli, F.; Damiani Scholten, J.; Mezzomo Collares, F. Ionic liquid as antibacterial agent for an experimental orthodontic adhesive. Dent. Mater. 2019, 35, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Borges, B.C.; Bezerra, G.V.; Mesquita Jde, A.; Pereira, M.R.; Aguiar, F.H.; Santos, A.J.; Pinheiro, I.V. Effect of irradiation times on the polymerization depth of contemporary fissure sealants with different opacities. Braz. Oral Res. 2011, 25, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, S.B.; Collares, F.M.; Leitune, V.C.; Schneider, L.F.; Ogliari, F.A.; Petzhold, C.L.; Samuel, S.M. Influence of hydroxyethyl acrylamide addition to dental adhesive resin. Dent. Mater. 2015, 31, 1579–1586. [Google Scholar] [CrossRef]
- Vidal, M.L.; Rego, G.F.; Viana, G.M.; Cabral, L.M.; Souza, J.P.B.; Silikas, N.; Schneider, L.F.; Cavalcante, L.M. Physical and chemical properties of model composites containing quaternary ammonium methacrylates. Dent. Mater. 2018, 34, 143–151. [Google Scholar] [CrossRef]
- Liang, Y.; Deng, Z.; Dai, X.; Tian, J.; Zhao, W. Micro-invasive interventions for managing non-cavitated proximal caries of different depths: A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2675–2684. [Google Scholar] [CrossRef]
- Yoshimura, T.; Chiba, N.; Matsuoka, K. Supra-long chain surfactants with double or triple quaternary ammonium headgroups. J. Colloid Interface Sci. 2012, 374, 157–163. [Google Scholar] [CrossRef]
- Wang, Y.; Costin, S.; Zhang, J.F.; Liao, S.; Wen, Z.T.; Lallier, T.; Yu, Q.; Xu, X. Synthesis, antibacterial activity, and biocompatibility of new antibacterial dental monomers. Am. J. Dent. 2018, 31, 17b–23b. [Google Scholar]
- Garcia, I.M.; Rodrigues, S.B.; de Souza Balbinot, G.; Visioli, F.; Leitune, V.C.B.; Collares, F.M. Quaternary ammonium compound as antimicrobial agent in resin-based sealants. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef]
- Garcia, I.M.; Rodrigues, S.B.; Leitune, V.C.B.; Collares, F.M. Antibacterial, chemical and physical properties of sealants with polyhexamethylene guanidine hydrochloride. Braz. Oral Res. 2019, 33, e019. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.H.S.; Garcia, I.M.; Motta, A.S.D.; Leitune, V.C.B.; Collares, F.M. Triclosan-loaded chitosan as antibacterial agent for adhesive resin. J. Dent. 2019, 83, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirstila, V.; Hakkinen, P.; Jentsch, H.; Vilja, P.; Tenovuo, J. Longitudinal analysis of the association of human salivary antimicrobial agents with caries increment and cariogenic micro-organisms: A two-year cohort study. J. Dent. Res. 1998, 77, 73–80. [Google Scholar] [CrossRef]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Gindri, I.M.; Siddiqui, D.A.; Bhardwaj, P.; Rodriguez, L.C.; Palmer, K.L.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Dicationic imidazolium-based ionic liquids: A new strategy for non-toxic and antimicrobial materials. RSC Adv. 2014, 4, 62594–62602. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity; ISO 10993-5:2009(E); ISO: Vernier, Geneva, Switzerland, 2009; Volume 1, p. 34. [Google Scholar]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Genari, B.; Leitune, V.C.B.; Jornada, D.S.; Camassola, M.; Arthur, R.A.; Pohlmann, A.R.; Guterres, S.S.; Collares, F.M.; Samuel, S.M.W. Antimicrobial effect and physicochemical properties of an adhesive system containing nanocapsules. Dent. Mater. 2017, 33, 735–742. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena Silva, P.A.; Garcia, I.M.; Nunes, J.; Visioli, F.; Castelo Branco Leitune, V.; Melo, M.A.; Collares, F.M. Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus mutans Grown over Dental Resins: An In Vitro Study. J. Funct. Biomater. 2020, 11, 9. https://doi.org/10.3390/jfb11010009
Mena Silva PA, Garcia IM, Nunes J, Visioli F, Castelo Branco Leitune V, Melo MA, Collares FM. Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus mutans Grown over Dental Resins: An In Vitro Study. Journal of Functional Biomaterials. 2020; 11(1):9. https://doi.org/10.3390/jfb11010009
Chicago/Turabian StyleMena Silva, Paola Andrea, Isadora Martini Garcia, Julia Nunes, Fernanda Visioli, Vicente Castelo Branco Leitune, Mary Anne Melo, and Fabrício Mezzomo Collares. 2020. "Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus mutans Grown over Dental Resins: An In Vitro Study" Journal of Functional Biomaterials 11, no. 1: 9. https://doi.org/10.3390/jfb11010009






