The Exacerbation of Aging and Oxidative Stress in the Epididymis of Sod1 Null Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Tissue Fixation
2.3. Histology
2.4. Immunofluorescence
2.5. Immunofluorescence Imaging and Quantification
2.6. Statistical Analyses
3. Results
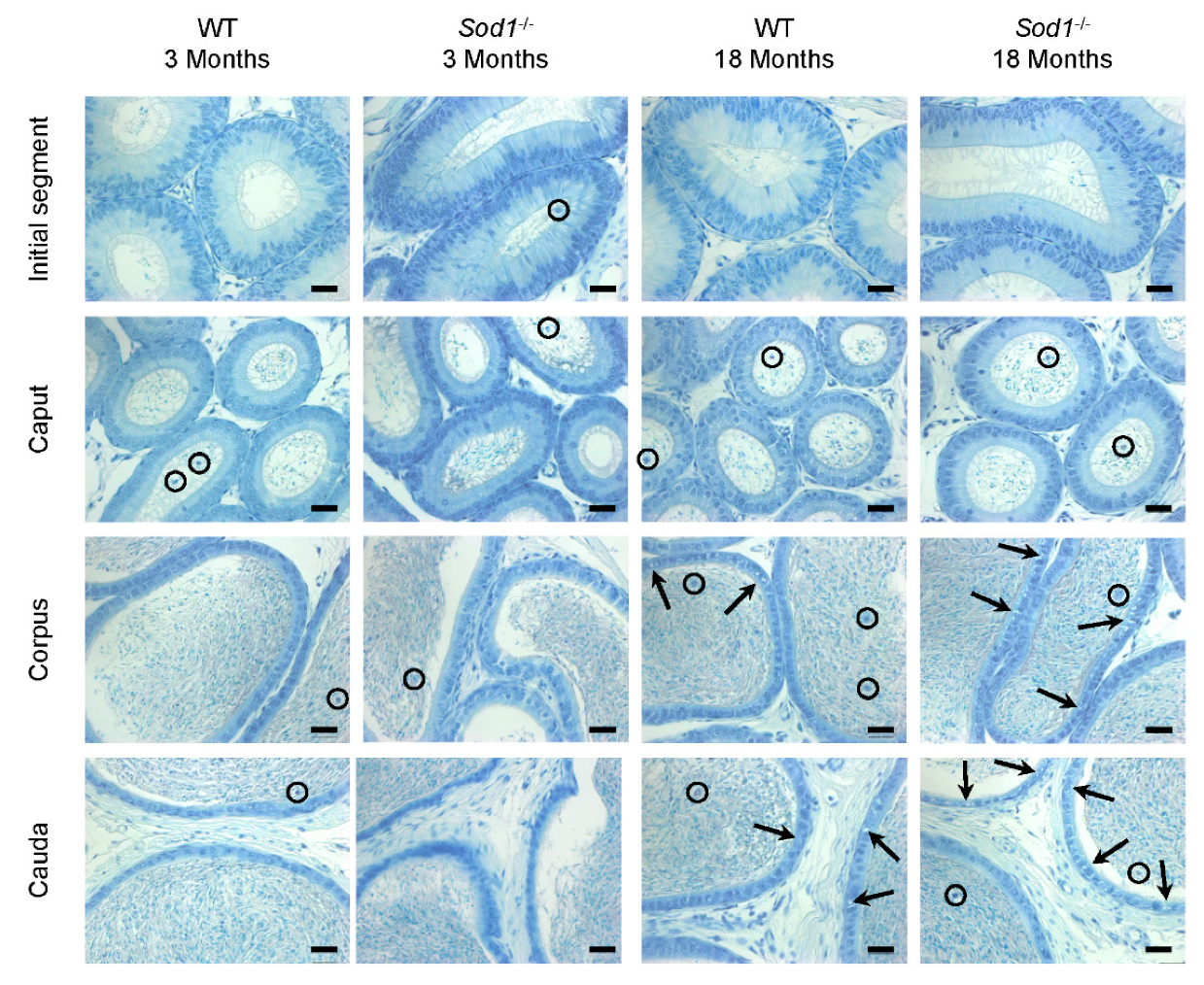
3.1. Histology
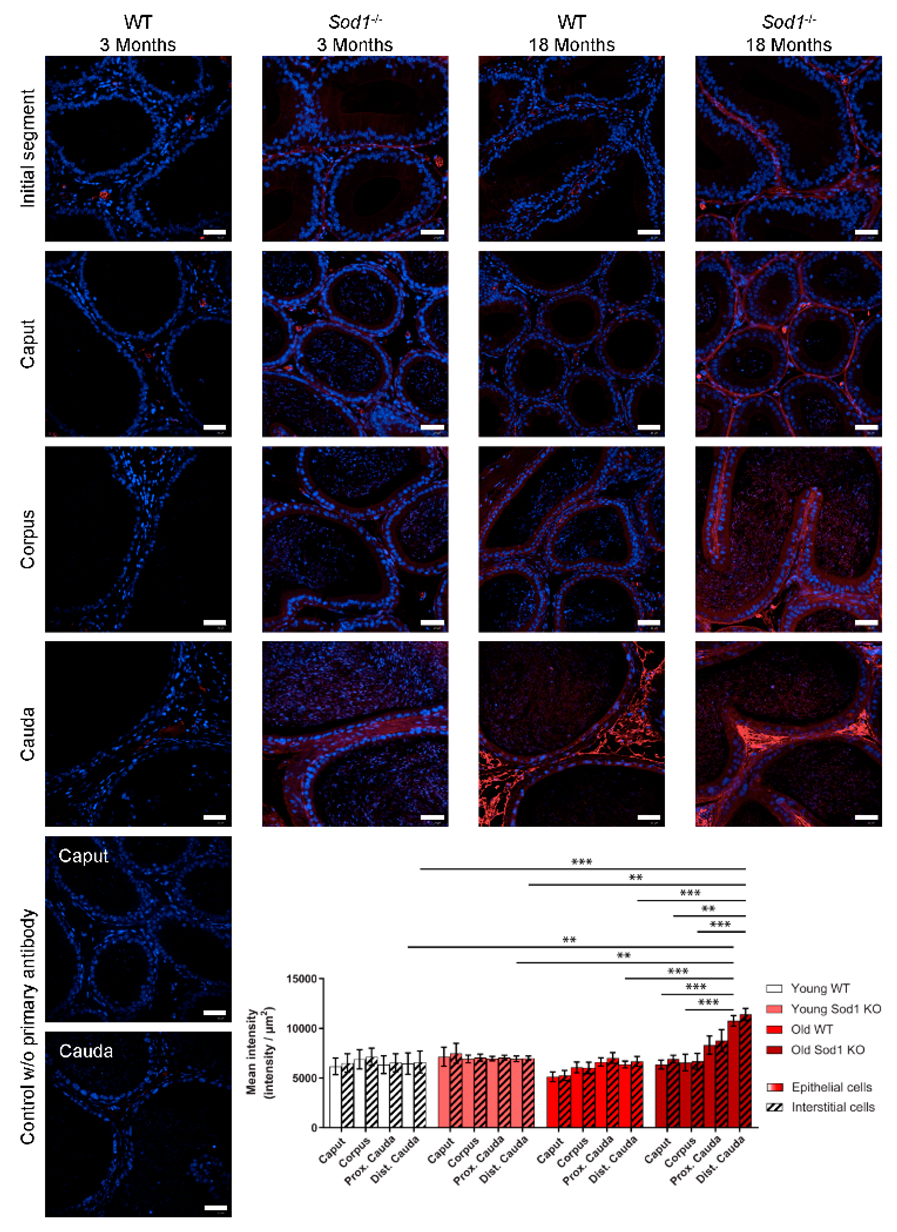
3.2. DNA Oxidation
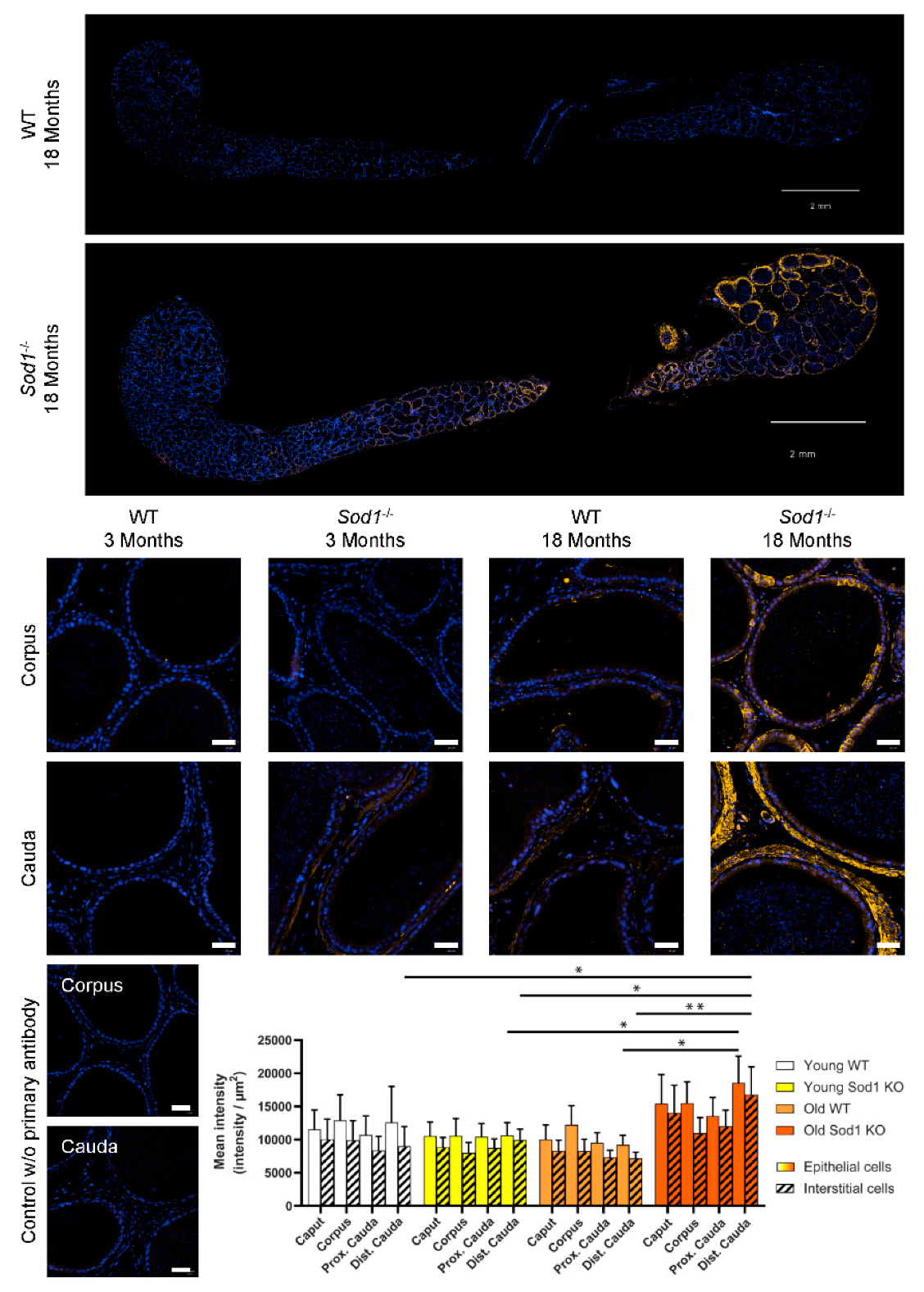
3.3. Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S. Oxidized LDL and Atherogenesis. Ann. New York Acad. Sci. 1999, 874, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Protein Oxidation. Ann. New York Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA Damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.W.; Fiore, C.; Zirkin, B.R. The Effect of Aging on the Seminiferous Epithelium of the Brown Norway Rat. J. Androl. 1993, 14, 110–117. [Google Scholar]
- Wang, C.; Hikim, A.S.; Ferrini, M.; Bonavera, J.J.; Vernet, L.; Leung, A.; Lue, Y.-H.; Gonzalez-Cadavid, N.F.; Swerdloff, R.S. Male Reproductive Ageing: Using the Brown Norway Rat as a Model for Man. Novartis Found. Symp. 2002, 242, 82–97. [Google Scholar]
- Robaire, B. Aging of the Epididymis. In The Epididymis: From Molecules to Clinical Practice; Robaire, B., Hinton, B.T., Eds.; Springer: Boston, MA, USA, 2002; pp. 285–296. [Google Scholar]
- Beattie, M.; Adekola, L.; Papadopoulos, V.; Chen, H.; Zirkin, B. Leydig Cell Aging and Hypogonadism. Exp. Gerontol. 2015, 68, 87–91. [Google Scholar] [CrossRef]
- Levy, S.; Robaire, B. Segment-Specific Changes with Age in the Expression of Junctional Proteins and the Permeability of the Blood-Epididymis Barrier in Rats. Boil. Reprod. 1999, 60, 1392–1401. [Google Scholar] [CrossRef]
- Serre, V. Segment-Specific Morphological Changes in Aging Brown Norway Rat Epididymis. Boil. Reprod. 1998, 58, 497–513. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and Aging: Causes, Consequences, and Therapeutic Avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, M.S.; King, M. Biological Theories of Aging. Dis. Mon. 2015, 61, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Brunk, U.T. Oxidative Stress, Accumulation of Biological ’Garbage’, and Aging. Antioxid. Redox Signal. 2006, 8, 197–204. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Sabatini, D.M. Redox Regulation of the Nutrient-Sensitive Raptor-mTOR Pathway and Complex. J. Boil. Chem. 2005, 280, 39505–39509. [Google Scholar] [CrossRef]
- Yoshida, S.; Hong, S.; Suzuki, T.; Nada, S.; Mannan, A.M.; Wang, J.; Okada, M.; Guan, K.-L.; Inoki, K. Redox Regulates Mammalian Target of Rapamycin Complex 1 (mTORC1) Activity by Modulating the TSC1/TSC2-Rheb GTPase Pathway. J. Boil. Chem. 2011, 286, 32651–32660. [Google Scholar] [CrossRef]
- Ramírez-Rangel, I.; Bracho-Valdés, I.; Vázquez-Macías, A.; Carretero-Ortega, J.; Reyes-Cruz, G.; Vazquez-Prado, J. Regulation of mTORC1 Complex Assembly and Signaling by GRp58/ERp57. Mol. Cell. Boil. 2011, 31, 1657–1671. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, B.; Shirwany, N.A.; Zou, M.-H. 2-Deoxy-D-Glucose Treatment of Endothelial Cells Induces Autophagy by Reactive Oxygen Species-Mediated Activation of the AMP-Activated Protein Kinase. PLOS ONE 2011, 6, e17234. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.D.P.S.; Melendez, J.A. Redox Control of Senescence and Age-Related Disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Weichhart, T. MTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Baker, J.; Hardy, M.P.; Zhou, J.; Bondy, C.; Lupu, F.; Bellvé, A.R.; Efstratiadis, A. Effects of an Igf1 Gene Null Mutation on Mouse Reproduction. Mol. Endocrinol. 1996, 10, 903–918. [Google Scholar] [PubMed]
- Hamzeh, M.; Robaire, B. Identification of Early Response Genes and Pathway Activated by Androgens in the Initial Segment and Caput Regions of the Regressed Rat Epididymis. Endocrinology 2010, 151, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- Balhorn, R. The Protamine Family of Sperm Nuclear Proteins. Genome Boil. 2007, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.S. Organization of Sperm DNA by the Nuclear Matrix. Am. J. Clin. Exp. Urol. 2018, 6, 87–92. [Google Scholar] [PubMed]
- Maiorino, M.; Roveri, A.; Benazzi, L.; Bosello, V.; Mauri, P.; Toppo, S.; Tosatto, S.C.E.; Ursini, F. Functional Interaction of Phospholipid Hydroperoxide Glutathione Peroxidase with Sperm Mitochondrion-associated Cysteine-rich Protein Discloses the Adjacent Cysteine Motif as a New Substrate of the Selenoperoxidase. J. Boil. Chem. 2005, 280, 38395–38402. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F. Dual Function of the Selenoprotein PHGPx During Sperm Maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef]
- Conrad, M.; Moreno, S.G.; Sinowatz, F.; Ursini, F.; Kölle, S.; Roveri, A.; Brielmeier, M.; Wurst, W.; Maiorino, M.; Bornkamm, G.W. The Nuclear Form of Phospholipid Hydroperoxide Glutathione Peroxidase Is a Protein Thiol Peroxidase Contributing to Sperm Chromatin Stability. Mol. Cell. Boil. 2005, 25, 7637–7644. [Google Scholar] [CrossRef]
- Noblanc, A.; Peltier, M.; Damon-Soubeyrand, C.; Kerchkove, N.; Chabory, E.; Vernet, P.; Saez, F.; Cadet, R.; Janny, L. and Pons-Rejraji, H. et al. Epididymis Response Partly Compensates for Spermatozoa Oxidative Defects in snGPx4 and GPx5 Double Mutant Mice. PLoS ONE 2012, 7, e38565. [Google Scholar] [CrossRef]
- Pfeifer, H. Identification of a Specific Sperm Nuclei Selenoenzyme Necessary for Protamine Thiol Cross-Linking during Sperm Maturation. FASEB J. 2001, 15, 1236–1238. [Google Scholar] [CrossRef]
- Puglisi, R.; Maccari, I.; Pipolo, S.; Conrad, M.; Mangia, F.; Boitani, C. The Nuclear Form of Glutathione Peroxidase 4 Is Associated with Sperm Nuclear Matrix and Is Required for Proper Paternal Chromatin Decondensation at Fertilization. J. Cell. Physiol. 2012, 227, 1420–1427. [Google Scholar] [CrossRef]
- Ellerman, D.A.; Myles, D.G.; Primàkoff, P. A Role for Sperm Surface Protein Disulfide Isomerase Activity in Gamete Fusion: Evidence for the Participation of ERp57. Dev. Cell 2006, 10, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, D.; Atreja, S. Signalling Events and Associated Pathways Related to the Mammalian Sperm Capacitation. Reprod. Domest. Anim. 2015, 50, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.C.P.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Boil. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; O’Flaherty, C. Sperm Activation: Role of Reactive Oxygen Species and Kinases. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 106–115. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Zini, A.; Gabriel, M.C. Human Sperm Chromatin Undergoes Physiological Remodeling During in Vitro Capacitation and Acrosome Reaction. J. Androl. 2012, 33, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; De Lamirande, E.; Gagnon, C. Positive Role of Reactive Oxygen Species in Mammalian Sperm Capacitation: Triggering and Modulation of Phosphorylation Events. Free. Radic. Boil. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- Jena, N.R. DNA Damage by Reactive Species: Mechanisms, Mutation and Repair. J. Biosci. 2012, 37, 503–517. [Google Scholar] [CrossRef]
- Iguchi, N.; Tobias, J.W.; Hecht, N.B. Expression Profiling Reveals Meiotic Male Germ Cell mRNAs that Are Translationally Up- and Down-Regulated. Proc. Natl. Acad. Sci. 2006, 103, 7712–7717. [Google Scholar] [CrossRef]
- Hao, S.-L.; Ni, F.-D.; Yang, W.-X. The Dynamics and Regulation of Chromatin Remodeling during Spermiogenesis. Gene 2019, 706, 201–210. [Google Scholar] [CrossRef]
- Cavé, T.; Desmarais, R.; Lacombe-Burgoyne, C.; Boissonneault, G. Genetic Instability and Chromatin Remodeling in Spermatids. Genes 2019, 10, 40. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of Reactive Oxygen Species, Lipid Peroxidation, and Human Sperm Function. Boil. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Irvine, D.S.; Aitken, R.J. Evaluation of a Spectrophotometric Assay for the Measurement of Malondialdehyde and 4-hydroxyalkenals in Human Spermatozoa: Relationships with Semen Quality and Sperm Function. Int. J. Androl. 1998, 21, 81–94. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Orchestrating the Antioxidant Defenses in the Epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Mccord, J.M.; Fridovich, I. The Utility of Superoxide Dismutase in Studying Free Radical Reactions. II. The Mechanism of the Mediation of Cytochrome c Reduction by a Variety of Electron Carriers. J. Boil. Chem. 1970, 245, 1374–1377. [Google Scholar]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X. Mutations in Cu/Zn Superoxide Dismutase Gene Are Associated with Familial Amyotrophic Lateral Sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef]
- Tsunoda, S.; Kawano, N.; Miyado, K.; Kimura, N.; Fujii, J. Impaired Fertilizing Ability of Superoxide Dismutase 1-Deficient Mouse Sperm During in Vitro Fertilization1. Boil. Reprod. 2012, 87, 121. [Google Scholar] [CrossRef]
- Brayton, C.F.; Treuting, P.M.; Ward, J.M. Pathobiology of Aging Mice and GEM: Background Strains and Experimental Design. Vet. Pathol. 2012, 49, 85–105. [Google Scholar] [CrossRef]
- Pettan-Brewer, C.; Treuting, P.M. Practical Pathology of Aging Mice. Pathobiol. Aging Age Relat. Dis. 2011, 1, 7202. [Google Scholar] [CrossRef]
- Elchuri, S.; Oberley, T.D.; Qi, W.; Eisenstein, R.S.; Jackson Roberts, L.; Van Remmen, H.; Epstein, C.J.; Huang, T.-T. CuZnSOD Deficiency Leads to Persistent and Widespread Oxidative Damage and Hepatocarcinogenesis Later in Life. Oncogene 2005, 24, 367–380. [Google Scholar] [CrossRef]
- Kruidenier, L. Attenuated Mild Colonic Inflammation and Improved Survival from Severe DSS-Colitis of Transgenic Cu/Zn-SOD Mice. Free. Radic. Boil. Med. 2003, 34, 753–765. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Dionne, L.; Guo, Q.; Kumar, T.R.; Lebovitz, R.M. Ovarian Function in Superoxide Dismutase 1 and 2 Knockout Mice. Endocrinology 1998, 139, 4008–4011. [Google Scholar] [CrossRef] [PubMed]
- Selvaratnam, J.S.; Robaire, B. Effects of Aging and Oxidative Stress on Spermatozoa of Superoxide-Dismutase 1- and Catalase-Null Mice1. Boil. Reprod. 2016, 95, 60. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Yasunami, M.; Carlson, E.J.; Gillespie, A.M.; Reaume, A.G.; Hoffman, E.K.; Chan, P.H.; Scott, R.W.; Epstein, C.J. Superoxide-Mediated Cytotoxicity in Superoxide Dismutase-Deficient Fetal Fibroblasts. Arch. Biochem. Biophys. 1997, 344, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Latendresse, J.R.; Warbrittion, A.R.; Jonassen, H.; Creasy, D.M. Fixation of Testes and Eyes Using a Modified Davidson’s fluid: Comparison with Bouin’s Fluid and Conventional Davidson’s Fluid. Toxicol. Pathol. 2002, 30, 524–533. [Google Scholar] [CrossRef]
- Selvaratnam, J.; Paul, C.; Robaire, B. Male Rat Germ Cells Display Age-Dependent and Cell-Specific Susceptibility in Response to Oxidative Stress Challenges1. Boil. Reprod. 2015, 93, 72. [Google Scholar] [CrossRef]
- Serre, V. Distribution of Immune Cells in the Epididymis of the Aging Brown Norway Rat Is Segment-Specific and Related to the Luminal Content. Boil. Reprod. 1999, 61, 705–714. [Google Scholar] [CrossRef]
- Da Silva, N.; Barton, C.R. Macrophages and Dendritic Cells in the Post-Testicular Environment. Cell Tissue Res. 2016, 363, 97–104. [Google Scholar] [CrossRef][Green Version]
- Calvo, A.; Pastor, L.M.; Roca, J.; Martínez, E.; Vázquez, J.M. Age-Related Changes in the Hamster Epididymis. Anat. Rec. 1999, 256, 335–346. [Google Scholar] [CrossRef]
- Robaire, B.; Hinton, B. The Epididymis. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T., Zeleznik, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 691–771. [Google Scholar]
- Weir, C.P.; Robaire, B. Spermatozoa Have Decreased Antioxidant Enzymatic Capacity and Increased Reactive Oxygen Species Production during Aging in the Brown Norway rat. J. Androl. 2007, 28, 229–240. [Google Scholar] [CrossRef]
| Target | Conjugation | Clonality | Dilution | Company | Catalog |
|---|---|---|---|---|---|
| Primary Antibodies | |||||
| 8-OHG (DNA/RNA) | None | Mouse Monoclonal (15A3) | 1/1000 | Novus Biologicals | NB110-96878 |
| 4-HNE | None | Rabbit polyclonal | 1/500 | Abcam | ab46545 |
| SRC, C-term | None | Mouse monoclonal | 1/250 | Santa Cruz | sc8056 |
| Secondary Antibodies | |||||
| Mouse whole IgG | Alexa Fluor 488 | Goat polyclonal | 1/500 | Invitrogen | a11029 |
| Rabbit whole IgG | Alexa Fluor 546 | Goat polyclonal | 1/1000 | Invitrogen | a11010 |
| Mouse whole IgG | Alexa Fluor 633 | Goat polyclonal | 1/2000 | Invitrogen | a21046 |
| Staining | 8-Hydroxyguanine | 4-Hydroxynonenal | ||||
|---|---|---|---|---|---|---|
| Source of Variation | % of Total Variation | p Value | Significance | % of Total Variation | p Value | Significance |
| Region | 10.23 | 0.0122 | * | 5.78 | 0.3546 | ns |
| Age | 1.07 | 0.1604 | ns | 2.18 | 0.0880 | ns |
| Genotype | 14.00 | <0.0001 | **** | 4.47 | 0.0153 | * |
| Region × Age | 12.50 | 0.0030 | ** | 0.31 | 0.9997 | ns |
| Region × Genotype | 5.38 | 0.1988 | ns | 1.84 | 0.9251 | ns |
| Age × Genotype | 5.37 | 0.0020 | ** | 8.69 | 0.0008 | *** |
| Region × Age × Genotype | 4.63 | 0.2897 | ns | 1.06 | 0.9833 | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noblanc, A.; Klaassen, A.; Robaire, B. The Exacerbation of Aging and Oxidative Stress in the Epididymis of Sod1 Null Mice. Antioxidants 2020, 9, 151. https://doi.org/10.3390/antiox9020151
Noblanc A, Klaassen A, Robaire B. The Exacerbation of Aging and Oxidative Stress in the Epididymis of Sod1 Null Mice. Antioxidants. 2020; 9(2):151. https://doi.org/10.3390/antiox9020151
Chicago/Turabian StyleNoblanc, Anaīs, Alicia Klaassen, and Bernard Robaire. 2020. "The Exacerbation of Aging and Oxidative Stress in the Epididymis of Sod1 Null Mice" Antioxidants 9, no. 2: 151. https://doi.org/10.3390/antiox9020151
APA StyleNoblanc, A., Klaassen, A., & Robaire, B. (2020). The Exacerbation of Aging and Oxidative Stress in the Epididymis of Sod1 Null Mice. Antioxidants, 9(2), 151. https://doi.org/10.3390/antiox9020151





