Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
2.3. Testes and Epididymes Homogenates Preparation
2.4. Sperm Motility and DNA Oxidation Determinations
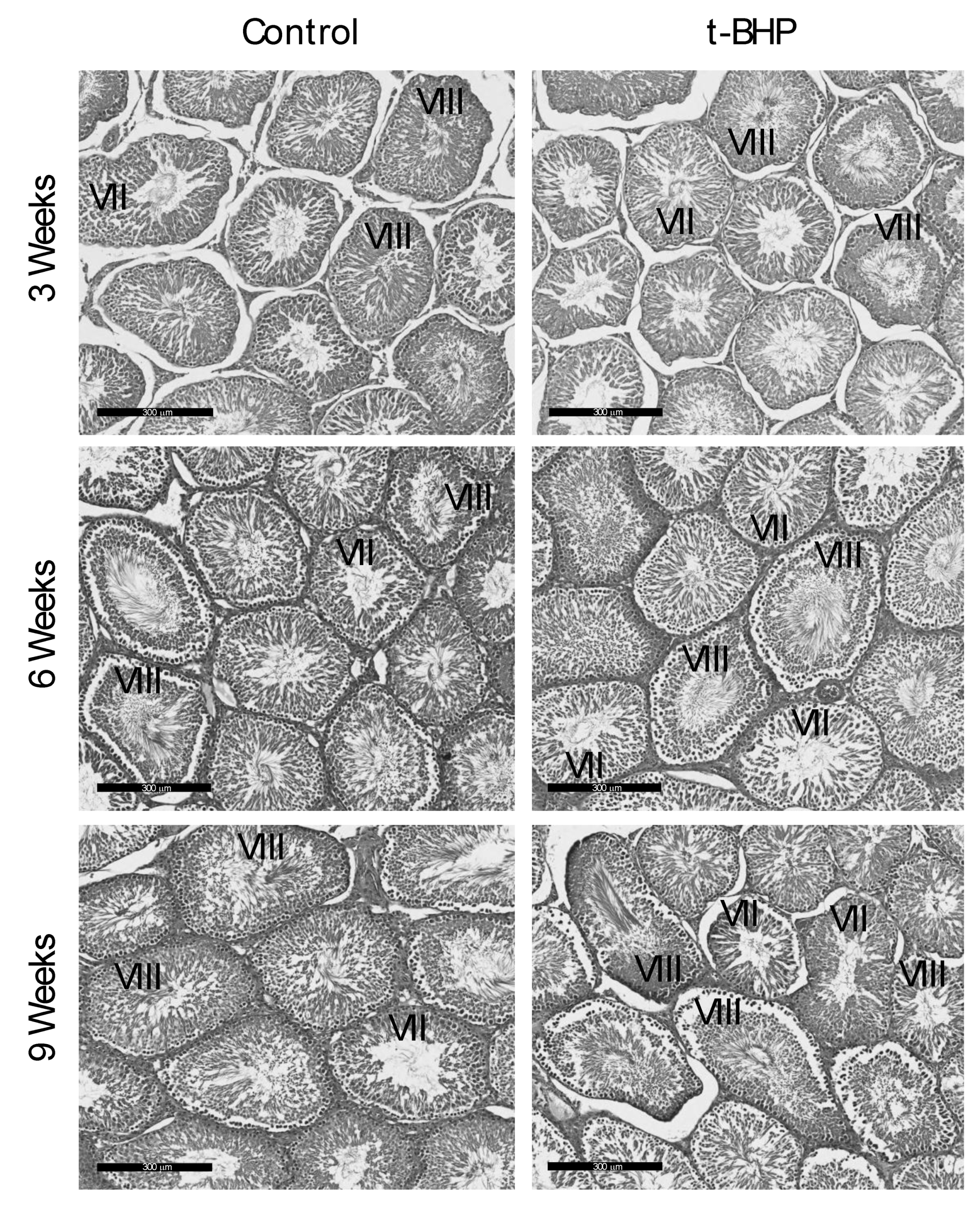
2.5. Testes Histological Analysis, and Sperm Count
2.6. SDS-PAGE and Immunoblotting
2.7. Statistical Analysis
3. Results
3.1. Low Sperm Motility and High DNA Oxidation Suggest Compromised Sperm Quality Due to Oxidative Stress
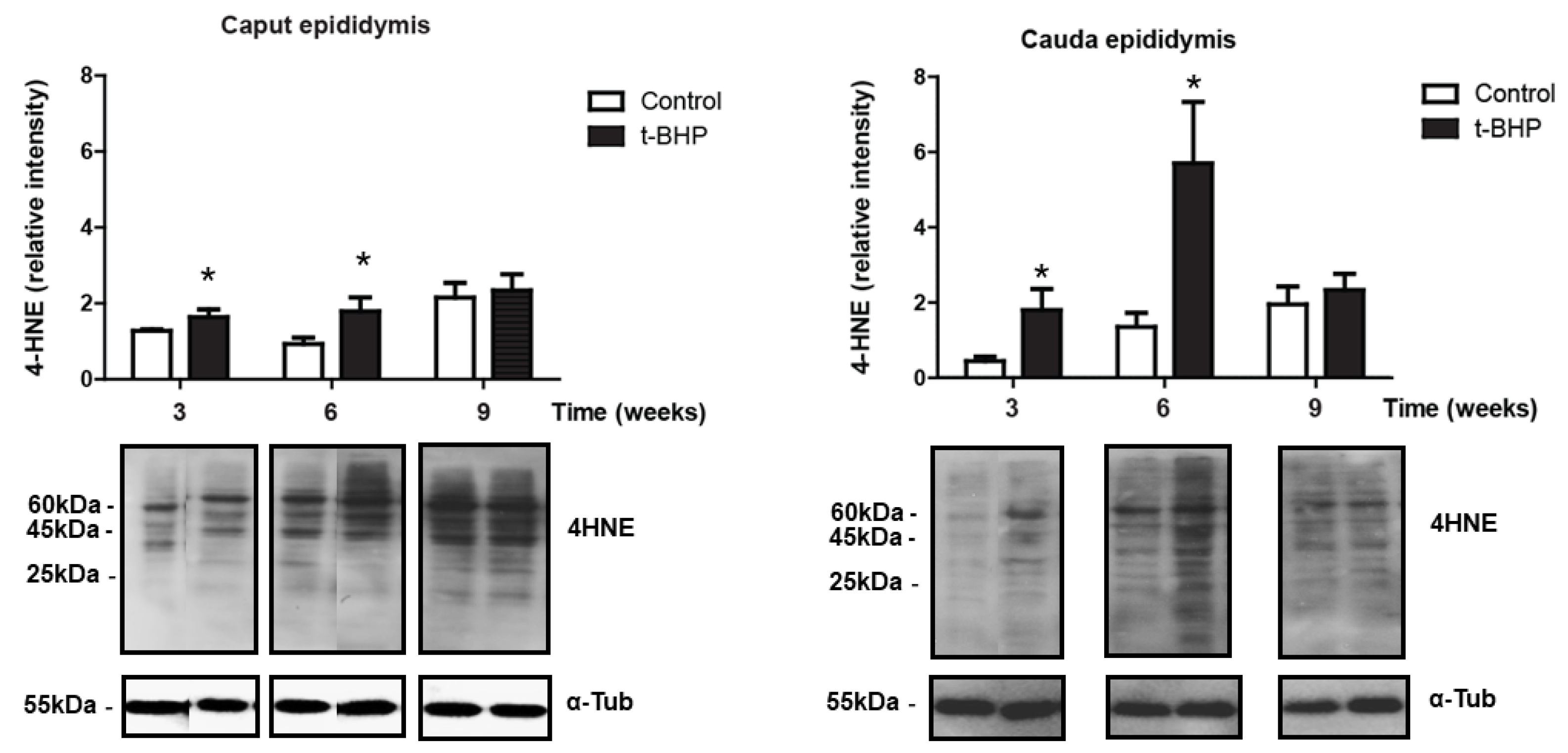
3.2. Lipid Peroxidation Increased in Caput and Cauda Epididymis of t-BHP Treated Male Rats
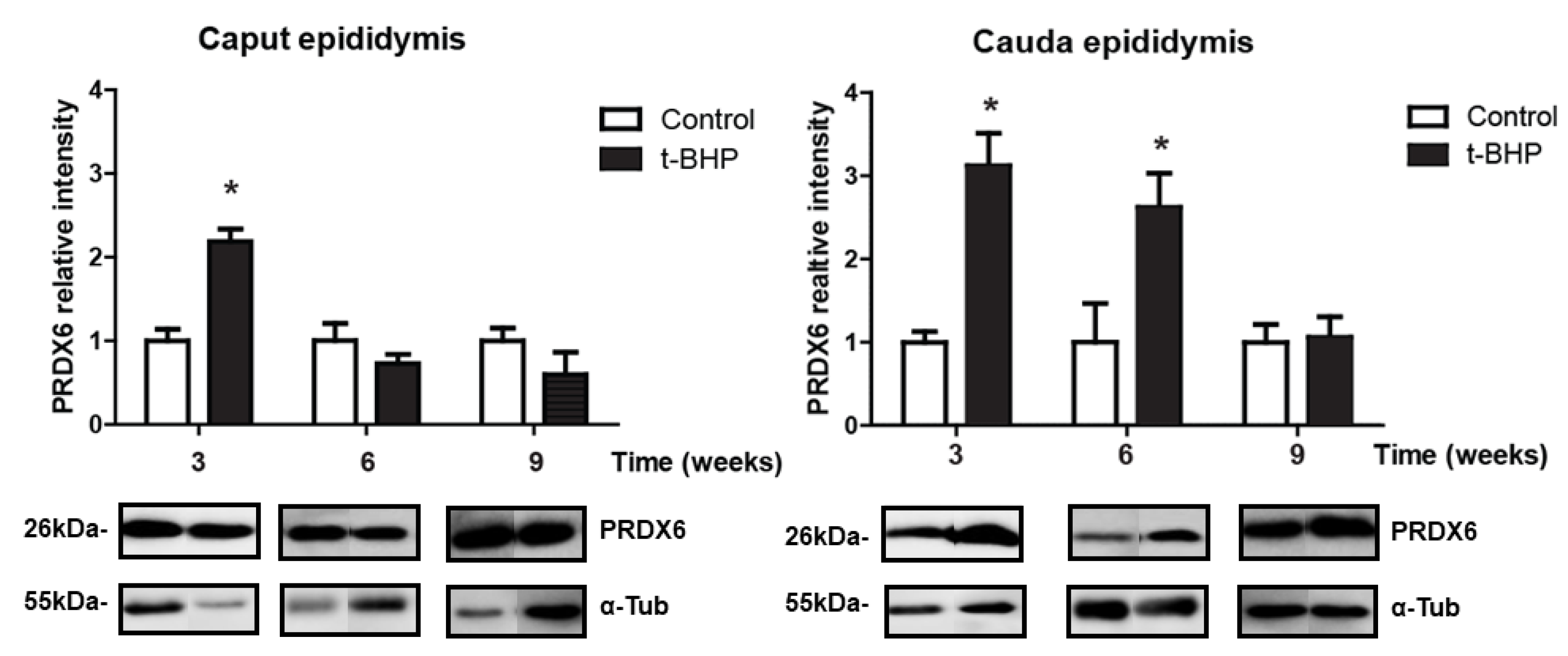
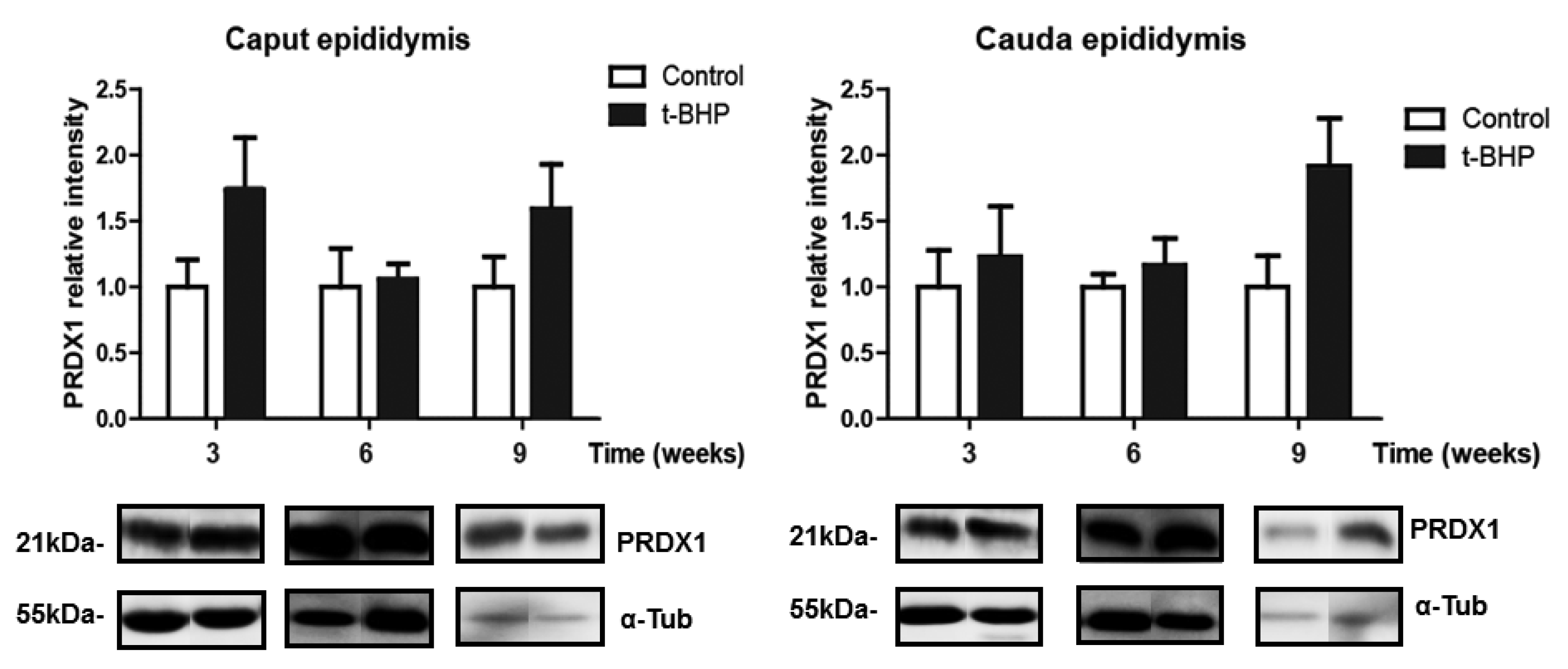
3.3. PRDX1 and PRDX6 Are Differentially Upregulated in Caput and Cauda Epididymis at Different Time Points
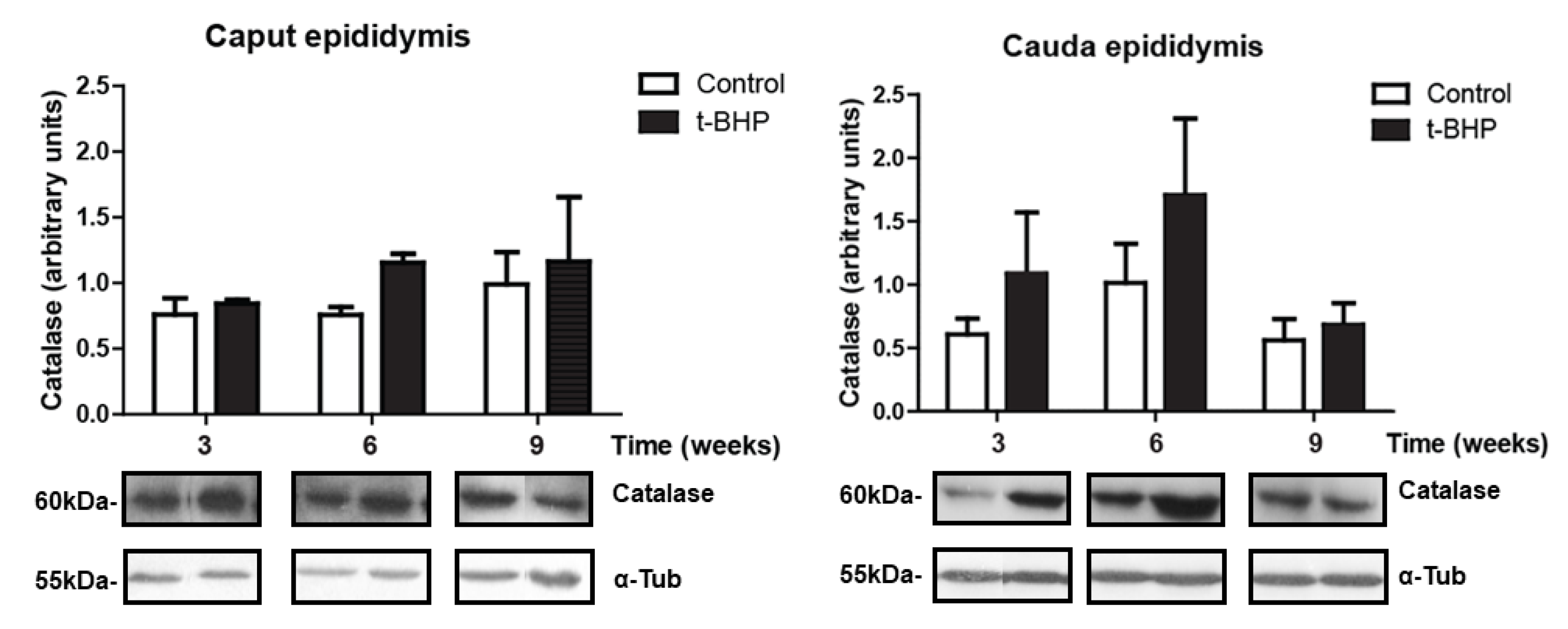
3.4. Catalase Expression Shows Trends of Increase, with Significant Individual Variation in the Epididymis
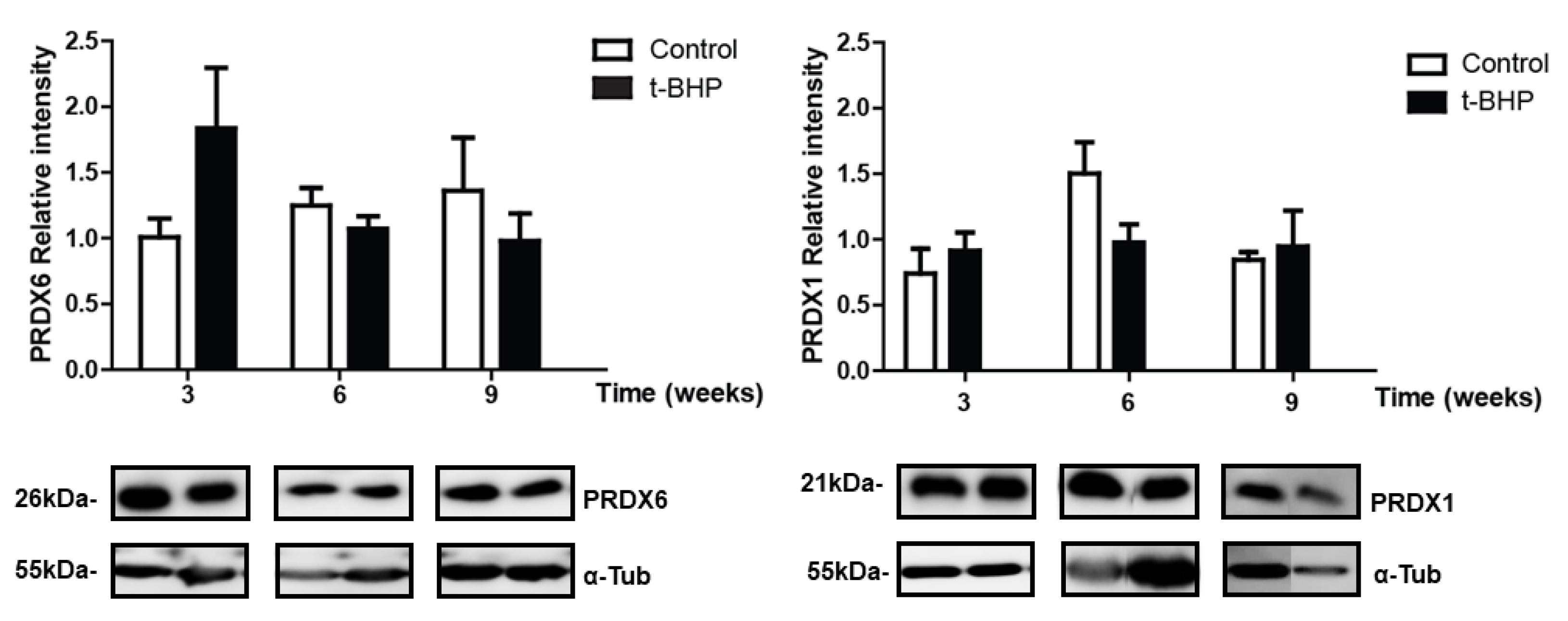
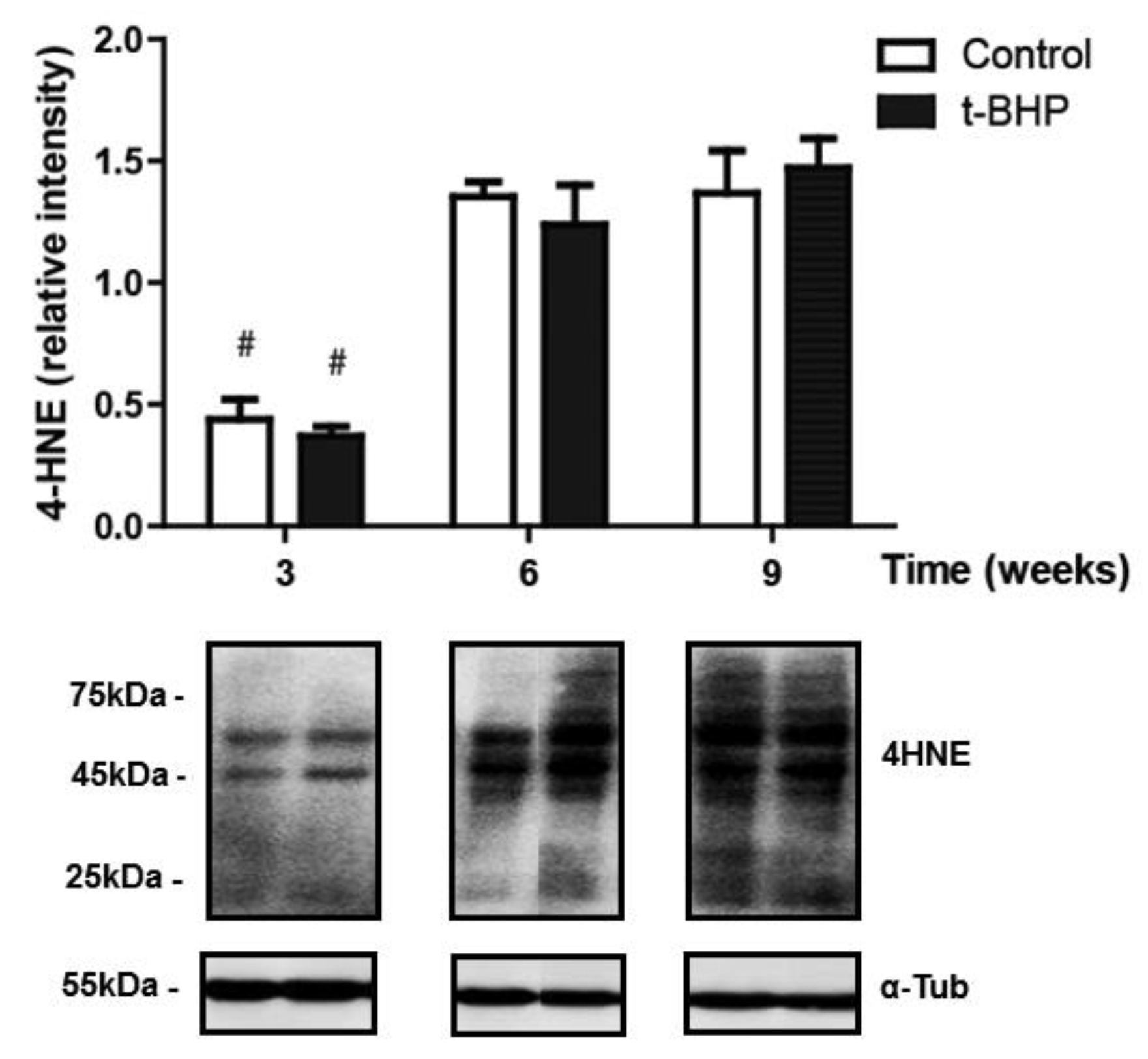
3.5. PRDXs, Catalase and Thioredoxin Expression Levels and Lipid Peroxidation Are Similar in Testis Despite the t-BHP Treatment
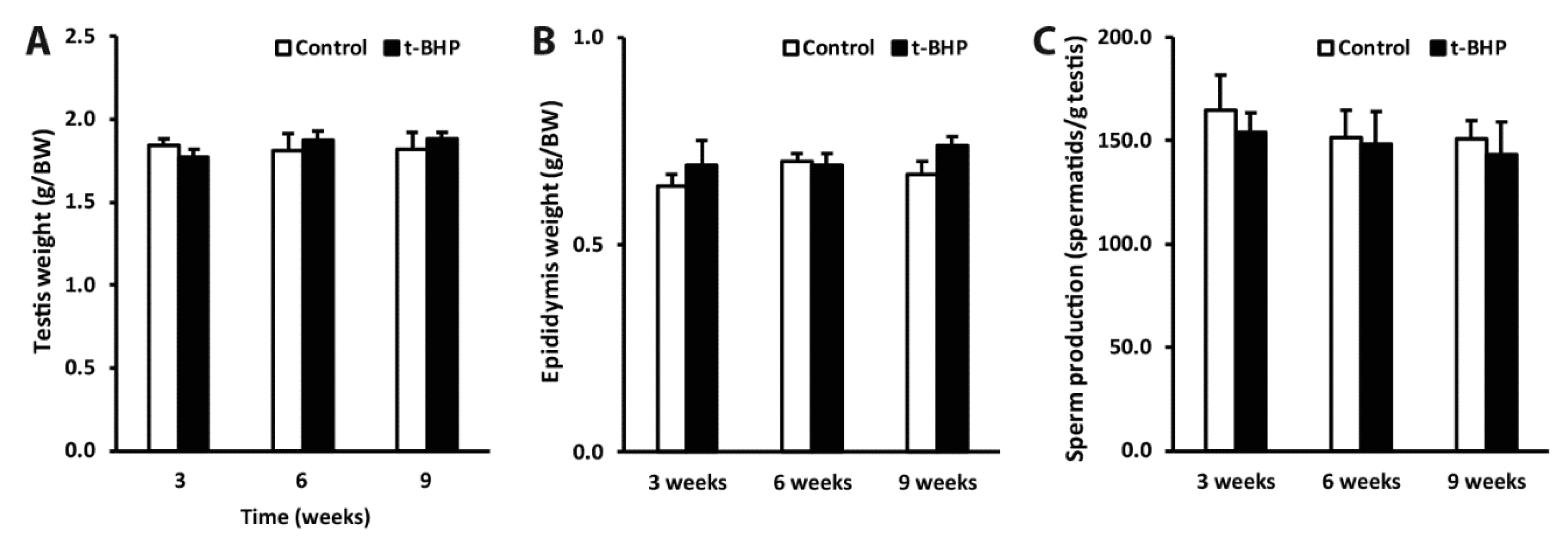
3.6. Reproductive Organs Weight, Spermatogenesis, and Sperm Production Were Not Affected by the t-BHP Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Towards more objectivity in diagnosis and management of male fertility. Int. J. Androl. 1997, 7, 1–53. [Google Scholar]
- Iwasaki, A.; Gagnon, C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil. Steril. 1992, 57, 409–416. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility: A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Williamson, R.C. Testicular torsion in Bristol: A 25-year review. Br. J. Surg. 1988, 75, 988–992. [Google Scholar] [CrossRef]
- Hasegawa, M.; Wilson, G.; Russell, L.D.; Meistrich, M.L. Radiation-induced cell death in the mouse testis: Relationship to apoptosis. Radiat. Res. 1997, 147, 457–467. [Google Scholar] [CrossRef]
- Brennemann, W.; Stoffel-Wagner, B.; Helmers, A.; Mezger, J.; Jager, N.; Klingmuller, D. Gonadal function of patients treated with cisplatin based chemotherapy for germ cell cancer. J. Urol. 1997, 158, 844–850. [Google Scholar] [CrossRef]
- Smith, R.; Kaune, H.; Parodi, D.; Madariaga, M.; Ríos, R.; Morales, I.; Castro, A. Increased sperm DNA damage in patients with varicocele: Relationship with seminal oxidative stress. Hum. Reprod. 2006, 21, 986–993. [Google Scholar] [CrossRef]
- Turner, T.T. The study of varicocele through the use of animal models. Hum. Reprod. Update 2001, 7, 78–84. [Google Scholar] [CrossRef]
- de Lamirande, E.; O’Flaherty, C. Sperm Capacitation as an Oxidative Event. In Studies on Men’s Health and Fertility, Oxidative Stress in Applied Basic Research and Clinical Practice; Aitken, J., Alvarez, J., Agawarl, A., Eds.; Springer: Berlin/Heidelber, Germany, 2012; pp. 57–94. [Google Scholar]
- Storey, B.T. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol. Hum. Reprod. 1997, 3, 203–213. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Sikka, S.C.; Rajasekaran, M.; Hellstrom, W.J. Role of oxidative stress and antioxidants in male infertility. J. Androl. 1995, 16, 464–468. [Google Scholar] [PubMed]
- O’Flaherty, C. The Enzymatic Antioxidant System of Human Spermatozoa. Adv. Androl. 2014, 2014, 1–15. [Google Scholar]
- O’Flaherty, C. Peroxiredoxins: Hidden players in the antioxidant defence of human spermatozoa. Basic Clin. Androl. 2014, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 activates maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Absence of Peroxiredoxin 6 Amplifies the Effect of Oxidant Stress on Mobility and SCSA/CMA3 Defined Chromatin Quality and Impairs Fertilizing Ability of Mouse Spermatozoa. Biol. Reprod. 2016, 94, 1–10. [Google Scholar] [CrossRef]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015, 5, 15–23. [Google Scholar] [CrossRef]
- Liu, Y.; O’Flaherty, C. In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality. Asian J. Androl. 2017, 19, 73–79. [Google Scholar]
- Miranda-Vizuete, A.; Sadek, C.M.; Jiménez, A.; Krause, W.J.; Sutovsky, P.; Oko, R. The Mammalian Testis-Specific Thioredoxin System. Antioxid. Redox Signal. 2004, 6, 25–40. [Google Scholar] [CrossRef]
- Chabory, E.; Damon, C.; Lenoir, A.; Henry-Berger, J.; Vernet, P.; Cadet, R.; Drevet, J.R. Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. J. Anim. Sci. 2009, 88, 1321–1331. [Google Scholar] [CrossRef]
- Robaire, B.; Hinton, B.T. Orgebin-Crist, The Epididymis. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Academic Press: St. Louis, MO, USA, 2006; pp. 1071–1148. [Google Scholar]
- Sommer, R.J.; Ippolito, D.L.; Peterson, R.E. In Uteroand Lactational Exposure of the Male Holtzman Rat to 2,3,7,8-Tetrachlorodibenzo-p-dioxin: Decreased Epididymal and Ejaculated Sperm Numbers without Alterations in Sperm Transit Rate. Toxicol. Appl. Pharmacol. 1996, 140, 146–153. [Google Scholar] [CrossRef]
- Sullivan, R.; Frenette, G.; Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 2007, 9, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.R. Induction of Oxidative Stress by Organic Hydroperoxides in Testis and Epididymal Sperm of Rats In Vivo. J. Androl. 2007, 28, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.E.R.; Sinha Hikim, A.; Clegg, E. Histological and Histopathological Evaluation of the Testis; Cache River Press: St. Louis, MO, USA, 1990. [Google Scholar]
- Robb, G.W.; Amann, R.P.; Killian, G.J. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. Reproduction 1978, 54, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Valli, H.; Phillips, B.T.; Shetty, G.; Byrne, J.A.; Clark, A.T.; Meistrich, M.L.; Orwig, K.E. Germline stem cells: Toward the regeneration of spermatogenesis. Fertil. Steril. 2014, 101, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Moskovtsev, S.I.; Alladin, N.; Lo, K.C.; Jarvi, K.; Mullen, J.B.M.; Librach, C.L. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst. Biol. Reprod. Med. 2012, 58, 142–148. [Google Scholar] [CrossRef][Green Version]
- Greco, E.; Scarselli, F.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum. Reprod. 2005, 20, 226–230. [Google Scholar] [CrossRef]
- Aguilar, C.; Meseguer, M.; García-Herrero, S.; Gil-Salom, M.; O’Connor, J.E.; Garrido, N. Relevance of testicular sperm DNA oxidation for the outcome of ovum donation cycles. Fertil. Steril. 2010, 94, 979–988. [Google Scholar] [CrossRef]
- Russell, L.D.; Peterson, R.N. Sertoli cell junctions: Morphological and functional correlates. Int. Rev. Cytol. 1985, 94, 177–211. [Google Scholar]
- Griswold, M.D.; McLean, D. The Sertoli Cell. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Academic Press: St. Louis, MO, USA, 2006; pp. 949–975. [Google Scholar]
- Yoganathan, T.; Eskild, W.; Hansson, V. Investigation of detoxification capacity of rat testicular germ cells and sertoli cells. Free Radic. Biol. Med. 1989, 7, 355–359. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Boisvert, A.; Manku, G.; Culty, M. Protective Role of Peroxiredoxins against Reactive Oxygen Species in Neonatal Rat Testicular Gonocytes. Antioxidants 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Bauché, F.; Fouchard, M.-H.; Jégou, B. Antioxidant system in rat testicular cells. FEBS Lett. 1994, 349, 392–396. [Google Scholar] [CrossRef]
- Syntin, P.; Robaire, B. Sperm Structural and Motility Changes During Aging in the Brown Norway Rat. J. Androl. 2001, 22, 235–244. [Google Scholar]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C.; Sorokina, E.M.; Li, H.; Zhou, S.; Raabe, T.; Feinstein, S.I. A novel LysoPhosphatidylcholine Acyl Transferase Activity is Expressed by Peroxiredoxin 6. J. Lipid Res. 2016, 31, 292–303. [Google Scholar] [CrossRef]
- Fisher, A.B.; Vasquez-Medina, J.P.; Dodia, C.; Sorokina, E.M.; Tao, J.Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018, 14, 41–46. [Google Scholar] [CrossRef]
- Fatemi, N.; Sanati, M.H.; Shamsara, M.; Moayer, F.; Zavarehei, M.J.; Pouya, A.; Gourabi, H. TBHP-induced oxidative stress alters microRNAs expression in mouse testis. J. Assist. Reprod. Genet. 2014, 31, 1287–1293. [Google Scholar] [CrossRef]
- Wykes, S.M.; Krawetz, S.A. The structural organization of sperm chromatin. J. Biol. Chem. 2003, 278, 29471–29477. [Google Scholar] [CrossRef]
- Bedford, J.M.; Bent, M.J.; Calvin, H. Variations in the structural character and stability of the nuclear chromatin in morphologically normal human spermatozoa. J. Reprod. Fertil. 1973, 33, 19–29. [Google Scholar] [CrossRef]
- Castillo, J.; Simon, L.; de Mateo, S.; Lewis, S.; Oliva, R. Protamine/DNA ratios and DNA damage in native and density gradient centrifuged sperm from infertile patients. J. Androl. 2011, 32, 324–332. [Google Scholar] [CrossRef]
- Codrington, A.M.; Hales, B.F.; Robaire, B. Exposure of male rats to cyclophosphamide alters the chromatin structure and basic proteome in spermatozoa. Hum. Reprod. 2007, 22, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Weijl, N.I.; Hopman, G.D.; Wipkink-Bakker, A.; Lentjes EG, W.M.; Berger, H.M.; Cleton, F.J.; Osanto, S. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann. Oncol. 1998, 9, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, D.; Ciafrè, S.A.; Pittaluga, M.; Savini, I.; Farace, M.G. Cellular responses to H2O2 and bleomycin-induced oxidative stress in L6C5 rat myoblasts. Free Radic. Biol. Med. 2003, 35, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Look, M.P.; Musch, E. Lipid Peroxides in the Polychemotherapy of Cancer Patients. Chemotherapy 1994, 40, 8–15. [Google Scholar] [CrossRef]
- O’flaherty, C.M.; Chan, P.T.; Hales, B.F.; Robaire, B. Sperm Chromatin Structure Components Are Differentially Repaired in Cancer Survivors. J. Androl. 2012, 33, 629–636. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Hales, B.F.; Chan, P.; Robaire, B. Impact of chemotherapeutics and advanced testicular cancer or Hodgkin lymphoma on sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 94, 1374–1379. [Google Scholar] [CrossRef]
- Xiao, M.; Zhong, H.; Xia, L.; Tao, Y.; Yin, H. Pathophysiology of mitochondrial lipid oxidation: Role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic. Biol. Med. 2017, 111, 316–327. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.Y.; Scarlata, E.; O’Flaherty, C. Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa. Antioxidants 2020, 9, 170. https://doi.org/10.3390/antiox9020170
Wu PY, Scarlata E, O’Flaherty C. Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa. Antioxidants. 2020; 9(2):170. https://doi.org/10.3390/antiox9020170
Chicago/Turabian StyleWu, Pei You, Eleonora Scarlata, and Cristian O’Flaherty. 2020. "Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa" Antioxidants 9, no. 2: 170. https://doi.org/10.3390/antiox9020170
APA StyleWu, P. Y., Scarlata, E., & O’Flaherty, C. (2020). Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa. Antioxidants, 9(2), 170. https://doi.org/10.3390/antiox9020170





