Methylglyoxal, Glycated Albumin, PAF, and TNF-α: Possible Inflammatory and Metabolic Biomarkers for Management of Gestational Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Anthropometry
2.3. Dietary Intervention
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Analysis
3. Results
Correlations
4. Discussion
- (1)
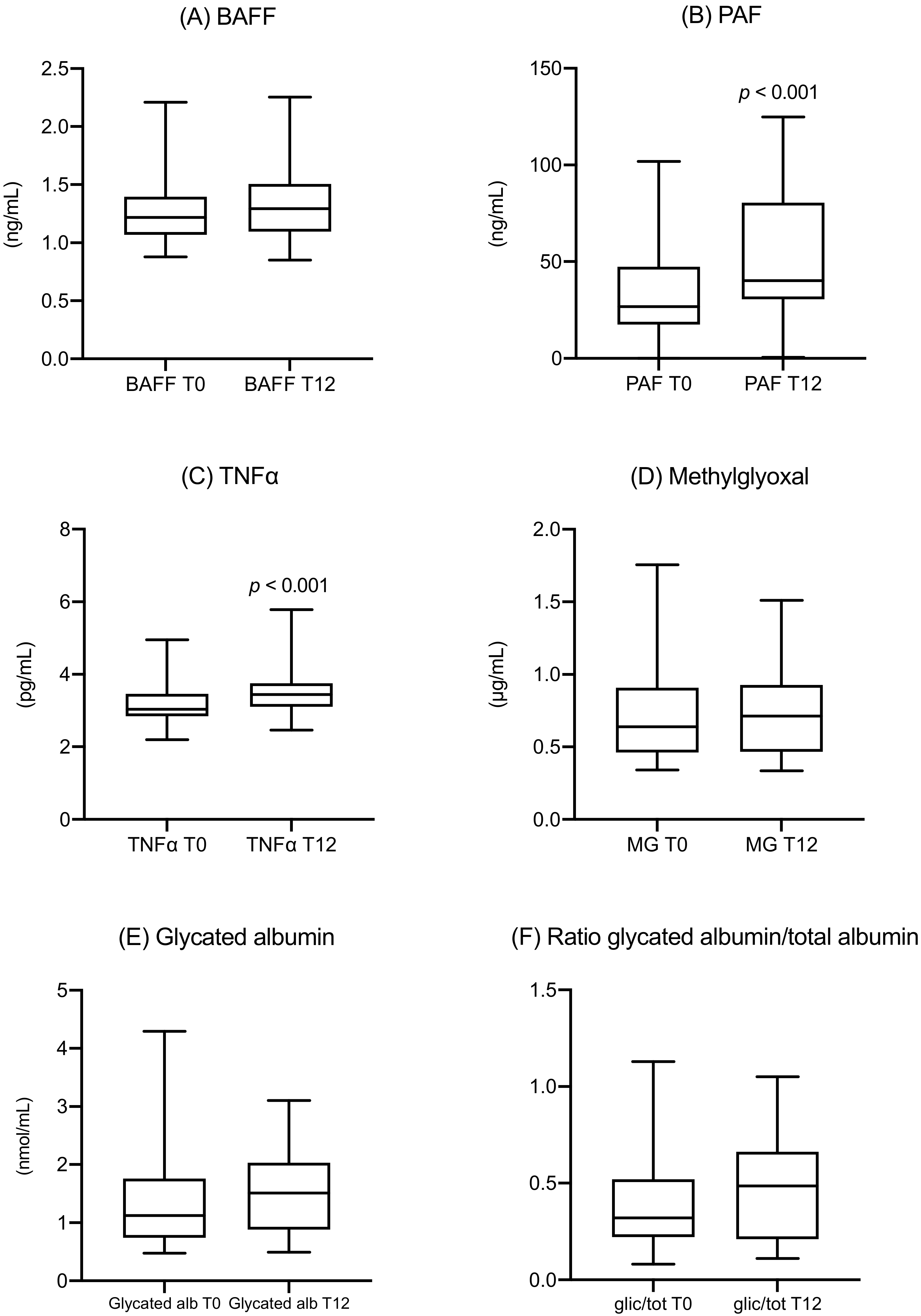
- After the diagnosis, the metabolic parameters of the women following the suggested diet substantially stabilized. The median values of fasting and postprandial glucose levels were normal both at T0 and after 12 weeks, while HbA1c showed a modest increase, but their values remained within the normal range. The same was observed for MGO and GA, which both showed a slight increase in their values but without any statistical significance. The only significant increase was observed in the triglycerides values, confirming the results already shown in literature [32,33]. Despite the stability of the standard glycemic parameters, this increase could reflect an obesogenic pathway [34] due to an inflammatory and metabolic effect on insulin resistance of PAF, TNF-alfa [35], MGO [36], and GA [37], that needs further investigation.
- (2)
- Maternal body weight increased after 12 weeks, while fat mass reduced. This result, also associated with a correct evaluation of the food diaries, suggests an excellent dietetic adherence and a positive effect of the dietary intervention on body composition.
- (3)
- Despite this metabolic stability, a significant increase of two inflammatory cytokines (PAF and TNF-α) was observed, corresponding to the proinflammatory conditions of gestational diabetes mellitus (GDM), acting even without metabolic impairment.
- (4)
- Despite normal HbA1c and fasting glycaemia levels, the metabolic biomarkers MGO and GA were significantly different compared with the general population. For MGO, this difference was evident since the time of diagnosis at around 26 weeks.
- (5)
- Some positive correlations were observed among inflammatory markers, metabolic parameters, and the anthropometric analysis. For example, a strict correlation between MGO and fetal overgrowth was evident, and a correlation between PAF, MGO, and the HOMA index was also observed.
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kalter-Leibovici, O.; Freedman, L.S.; Olmer, L.; Liebermann, N.; Heymann, A.; Tal, O.; Lerner-Geva, L.; Melamed, N.; Hod, M. Screening and diagnosis of gestational diabetes mellitus: Critical appraisal of the new International Association of Diabetes in Pregnancy Study Group recommendations on a national level. Diabetes Care 2012, 35, 1894–1896. [Google Scholar] [CrossRef] [PubMed]
- Gümüş, P.; Özçaka, Ö.; Ceyhan-Öztürk, B.; Akcali, A.; Lappin, D.F.; Buduneli, N. Evaluation of biochemical parameters and local and systemic levels of osteoactive and B-cell stimulatory factors in gestational diabetes in the presence or absence of gingivitis. J. Periodontol. 2015, 86, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.L.; Lombard, C.B.; East, C.; Boyle, J.; Teede, H.J. Risk stratification in early pregnancy for women at increased risk of gestational diabetes. Diabetes Res. Clin. Pract. 2015, 107, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Malamitsi-Puchner, A. Reviews: Adipocytokines in normal and complicated pregnancies. Reprod. Sci. 2009, 16, 921–937. [Google Scholar] [CrossRef]
- Cieślak, M.; Wojtczak, A.; Cieślak, M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim. Pol. 2015, 62, 15–21. [Google Scholar] [CrossRef]
- Stohl, H.E.; Lee, R.H.; Manetta, J.; Kikly, K.; Korst, L.M.; Stohl, W. Maternal Serum B-Cell Activating Factor Levels: Candidate Early Biomarker for Hypertensive Disorders of Pregnancy. Hypertension 2017, 70, 1007–1013. [Google Scholar] [CrossRef]
- Tay, J.; Costanzi, A.; Basello, K.; Piuri, G.; Ferrazzi, E.; Speciani, A.F.; Lees, C.C. Maternal Serum B Cell activating factor in hypertensive and normotensive pregnancies. Pregnancy Hypertens. 2018, 13, 58–61. [Google Scholar] [CrossRef]
- Sankaralingam, S.; Xu, H.; Jiang, Y.; Sawamura, T.; Davidge, S.T. Evidence for increased methylglyoxal in the vasculature of women with preeclampsia: Role in upregulation of LOX-1 and arginase. Hypertension 2009, 54, 897–904. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Choi, B.-H.; Cheon, H.-G.; Do, M.-S. B cell activation factor (BAFF) is a novel adipokine that links obesity and inflammation. Exp. Mol. Med. 2009, 41, 208–216. [Google Scholar] [CrossRef]
- Hamada, M.; Abe, M.; Miyake, T.; Kawasaki, K.; Tada, F.; Furukawa, S.; Matsuura, B.; Hiasa, Y.; Onji, M. B cell-activating factor controls the production of adipokines and induces insulin resistance. Obesity 2011, 19, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Kim, D.-H.; Do, M.-S. B-cell-activating factor is a regulator of adipokines and a possible mediator between adipocytes and macrophages. Exp. Mol. Med. 2013, 45, e4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lundell, A.-C.; Nordström, I.; Andersson, K.; Lundqvist, C.; Telemo, E.; Nava, S.; Kaipe, H.; Rudin, A. IFN type I and II induce BAFF secretion from human decidual stromal cells. Sci. Rep. 2017, 7, 39904–39913. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.M.; Lee, J.; Pilch, P.F. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 1997, 272, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, H.M.; Lappas, M.; Georgiou, G.M.; Marita, A.; Bryant, V.J.; Hiscock, R.; Permezel, M.; Khalil, Z.; Rice, G.E. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008, 45, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kinalski, M.; Telejko, B.; Kuźmicki, M.; Krętowski, A.; Kinalska, I. Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes. Horm. Metab. Res. 2005, 37, 450–454. [Google Scholar] [CrossRef]
- Fan, P.; Liu, X.-H.; He, G.-L.; Zhang, S.; Zhang, J.-X.; Bai, H. Maternal and fetal plasma platelet-activating factor acetylhydrolase activity and distribution in pre-eclampsia. Pediatr. Res. 2012, 72, 426–431. [Google Scholar] [CrossRef]
- Mericq, V.; Piccardo, C.; Cai, W.; Chen, X.; Zhu, L.; Striker, G.E.; Vlassara, H.; Uribarri, J. Maternally transmitted and food-derived glycotoxins: A factor preconditioning the young to diabetes? Diabetes Care 2010, 33, 2232–2237. [Google Scholar] [CrossRef]
- Yang, G.; Cancino, G.I.; Zahr, S.K.; Guskjolen, A.; Voronova, A.; Gallagher, D.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. A Glo1-Methylglyoxal Pathway that Is Perturbed in Maternal Diabetes Regulates Embryonic and Adult Neural Stem Cell Pools in Murine Offspring. Cell Rep. 2016, 17, 1022–1036. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Rajaraman, B.; Ravi, V.; Rajagopal, R.; Ganeshprasad, A.; Kuppuswamy, A.A.; Pathak, A.; Dhevasena, C.S.; Swaminathan, K.; Sundaresan, M.; et al. Association of advanced glycation end products (AGEs) with endothelial dysfunction, oxidative stress in gestational diabetes mellitus (GDM). Int. J. Diabetes Dev. Ctries. 2019, 52, 707. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Chan, W.-H. Methylglyoxal has injurious effects on maturation of mouse oocytes, fertilization, and fetal development, via apoptosis. Toxicol. Lett. 2010, 193, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Garlick, R.L.; Mazer, J.S. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J. Biol. Chem. 1983, 258, 6142–6146. [Google Scholar] [PubMed]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Nouvenne, A.; Lippi, G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J. Diabetes Sci. Technol. 2015, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, D.; Maruyama, A.; Imanishi, T.; Sugiyama, Y.; Ichihashi, K. Complications in Infants of Diabetic Mothers Related to Glycated Albumin and Hemoglobin Levels During Pregnancy. Pediatr. Neonatol. 2016, 57, 496–500. [Google Scholar] [CrossRef]
- Shimizu, I.; Hiramatsu, Y.; Omori, Y.; Nakabayashi, M. JGA (Japan Glycated Albumin) Study Group Comparison of HbA1c and glycated albumin as a control marker for newborn complications in diabetic women in a multicentre study in Japan (Japan glycated albumin study group: Study 2). Ann. Clin. Biochem. 2018, 55, 639–646. [Google Scholar] [CrossRef]
- Sugawara, D.; Sato, H.; Ichihashi, K.; Nagai, K.; Kawano, A. Glycated albumin level during late pregnancy as a predictive factor for neonatal outcomes of women with diabetes. J. Matern. Fetal Neonatal Med. 2018, 31, 2007–2012. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics: Stanningley, UK, 1991. [Google Scholar]
- Società Italiana di Nutrizione (SINU). Recommended Assumption Levels of Energy and Nutrients for Italian Population—Livelli di Assunzione Raccomandata di Nutrienti per la Popolazione Italiana (L.A.R.N), 4th ed.; SICS Editore: Rome, Italy, 2014. [Google Scholar]
- Healthy Eating Plate. Available online: https://www.hsph.harvard.edu/nutritionsource/healthy-eating-plate/ (accessed on 17 December 2019).
- Liang, Z.; Wu, Y.; Zhu, X.; Fang, Q.; Chen, D. Insulin resistance and lipid profile during an oral glucose tolerance test in women with and without gestational diabetes mellitus. J. Obstet. Gynaecol. 2016, 36, 337–339. [Google Scholar] [CrossRef]
- Barbour, L.A.; Hernandez, T.L. Maternal Non-Glycemic Contributors to Fetal Growth in Obesity and Gestational Diabetes: Spotlight on Lipids. Curr. Diabetes Rep. 2018, 18, 37. [Google Scholar] [CrossRef]
- O’Malley, E.G.; Reynolds, C.M.E.; Killalea, A.; O’Kelly, R.; Sheehan, S.R.; Turner, M.J. Maternal obesity and dyslipidemia associated with gestational diabetes mellitus (GDM). Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Khosrowbeygi, A.; Rezvanfar, M.R.; Ahmadvand, H. Tumor necrosis factor- α, adiponectin and their ratio in gestational diabetes mellitus. Casp. J. Intern. Med. 2018, 9, 71–79. [Google Scholar]
- Rodrigues, T.; Matafome, P.; Sereno, J.; Almeida, J.; Castelhano, J.; Gamas, L.; Neves, C.; Gonçalves, S.; Carvalho, C.; Arslanagic, A.; et al. Methylglyoxal-induced glycation changes adipose tissue vascular architecture, flow and expansion, leading to insulin resistance. Sci. Rep. 2017, 7, 1698–1713. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Murai, J.; Saito, H.; Mukai, M.; Kasayama, S. Serum glycated albumin, but not glycated hemoglobin, is low in relation to glycemia in men with hypertriglyceridemia. J. Diabetes Investig. 2010, 1, 202–207. [Google Scholar] [CrossRef] [PubMed]
| Anthropometric Data | Before Pregnancy | p | |
|---|---|---|---|
| Height (cm) | 163 (160–168) | - | |
| Pre-gestational weight (kg) | 62.8 (55.6–69.6) | - | |
| Pre-gestational BMI (kg/m2) | 23.3 (21.0–26.3) | - | |
| At diagnosis | After 12 weeks of diet | ||
| Weight (kg) | 71.0 (63.5–78.5) | 78.0 (64.6–82.8) | <0.001 |
| Arm circumference (cm) | 29.0 (26.9–30.1) | 28.8 (28.0–31.3) | ns |
| Wrist circumference (cm) | 15.0 (14.3–16.0) | 15 (14.3–16.0) | ns |
| Waist circumference (cm) | 96.0 (87.5–100.0) | 104.0 (97.9–107.1) | <0.001 |
| Bicipital skinfold (mm) | 9.0 (7.8–13.3) | 10.7 (7.2–12.8) | 0.05 |
| Tricipital skinfold (mm) | 21.6 (18.0–28.7) | 20.1 (16.8–25.8) | 0.001 |
| Subscapular skinfold (mm) | 18.40 (13.40–25.20) | 14.4 (12.3–24.0) | 0.02 |
| Delivery and newborn details | |||
| Gestational age at birth (weeks+days) | 39+5 (39+0–39+6) | - | |
| Birth weight (g) | 3170 (3040–3460) | - | |
| Birth weight centile | 41.5 (22.5–67.8) | - | |
| APGAR 1’ | 9 (9–9) | - | |
| APGAR 5’ | 10 (10–10) | - | |
| Metabolic Data | At Diagnosis | After 12 Weeks of Diet | p |
|---|---|---|---|
| Fasting blood glucose (mg/dL) | 85.4 (79.4–90.8) | 80.0 (73.0–90.0) | ns |
| Post prandial blood glucose (mg/dL) | 94.4 (88.4–103.9) | 97.1 (92.7–100.7) | ns |
| Glycated hemoglobin (mmol/mol) | 30.5 (28.8–32.0) | 33.0 (31.8–35.3) | <0.001 |
| Insulin (μU/mL) | 9.3 (5.5–14.3) | 9.7 (7.4–15.3) | ns |
| HOMA index | 1.54 (0.88–2.31) | 1.45 (0.70–2.30) | ns |
| Cortisol at 08:00 (μg/dL) | 27.6 (21.1–30.9) | 27.0 (23.0–32.4) | ns |
| Total cholesterol (mg/dL) | 258 (221–279) | 267 (232–301) | ns |
| HDL cholesterol (mg/dL) | 79 (65–87) | 75 (66–87) | ns |
| LDL cholesterol (mg/dL) | 142 (109–167) | 146 (114–164) | ns |
| Triglycerides (mg/dL) | 185 (150–208) | 227 (221–282) | <0.001 |
| CRP (mg/dL) | 0.42 (0.17–0.63) | 0.35 (0.23–0.60) | ns |
| Creatinine (mg/dl) | 0.47 (0.42–0.62) | 0.56 (0.50–0.67) | ns |
| Ferritin (ng/ml) | 18 (12–35) | 23 (16–32) | ns |
| GDM Women | GDM Women | ||||
|---|---|---|---|---|---|
| General Population | At Diagnosis | p | After 12 Weeks of Diet | p | |
| MGO (μg/mL) | 0.25 (0.19–0.28) | 0.64 (0.46–0.90) | <0.001 | 0.71 (0.47–0.93) | <0.001 |
| GA (nmol/mL) | 0.95 (0.63–1.4) | 1.12 (0.74–1.76) | ns | 1.51 (0.88–2.03) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piuri, G.; Basello, K.; Rossi, G.; Soldavini, C.M.; Duiella, S.; Privitera, G.; Spadafranca, A.; Costanzi, A.; Tognon, E.; Cappelletti, M.; et al. Methylglyoxal, Glycated Albumin, PAF, and TNF-α: Possible Inflammatory and Metabolic Biomarkers for Management of Gestational Diabetes. Nutrients 2020, 12, 479. https://doi.org/10.3390/nu12020479
Piuri G, Basello K, Rossi G, Soldavini CM, Duiella S, Privitera G, Spadafranca A, Costanzi A, Tognon E, Cappelletti M, et al. Methylglyoxal, Glycated Albumin, PAF, and TNF-α: Possible Inflammatory and Metabolic Biomarkers for Management of Gestational Diabetes. Nutrients. 2020; 12(2):479. https://doi.org/10.3390/nu12020479
Chicago/Turabian StylePiuri, Gabriele, Katia Basello, Gabriele Rossi, Chiara Maria Soldavini, Silvia Duiella, Giulia Privitera, Angela Spadafranca, Andrea Costanzi, Emiliana Tognon, Mattia Cappelletti, and et al. 2020. "Methylglyoxal, Glycated Albumin, PAF, and TNF-α: Possible Inflammatory and Metabolic Biomarkers for Management of Gestational Diabetes" Nutrients 12, no. 2: 479. https://doi.org/10.3390/nu12020479
APA StylePiuri, G., Basello, K., Rossi, G., Soldavini, C. M., Duiella, S., Privitera, G., Spadafranca, A., Costanzi, A., Tognon, E., Cappelletti, M., Corsetto, P. A., Rizzo, A. M., Speciani, A. F., & Ferrazzi, E. (2020). Methylglyoxal, Glycated Albumin, PAF, and TNF-α: Possible Inflammatory and Metabolic Biomarkers for Management of Gestational Diabetes. Nutrients, 12(2), 479. https://doi.org/10.3390/nu12020479







