Dietary Platycodon grandiflorus Attenuates Hepatic Insulin Resistance and Oxidative Stress in High-Fat-Diet Induced Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PG
2.2. Animal Experiment
2.3. Glucose Tolerance Test
2.4. Biochemical Analysis
2.5. Measurement of Antioxidant Defenses
2.6. Histological Analysis
2.7. Real-Time Quantitative PCR
2.8. Western Blot
2.9. Statistical Analysis
3. Results
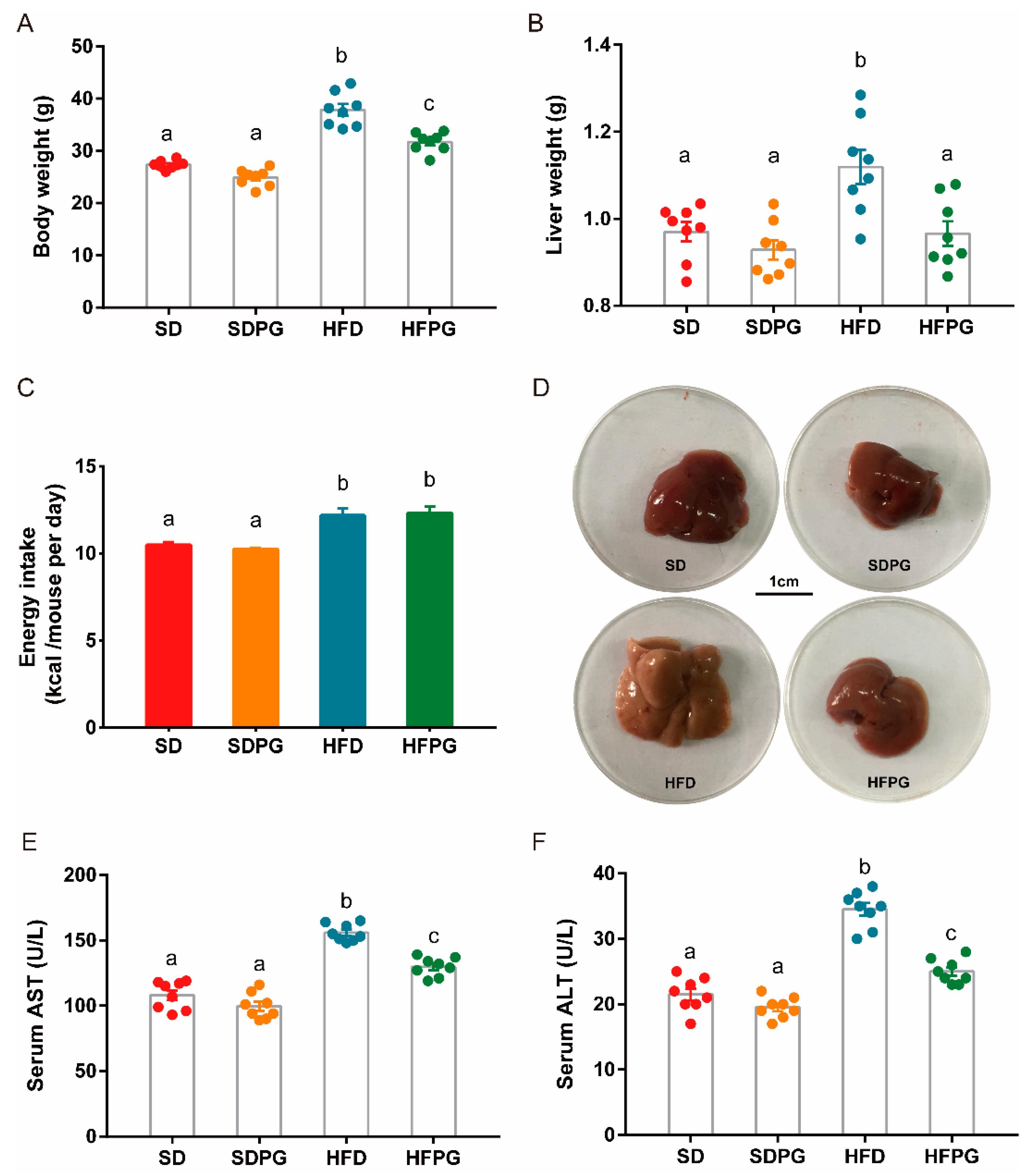
3.1. PG Attenuated Lipid Accumulation and Liver Injuries in HFD-Fed Mice
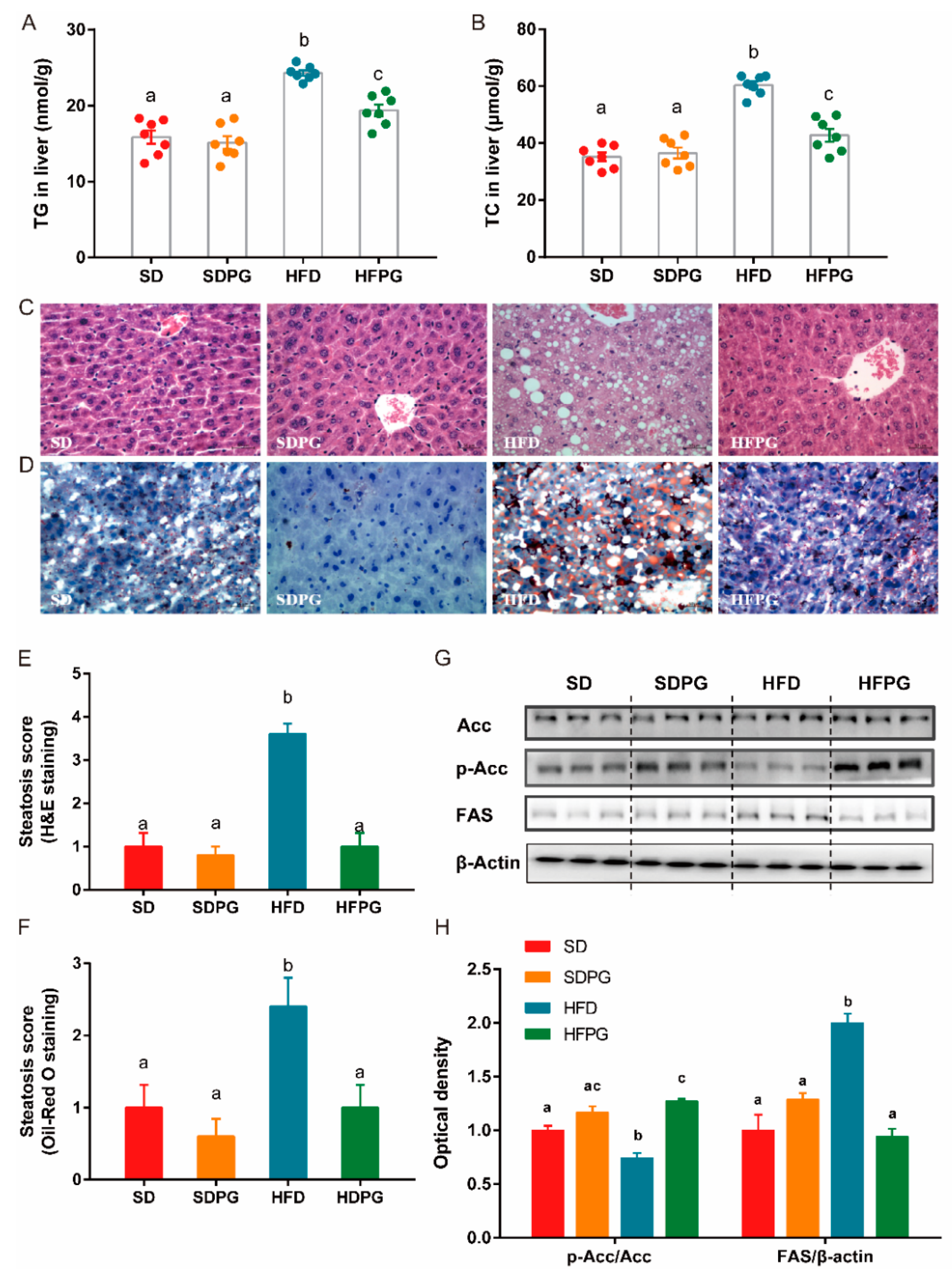
3.2. PG Reduced Steatosis in Liver and Regulated Lipid Metabolism Genes Expression
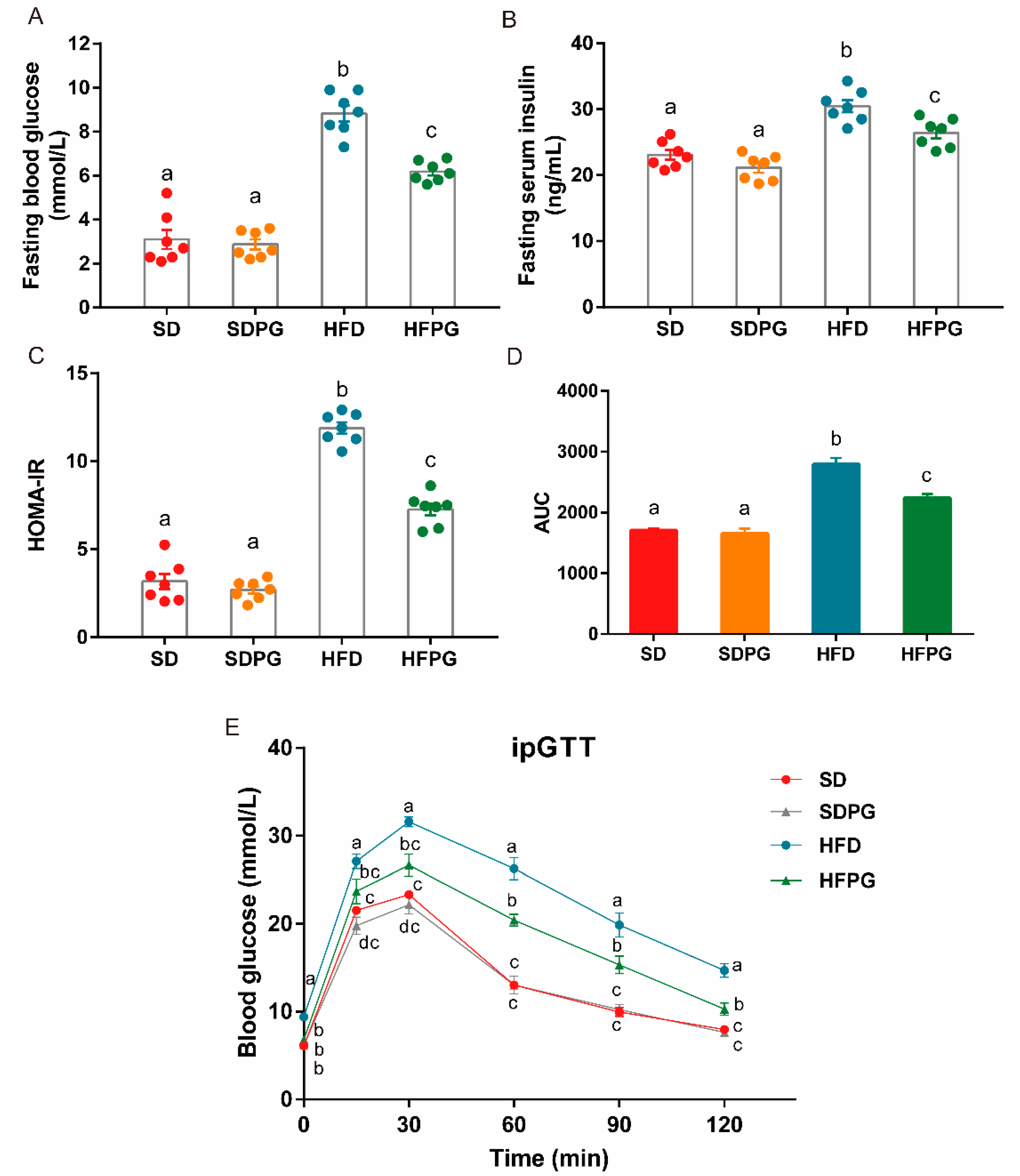
3.3. PG Improved Insulin Sensitivity and Glucose Tolerance in HFD-Fed Mice
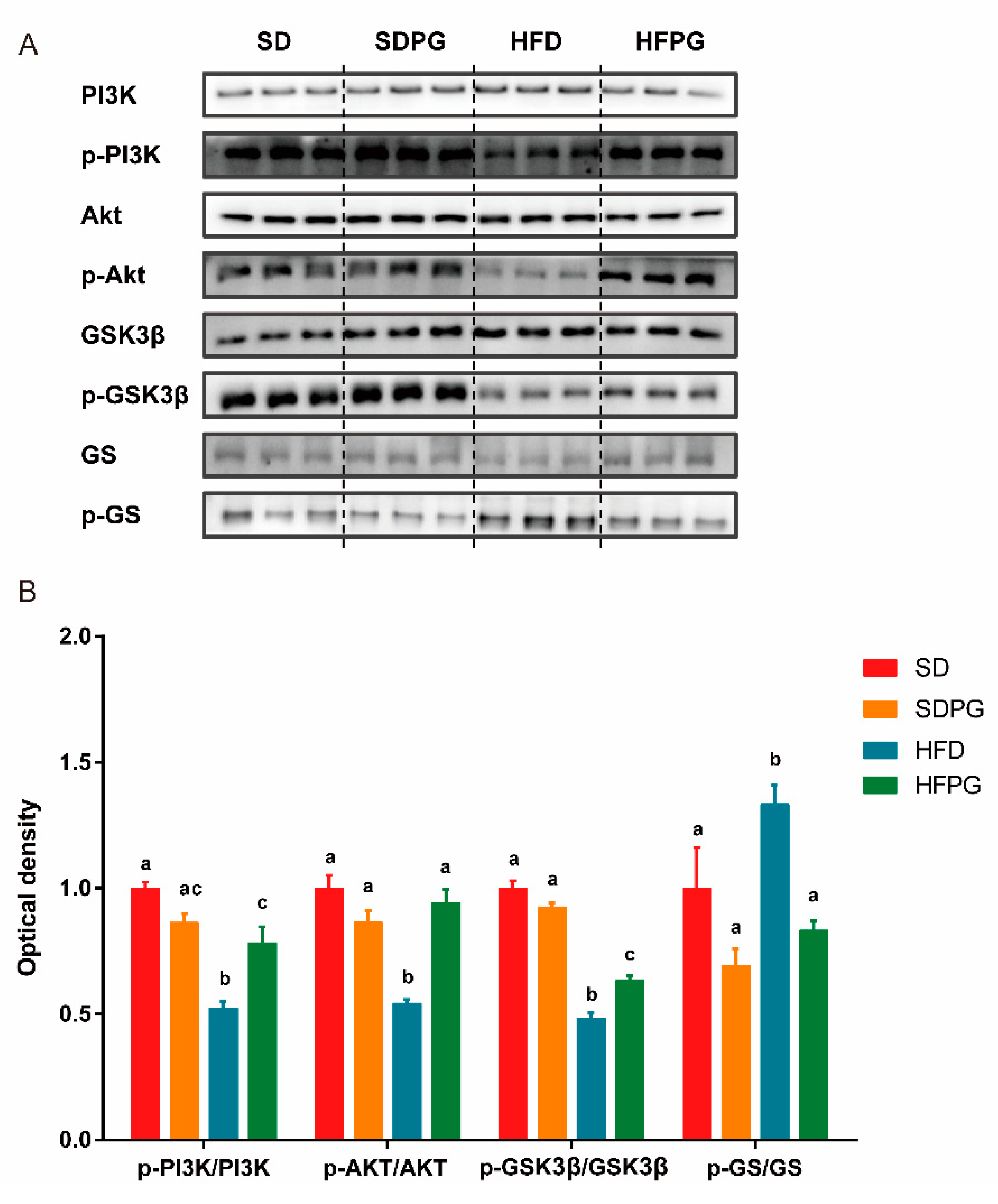
3.4. PG Enhanced Hepatic Insulin Signaling Pathway PI3K/Akt/ GSK3ΒIn HFD Mice
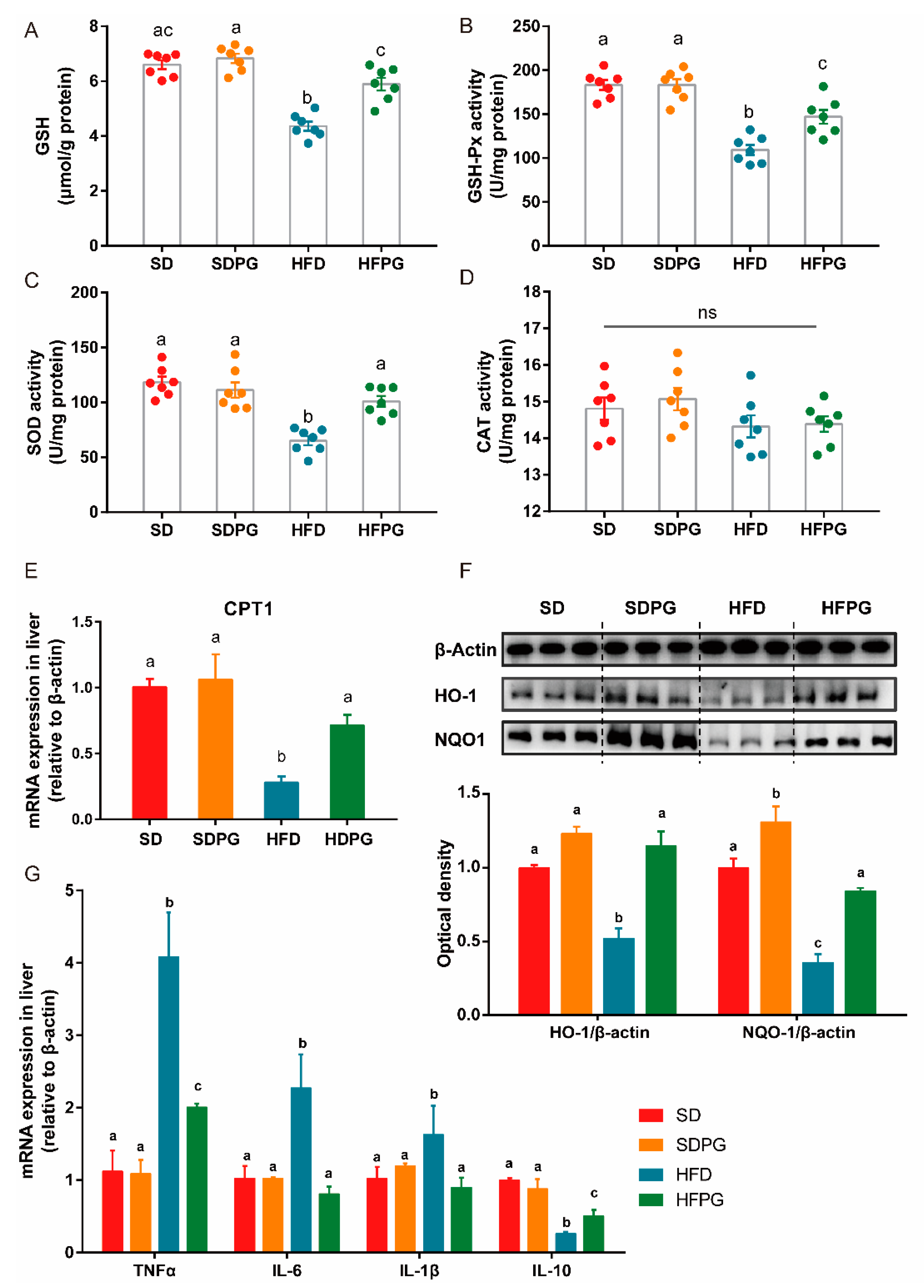
3.5. PG Improved Hepatic Antioxidant Capacity and Reduced Inflammation in HFD-Fed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “Hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, H.J.; Rhyu, D.Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef]
- Kanai, F.; Ito, K.; Todaka, M.; Hayashi, H.; Kamohara, S.; Ishii, K.; Okada, T.; Hazeki, O.; Ui, M.; Ebina, Y. Insulin-Stimulated GLUT4 Translocation Is Relevant to the Phosphorylation of IRS-1 and the Activity of PI3 Kinase. Biochem. Biophys. Res. Commun. 1993, 195, 762–768. [Google Scholar] [CrossRef]
- Tuttle, R.L.; Gill, N.S.; Pugh, W.; Lee, J.-P.; Koeberlein, B.; Furth, E.E.; Polonsky, K.S.; Naji, A.; Birnbaum, M.J. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 2001, 7, 1133–1137. [Google Scholar] [CrossRef]
- Engin, A. Non-alcoholic fatty liver disease. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 960, pp. 443–467. [Google Scholar]
- Pirgon, Ö.; Bilgin, H.; Çekmez, F.; Kurku, H.; Dündar, B.N. Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 33–39. [Google Scholar]
- Videla, L.A.; Rodrigo, R.; Araya, J.; Poniachik, J. Insulin resistance and oxidative stress interdependency in non-alcoholic fatty liver disease. Trends Mol. Med. 2006, 12, 555–558. [Google Scholar] [CrossRef]
- Rector, R.S.; Thyfault, J.P.; Uptergrove, G.M.; Morris, E.M.; Naples, S.P.; Borengasser, S.J.; Mikus, C.R.; Laye, M.J.; Laughlin, M.H.; Booth, F.W.; et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J. Hepatol. 2010, 52, 727–736. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, M.-G.; Jiang, C.-H.; Sheng, X.-P.; Yang, H.-M.; Liu, Y.; Yao, X.-M.; Zhang, J.; Yin, Z.-Q. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates insulin resistance and hepatic steatosis via PI3K/Akt/GSK3β pathway. Phytomedicine 2020, 66, 153130. [Google Scholar] [CrossRef]
- Feng, X.; Yu, W.; Li, X.; Zhou, F.; Zhang, W.; Shen, Q.; Li, J.; Zhang, C.; Shen, P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017, 136, 136–149. [Google Scholar] [CrossRef]
- Park, H.M.; Park, K.T.; Park, E.C.; Kim, S.I.; Choi, M.S.; Liu, K.H.; Lee, C.H. Mass spectrometry-based metabolomic and lipidomic analyses of the effects of dietary platycodon grandiflorum on liver and serum of obese mice under a high-fat diet. Nutrients 2017, 9, 71. [Google Scholar] [CrossRef]
- Kim, H.L.; Park, J.; Jung, Y.; Ahn, K.S.; Um, J.Y. Platycodin D, a novel activator of AMP-activated protein kinase, attenuates obesity in db/db mice via regulation of adipogenesis and thermogenesis. Phytomedicine 2019, 52, 254–263. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Yan, P.; Cheng, G.; Wang, C.; Geng, N.; Wang, X.; Liu, J. Effects of polysaccharides from platycodon grandiflorum on immunity-enhancing activity in vitro. Molecules 2017, 22, 1918. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, Y.P.; Kim, D.H.; Han, E.H.; Chung, Y.C.; Roh, S.H.; Jeong, H.G. Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Biosci. Biotechnol. Biochem. 2006, 70, 858–864. [Google Scholar] [CrossRef]
- Lee, K.J.; Choi, C.Y.; Chung, Y.C.; Kim, Y.S.; Ryu, S.Y.; Roh, S.H.; Jeong, H.G. Protective effect of saponins derived from roots of Platycodon grandiflorum on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Toxicol. Lett. 2004, 147, 271–282. [Google Scholar] [CrossRef]
- Jeong, C.H.; Choi, G.N.; Kim, J.H.; Kwak, J.H.; Kim, D.O.; Kim, Y.J.; Heo, H.J. Antioxidant activities from the aerial parts of Platycodon grandiflorum. Food Chem. 2010, 118, 278–282. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Kim, H.G.; Lee, H.-S.; Chung, Y.C.; Jeong, H.G. Saponins from Platycodon grandiflorum inhibit hepatic lipogenesis through induction of SIRT1 and activation of AMP-activated protein kinase in high-glucose-induced HepG2 cells. Food Chem. 2013, 140, 115–123. [Google Scholar] [CrossRef]
- Khanal, T.; Choi, J.H.; Hwang, Y.P.; Chung, Y.C.; Jeong, H.G. Protective effects of saponins from the root of Platycodon grandiflorum against fatty liver in chronic ethanol feeding via the activation of AMP-dependent protein kinase. Food Chem. Toxicol. 2009, 47, 2749–2754. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Kim, S.J.; Lee, H.S.; Chung, Y.C.; Kim, E.J.; Lee, Y.C.; Jeong, H.G. Saponins from the roots of Platycodon grandiflorum ameliorate high fat diet-induced non-alcoholic steatohepatitis. Biomed. Pharmacother. 2017, 86, 205–212. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. Gut microbiota promotes production of aromatic metabolites through degradation of barley leaf fiber. J. Nutr. Biochem. 2018, 58, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell. Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Wong, V.W.S.; Chitturi, S. NAFLD in Asia—As common and important as in the West. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Hur, H.J.; Hwang, J.T.; Sung, M.J.; Yang, H.J.; Kim, H.J.; Park, J.H.; Kwon, D.Y.; Kim, M.S. Long-term consumption of Platycodi radix ameliorates obesity and insulin resistance via the activation of AMPK pathways. Evid. Based Complement. Altern. Med. 2012, 2012, 759143. [Google Scholar] [CrossRef]
- Nordlie, R.C.; Foster, J.D.; Lange, A.J. Regulation of glucose producing by the liver. Annu. Rev. Nutr. 1999, 19, 379–406. [Google Scholar] [CrossRef]
- Krycer, J.R.; Sharpe, L.J.; Luu, W.; Brown, A.J. The Akt-SREBP nexus: Cell signaling meets lipid metabolism. Trends Endocrinol. Metab. 2010, 21, 268–276. [Google Scholar] [CrossRef]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. SREBP Activity Is Regulated by mTORC1 and Contributes to Akt-Dependent Cell Growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef]
- Gu, Y.; Gao, L.; Han, Q.; Li, A.; Yu, H.; Liu, D.; Pang, Q. GSK-3β at the Crossroads in Regulating Protein Synthesis and Lipid Deposition in Zebrafish. Cell 2019, 8, 205. [Google Scholar] [CrossRef]
- Zheng, T.; Yang, X.; Wu, D.; Xing, S.; Bian, F.; Li, W.; Chi, J.; Bai, X.; Wu, G.; Chen, X.; et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3β pathway. Br. J. Pharmacol. 2015, 172, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Seppälä-Lindroos, A.; Vehkavaara, S.; Häkkinen, A.-M.; Goto, T.; Westerbacka, J.; Sovijärvi, A.; Halavaara, J.; Yki-Järvinen, H. Fat Accumulation in the Liver Is Associated with Defects in Insulin Suppression of Glucose Production and Serum Free Fatty Acids Independent of Obesity in Normal Men. J. Clin. Endocrinol. Metab. 2002, 87, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, K.M.; Kahn, S.E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jin, S.W.; Kim, H.G.; Khanal, T.; Hwang, Y.P.; Lee, K.J.; Choi, C.Y.; Chung, Y.C.; Lee, Y.C.; Jeong, H.G. Platycodi Radix attenuates dimethylnitrosamine-induced liver fibrosis in rats by inducing Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 2013, 56, 231–239. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Abu-Elheiga, L.; Matzuk, M.M.; Abo-Hashema, K.A.H.; Wakil, S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-coa carboxylase 2. Science 2001, 291, 2613–2616. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, R.; Sun, H.; Peng, J.; Ma, X.; Wang, C.Y.; Fu, Y.; Bao, L.; Jin, Y. Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARγ/PGC-1α-Nrf2 pathway. Free Radic. Res. 2018, 52, 198–211. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Ji, H.; Zhang, Z.; Chen, J.; Tan, Y.; Wintergerst, K.; Zheng, Y.; Sun, J.; Cai, L. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci. Rep. 2016, 6, 30252. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Xu, M.; Wang, X.; Wang, C.; Lian, Y.; Mehmood, A.; Dai, H. Stevia residue extract ameliorates oxidative stress in D-galactose-induced aging mice via Akt/Nrf2/HO-1 pathway. J. Funct. Foods 2019, 52, 587–595. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, K.M.; Kim, S.W.; Hwang, O.; Choi, H.J. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: Novel cytoprotective mechanism against oxidative damage. Pharmacol. Res. 2008, 57, 325–331. [Google Scholar] [CrossRef]
| Serum Parameters | SD | SDPG | HFD | HFPG |
|---|---|---|---|---|
| TG (mg/dL) | 0.27 ± 0.02 a | 0.26 ± 0.04 a | 0.42 ± 0.03 b | 0.28 ± 0.03 a |
| TC (mg/dL) | 3.02 ± 0.23 a | 2.93 ± 0.2 a | 5.2 ± 0.16 b | 3.89 ± 0.27 c |
| HDL-C (mmol/L) | 2.52 ± 0.19 a | 3.77 ± 0.12 a | 3.77 ± 0.12 b | 3.13 ± 0.18 c |
| LDL-C (mmol/L) | 0.42 ± 0.03 a | 0.38 ± 0.12 a | 1.18 ± 0.12 b | 0.7 ± 0.1 a |
| LDL-C/HDL-C | 0.18 ± 0.03 a | 0.17 ± 0.02 a | 0.31 ± 0.03 b | 0.22 ± 0.02 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, W.; Wang, P.; Wang, X.; Zhou, X.; Hu, X.; Chen, F. Dietary Platycodon grandiflorus Attenuates Hepatic Insulin Resistance and Oxidative Stress in High-Fat-Diet Induced Non-Alcoholic Fatty Liver Disease. Nutrients 2020, 12, 480. https://doi.org/10.3390/nu12020480
Ke W, Wang P, Wang X, Zhou X, Hu X, Chen F. Dietary Platycodon grandiflorus Attenuates Hepatic Insulin Resistance and Oxidative Stress in High-Fat-Diet Induced Non-Alcoholic Fatty Liver Disease. Nutrients. 2020; 12(2):480. https://doi.org/10.3390/nu12020480
Chicago/Turabian StyleKe, Weixin, Pan Wang, Xuehua Wang, Xiaolu Zhou, Xiaosong Hu, and Fang Chen. 2020. "Dietary Platycodon grandiflorus Attenuates Hepatic Insulin Resistance and Oxidative Stress in High-Fat-Diet Induced Non-Alcoholic Fatty Liver Disease" Nutrients 12, no. 2: 480. https://doi.org/10.3390/nu12020480
APA StyleKe, W., Wang, P., Wang, X., Zhou, X., Hu, X., & Chen, F. (2020). Dietary Platycodon grandiflorus Attenuates Hepatic Insulin Resistance and Oxidative Stress in High-Fat-Diet Induced Non-Alcoholic Fatty Liver Disease. Nutrients, 12(2), 480. https://doi.org/10.3390/nu12020480





