Comparison of an Offline SPE–GC–MS and Online HS–SPME–GC–MS Method for the Analysis of Volatile Terpenoids in Wine
Abstract
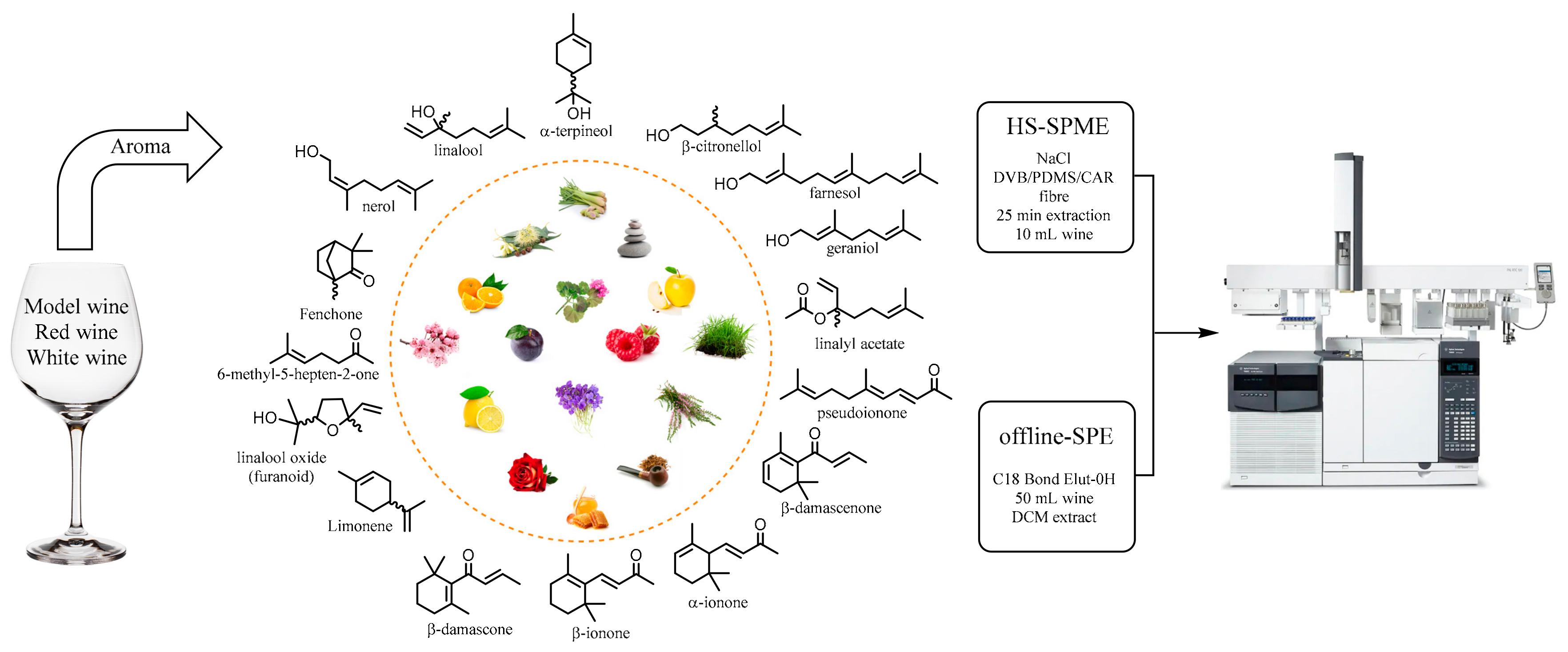
1. Introduction
2. Results and Discussion
2.1. Sample Preparation
2.2. Comparison of the Figures of Merit for Method Testing Parameters
2.3. Internal Standard Comparison
2.4. Volatile Terpenoid Quantification in White Wine
3. Materials and Methods
3.1. Chemicals
3.2. Method of cis/trans Ratio Determination in Standard Mixtures
3.3. Method for Dearomatized Wines
3.4. Sample Preparation
3.4.1. Offline SPE
3.4.2. Online HS–SPME
3.5. GC–MS Instrumental Parameters
3.6. Method Performance Parameters
3.6.1. Selectivity
3.6.2. Linearity
3.6.3. Limits of Detection and Limits of Quantitation
3.6.4. Accuracy (Recovery Test)
3.6.5. Precision (Repeatability Test)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Dziadas, M.; Jeleń, H.H. Analysis of terpenes in white wines using SPE-SPME-GC/MS approach. Anal. Chim. Acta 2010, 677, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Louw, L.; Tredoux, A.G.J.; van Rensburg, P.; Kidd, M.; Naes, T.; Nieuwoudt, H.H. Fermentation-derived aroma compounds in varietal young wines from South Africa. S. Afr. J. Enol. Vitic. 2010, 31, 213–225. [Google Scholar] [CrossRef][Green Version]
- Cocito, C.; Delfini, C. Simultaneous determination by GC of free and combined fatty acids and sterols in grape musts and yeasts as silanized compounds. Food Chem. 1994, 50, 297–305. [Google Scholar] [CrossRef]
- Shinohara, T. Agricultural and Biological Chemistry Gas Chromatographic Analysis of Volatile Fatty Acids in Wines Gas Chromatographic Analysis of Volatile Fatty Acids in Wines. Agric. Biol. Chem. 1985, 497, 5–7. [Google Scholar]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C 6 -alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Ryan, D.; Watkins, P.; Smith, J.; Allen, M.; Marriott, P. Analysis of methoxypyrazines in wine using headspace solid phase microextraction with isotope dilution and comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2005, 28, 1075–1082. [Google Scholar] [CrossRef]
- Mafata, M.; Stander, M.; Thomachot, B.; Buica, A. Measuring Thiols in Single Cultivar South African Red Wines Using 4,4-Dithiodipyridine (DTDP) Derivatization and Ultraperformance Convergence Chromatography-Tandem Mass Spectrometry. Foods 2018, 7, 138. [Google Scholar] [CrossRef]
- De Vries, C.J.; Mokwena, L.M.; Buica, A.; McKay, M. Determination of Volatile Phenol in Cabernet Sauvignon Wines, Made from Smoke-affected Grapes, by using HS-SPME GC-MS. S. Afr. J. Enol. Vitic. 2016, 37, 15–21. [Google Scholar] [CrossRef]
- Araujo, L.D.; Vannevel, S.; Buica, A.; Callerot, S.; Fedrizzi, B.; Kilmartin, P.A.; du Toit, W.J. Indications of the prominent role of elemental sulfur in the formation of the varietal thiol 3-mercaptohexanol in Sauvignon blanc wine. Food Res. Int. 2017, 98, 79–86. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Sánchez-Palomo, E.; Pérez-Coello, M.S. Fast screening method for volatile compounds of oak wood used for aging wines by headspace SPME-GC-MS (SIM). J. Agric. Food Chem. 2004, 52, 6857–6861. [Google Scholar] [CrossRef] [PubMed]
- Marchand, S.; De Revel, G.; Bertrand, A. Approaches to wine aroma: Release of aroma compounds from reactions between cysteine and carbonyl compounds in wine. J. Agric. Food Chem. 2000, 48, 4890–4895. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Wood, C.; Siebert, T.E.; Parker, M.; Capone, D.L.; Elsey, G.M.; Pollnitz, A.P.; Eggers, M.; Meier, M.; Vössing, T.; Widder, S.; et al. From wine to pepper: Rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. J. Agric. Food Chem. 2008, 56, 3738–3744. [Google Scholar] [CrossRef]
- Câmara, J.S.; Herbert, P.; Marques, J.C.; Alves, M.A. Varietal flavour compounds of four grape varieties producing Madeira wines. Anal. Chim. Acta 2004, 513, 203–207. [Google Scholar] [CrossRef]
- Serra, S. Recent advances in the synthesis of carotenoid-derived flavours and fragrances. Molecules 2015, 20, 12817–12840. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Palma, M.; Barroso, C.G. Determination of terpenoids in wines by solid phase extraction and gas chromatography. Anal. Chim. Acta 2004, 513, 209–214. [Google Scholar] [CrossRef]
- Cataldo, F. Thermal depolymerization and pyrolysis of cis-1,4-polyisoprene: Preparation of liquid polyisoprene and terpene resin. J. Anal. Appl. Pyrolysis 1998, 44, 121–130. [Google Scholar] [CrossRef]
- Skinkis, P.A.; Bordelon, B.P.; Butz, E.M. Effects of sunlight exposure on berry and wine monoterpenes and sensory characteristics of traminette. Am. J. Enol. Vitic. 2010, 61, 147–156. [Google Scholar]
- Fariña, L.; Boido, E.; Carrau, F.; Versini, G.; Dellacassa, E. Terpene compounds as possible precursors of 1,8-cineole in red grapes and wines. J. Agric. Food Chem. 2005, 53, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Ebeler, S.E. Analytical chemistry: Unlocking the secrets of wine flavor. Food Rev. Int. 2001, 17, 45–64. [Google Scholar] [CrossRef]
- Polášková, P.; Herszage, J.; Ebeler, S.E. Wine flavor: Chemistry in a glass. Chem. Soc. Rev. 2008, 37, 2478–2489. [Google Scholar] [CrossRef]
- De Villiers, A.; Alberts, P.; Tredoux, A.G.J.; Nieuwoudt, H.H. Analytical techniques for wine analysis: An African perspective; a review. Anal. Chim. Acta 2012, 730, 2–23. [Google Scholar] [CrossRef]
- Williams, P.J.; Strauss, C.R.; Wilson, B. Classification of the Monoterpenoid Composition of Muscat Grapes. Am. J. Enol. Vitic. 1981, 32, 230–235. [Google Scholar]
- Kishimoto, T.; Wanikawa, A.; Kagami, N.; Kawatsura, K. Analysis of hop-derived terpenoids in beer and evaluation of their behavior using the stir bar-sorptive extraction method with GC-MS. J. Agric. Food Chem. 2005, 53, 4701–4707. [Google Scholar] [CrossRef]
- Câmara, J.S.; Arminda Alves, M.; Marques, J.C. Development of headspace solid-phase microextraction-gas chromatography-mass spectrometry methodology for analysis of terpenoids in Madeira wines. Anal. Chim. Acta 2006, 555, 191–200. [Google Scholar] [CrossRef]
- Andujar-Ortiz, I.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Pozo-Bayón, M.A. Analytical performance of three commonly used extraction methods for the gas chromatography-mass spectrometry analysis of wine volatile compounds. J. Chromatogr. A 2009, 1216, 7351–7357. [Google Scholar] [CrossRef]
- Gamero, A.; Wesselink, W.; de Jong, C. Comparison of the sensitivity of different aroma extraction techniques in combination with gas chromatography-mass spectrometry to detect minor aroma compounds in wine. J. Chromatogr. A 2013, 1272, 1–7. [Google Scholar] [CrossRef]
- Tufariello, M.; Pati, S.; D’Amico, L.; Bleve, G.; Losito, I.; Grieco, F. Quantitative issues related to the headspace-SPME-GC/MS analysis of volatile compounds in wines: The case of Maresco sparkling wine. LWT 2019, 108, 268–276. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Lanaridis, P.; Salaha, M.J.; Tzourou, I.; Tsoutsouras, E.; Karagiannis, S. Volatile compounds in grapes and wines from two Muscat varieties cultivated in Greek Islands. J. Int. Sci. Vigne Vin. 2002, 36, 39–47. [Google Scholar] [CrossRef]
- Bavčar, D.; Česnik, H.B. Validation of the method for the determination of some wine volatile compounds. Acta Agric. Slov. 2011, 97, 285–293. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Graña, M.; Oliveira, J.M. Determination of odorants in varietal wines from international grape cultivars (Vitis vinifera) grown in NW Spain. S. Afr. J. Enol. Vitic. 2013, 34, 212–222. [Google Scholar] [CrossRef]
- Michlmayr, H.; Nauer, S.; Brandes, W.; Schümann, C.; Kulbe, K.D.; Del Hierro, A.M.; Eder, R. Release of wine monoterpenes from natural precursors by glycosidases from Oenococcus oeni. Food Chem. 2012, 135, 80–87. [Google Scholar] [CrossRef]
- Park, S.K.; Morrison, J.C.; Adams, D.O.; Noble, A.C. Distribution of Free and Glycosidically Bound Monoterpenes in the Skin and Mesocarp of Muscat of Alexandria Grapes during Development. J. Agric. Food Chem. 1991, 39, 514–518. [Google Scholar] [CrossRef]
- Baron, M.; Prusova, B.; Tomaskova, L.; Kumsta, M.; Sochor, J. Terpene content of wine from the aromatic grape variety “Irsai Oliver” (Vitis vinifera L.) depends on maceration time. Open Life Sci. 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Zalacain, A.; Marín, J.; Alonso, G.L.; Salinas, M.R. Analysis of wine primary aroma compounds by stir bar sorptive extraction. Talanta 2007, 71, 1610–1615. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and Sensory Studies on Tomato Paste Volatiles. J. Agric. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, J.; Xu, Y. Different influences of β-glucosidases on volatile compounds and anthocyanins of Cabernet Gernischt and possible reason. Food Chem. 2013, 140, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M.; Ubeda, C.; Ríos-Reina, R.; Morales, M.L.; Troncoso, A.M. Recent developments in the analysis of musty odour compounds in water and wine: A review. J. Chromatogr. A 2016, 1428, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Sadoughi, N.; Schmidtke, L.M.; Antalick, G.; Blackman, J.W.; Steel, C.C. Gas chromatography-mass spectrometry method optimized using response surface modeling for the quantitation of fungal off-flavors in grapes and wine. J. Agric. Food Chem. 2015, 63, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Kaack, K.; Christensen, L.P.; Hughes, M.; Eder, R. Relationship between sensory quality and volatile compounds of elderflower (Sambucus nigra L.) extracts. Eur. Food Res. Technol. 2006, 223, 57–70. [Google Scholar] [CrossRef]
- Pino, J.A.; Queris, O. Analysis of volatile compounds of mango wine. Food Chem. 2011, 125, 1141–1146. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Selli, S.; Canbas, A.; Varlet, V.; Kelebek, H.; Prost, C.; Serot, T. Characterization of the most odor-active volatiles of orange wine made from a Turkish cv. Kozan (Citrus sinensis L. Osbeck). J. Agric. Food Chem. 2008, 56, 227–234. [Google Scholar] [CrossRef]
- Elsharif, S.A.; Banerjee, A.; Buettner, A. Structure-odor relationships of linalool, linalyl acetate and their corresponding oxygenated derivatives. Front. Chem. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Rouseff, R.; Valim, M.F.; Naim, M. Identification and aroma impact of norisoprenoids in orange juice. J. Agric. Food Chem. 2005, 53, 393–397. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Hirvi, T.; Honkanen, E. The aroma of blueberries. J. Sci. Food Agric. 1983, 34, 992–996. [Google Scholar] [CrossRef]
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zea, L.; Moyano, L.; Moreno, J.; Cortes, B.; Medina, M. Discrimination of the aroma fraction of Sherry wines obtained by oxidative and biological ageing. Food Chem. 2001, 75, 79–84. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 1–25. [Google Scholar] [CrossRef]
- Vilanova, M.; Sieiro, C. Determination of free and bound terpene compounds in Albariño wine. J. Food Compos. Anal. 2006, 19, 694–697. [Google Scholar] [CrossRef]
- Song, M.; Fuentes, C.; Loos, A.; Tomasino, E. Free monoterpene isomer profiles of Vitis vinifera L. Cv. white wines. Foods 2018, 7, 27. [Google Scholar] [CrossRef]
- Capone, D.L.; Van Leeuwen, K.; Taylor, D.K.; Jeffery, D.W.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A. Evolution and occurrence of 1,8-cineole (Eucalyptol) in Australian wine. J. Agric. Food Chem. 2011, 59, 953–959. [Google Scholar] [CrossRef]
- Pedersen, D.S.; Capone, D.L.; Skouroumounis, G.K.; Pollnitz, A.P.; Sefton, M.A. Quantitative analysis of geraniol, nerol, linalool, and α-terpineol in wine. Anal. Bioanal. Chem. 2003, 375, 517–522. [Google Scholar] [CrossRef]
- Williams, C.; Ferreira, M.; Monflier, E.; Mapolie, S.F.; Smith, G.S. Synthesis and hydroformylation evaluation of Fréchet-type organometallic dendrons with N,O-salicylaldimine Rh(i) complexes at the focal point. Dalt. Trans. 2018, 47. [Google Scholar] [CrossRef]
- Prosen, H.; Janeš, L.; Strlič, M.; Rusjan, D.; Kočar, D. Analysis of free and bound aroma compounds in grape berries using headspace solid-phase microextraction with GC-MS and a preliminary study of solid-phase extraction with LC-MS. Acta Chim. Slov. 2007, 54, 25–32. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound | CAS Number | OTDs (µg/L) | Odor Descriptor |
|---|---|---|---|
| Limonene | 5989-27-5 | 15 [39] | citrus [13] |
| 6-Methyl-5-hepten-2-one | 110-93-0 | 50 [40] | citrus-like [41] |
| Fenchone | 7787-20-4 | 110 [42] | muddy [43], earthy [42] |
| cis-Linalool oxide (furanoid) | 1365-19-1 | - | elderflower, leaves, sweet [44] |
| trans-Linalool oxide (furanoid) | 34995-77-2 | 320 [45] | woody [45] |
| Linalool | 78-70-6 | 25 [46] | Floral [47], lavender [13] |
| Linalyl acetate | 115-95-7 | 110 [48] | floral, sweet, mint, caraway-like [48] |
| α-terpineol | 98-55-5 | 250 [46] | oily, anise, spicy [13] |
| β-Citronellol | 106-22-9 | 18 [39] | citrus, floral [47] |
| Nerol | 106-25-2 | 15 [39] | orange, floral, lemongrass [49] |
| β-Damascone | 23726-91-2 | - | fruity, floral, plum, rose [18] |
| β-Damascenone | 23696-85-7 | 0.05 [50] | tobacco, apple, floral, rose [49] |
| Geraniol | 106-24-1 | 30 [50] | floral [47], geranium [49] |
| α-Ionone | 127-41-3 | 2.6 [46] | floral [49] |
| β-Ionone | 14901-07-6 | 0.09 [46] | raspberry, floral [49] |
| cis-PseudoIonone | 13927-47-4 | 800 [40] | - |
| trans-PseudoIonone | 3548-78-5 | 800 [40] | - |
| Farnesol (Z,E) | 3790-71-4 | - | - |
| Farnesol (E,Z) | 3879-60-5 | - | - |
| Farnesol (E,E) | 106-28-5 | 20 [45] | floral, oily, blueberry [51,52,53] |
| Compound | Calibration Range (µg/L) | R2 | Repeatability (RSD%) (n = 3) | Accuracy (%), Recovery Tests at 20 µg/L (n = 3) | LOD (µg/L) | LOQ (µg/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HS–SPME | ||||||||||||||||||
| MW | WW | RW | MW | WW | RW | MW | WW | RW | MW | WW | RW | MW | WW | RW | MW | WW | RW | |
| Limonene | 1–20 | 5–100 | 1–20 | 0.9806 | 0.9975 | 0.9946 | 1.9 | 8.1 | 6.4 | 112 | 108 | 110 | 0.0002 | 0.0019 | 0.0053 | 0.0008 | 0.0063 | 0.0178 |
| 6-Methyl-5-hepten-2-one | 1–50 | 1–50 | 1–50 | 0.9967 | 0.9992 | 0.9988 | 1.5 | 2.4 | 1.8 | 105 | 98 | 104 | 0.0108 | 0.0209 | 0.2143 | 0.0361 | 0.0697 | 0.7144 |
| Fenchone | 1–50 | 1–50 | 1–50 | 0.9966 | 0.9987 | 0.9979 | 1.7 | 5.1 | 0.5 | 105 | 96 | 104 | 0.0036 | 0.0100 | 0.0208 | 0.0120 | 0.0334 | 0.0693 |
| cis-Linalool oxide | 0.57–54 | 0.57–57 | 0.57–57 | 0.9991 | 0.9993 | 0.9996 | 1.8 | 1.2 | 2.3 | 104 | 88 | 102 | 0.0533 | 0.0687 | 0.1910 | 0.1777 | 0.2289 | 0.6366 |
| trans-Linalool oxide | 0.43–43 | 0.43–43 | 0.43–43 | 0.9997 | 0.9995 | 0.9986 | 1.3 | 8.7 | 5.1 | 97 | 91 | 95 | 0.0298 | 0.0456 | 0.1804 | 0.0994 | 0.1521 | 0.6015 |
| Linalool | 1–50 | 1–50 | 1–50 | 0.9916 | 0.9961 | 0.9975 | 1.9 | 2.7 | 0.6 | 108 | 94 | 104 | 0.0154 | 0.0164 | 0.0584 | 0.0514 | 0.0545 | 0.1948 |
| Linalyl acetate | 5–100 | 5–100 | 10–100 | 0.9798 | 0.9913 | 0.9976 | 11.5 | 12.4 | 16.6 | 107 | 91 | 108 | 0.0157 | 0.0301 | 0.4197 | 0.0522 | 0.1003 | 1.3989 |
| α-Terpineol | 1–100 | 1–100 | 1–100 | 0.9974 | 0.9988 | 0.9997 | 1.2 | 2.8 | 0.7 | 104 | 87 | 100 | 0.0019 | 0.0076 | 0.0441 | 0.0064 | 0.0254 | 0.1470 |
| β-Citronellol | 1–100 | 1–100 | 1–100 | 0.9970 | 0.9996 | 0.9985 | 4.1 | 9.0 | 1.4 | 101 | 102 | 94 | 0.0218 | 0.0453 | 0.1477 | 0.0726 | 0.1512 | 0.4924 |
| Nerol | 1–100 | 1–100 | 1–100 | 0.9985 | 0.9991 | 0.9965 | 5.0 | 1.6 | 1.2 | 99 | 97 | 90 | 0.1049 | 0.2163 | 0.6350 | 0.3495 | 0.7210 | 2.1166 |
| β-Damascone | 1–100 | 1–100 | 1–100 | 0.9898 | 0.9983 | 0.9999 | 3.8 | 2.1 | 1.2 | 106 | 95 | 101 | 0.0005 | 0.0015 | 0.0043 | 0.0017 | 0.0051 | 0.0145 |
| β-Damascenone | 1–100 | 1–100 | 1–100 | 0.9919 | 0.9957 | 0.9994 | 3.9 | 1.3 | 1.2 | 105 | 98 | 103 | 0.0007 | 0.0019 | 0.0133 | 0.0024 | 0.0062 | 0.0442 |
| Geraniol | 1–100 | 1–100 | 1–100 | 0.9908 | 0.9978 | 0.9993 | 5.1 | 1.8 | 1.6 | 105 | 107 | 103 | 0.0972 | 1.1069 | 0.8291 | 0.3240 | 3.6898 | 2.7636 |
| α-Ionone | 1–100 | 1–100 | 1–100 | 0.9917 | 0.9988 | 0.9997 | 3.8 | 1.8 | 1.1 | 105 | 97 | 99 | 0.0007 | 0.0042 | 0.0067 | 0.0025 | 0.0141 | 0.0223 |
| β-Ionone | 1–100 | 1–100 | 1–100 | 0.9981 | 0.9998 | 0.9966 | 4.7 | 1.7 | 1.3 | 110 | 99 | 91 | 0.0006 | 0.0020 | 0.0069 | 0.0020 | 0.0066 | 0.0231 |
| cis-Pseudoionone | 0.28–27.6 | 0.28–13.82 | 0.28–13.82 | 0.9931 | 0.9919 | 0.9876 | 5.4 | 1.1 | 3.1 | 89 | 100 | 90 | 0.0073 | 0.0349 | 0.1786 | 0.0244 | 0.1162 | 0.5954 |
| trans-Pseudoionone | 0.73–72.8 | 0.73–36.4 | 0.73–36.4 | 0.9957 | 0.9977 | 0.9909 | 7.0 | 0.7 | 4.6 | 93 | 103 | 92 | 0.0101 | 0.0465 | 0.2582 | 0.0338 | 0.1550 | 0.8606 |
| Farnesol (Z,E) | 0.088–8.79 | 0.18–4.4 | 0.18–4.4 | 0.9962 | 0.9907 | 0.9434 | 3.3 | 4.6 | 6.2 | 98 | 106 | 87 | 0.0631 | 0.0322 | 0.1842 | 0.2104 | 0.1073 | 0.6141 |
| Farnesol (E,Z) | 0.35–35 | 0.70–17.5 | 0.35–17.5 | 0.9952 | 0.9967 | 0.9830 | 3.2 | 2.5 | 8.0 | 98 | 107 | 87 | 0.0387 | 0.0473 | 0.2436 | 0.1290 | 0.1575 | 0.8118 |
| Farnesol (E,E) | 0.57–56.7 | 0.57–56.7 | 0.57–28.34 | 0.9951 | 0.9958 | 0.9907 | 2.6 | 3.4 | 5.8 | 98 | 109 | 82 | 0.0207 | 0.0419 | 0.2773 | 0.0692 | 0.1395 | 0.9245 |
| SPE | ||||||||||||||||||
| MW | WW | RW | MW | WW | RW | MW | WW | RW | MW | WW | RW | MW | WW | RW | MW | WW | RW | |
| Limonene | 5–100 | 5–100 | 5–100 | 0.9997 | 0.9934 | 0.9853 | 8.4 | 7.9 | 8.3 | 104 | 119 | 119 | 0.0226 | 0.0399 | 0.0504 | 0.0755 | 0.1330 | 0.1680 |
| 6-Methyl-5-hepten-2-one | 5–500 | 5–500 | 5–500 | 0.9993 | 0.9993 | 0.9987 | 7.0 | 3.8 | 5.9 | 101 | 98 | 102 | 0.0098 | 0.0137 | 0.0135 | 0.0328 | 0.0457 | 0.0450 |
| Fenchone | 5–500 | 5–500 | 5–500 | 0.9994 | 0.9991 | 0.9989 | 6.4 | 4.6 | 5.4 | 98 | 98 | 101 | 0.0123 | 0.0139 | 0.0146 | 0.0412 | 0.0464 | 0.0488 |
| cis-Linalool oxide | 2.87–287 | 2.87–287 | 2.87–287 | 0.9997 | 0.998 | 0.9993 | 3.8 | 4.0 | 6.1 | 100 | 93 | 90 | 0.0349 | 0.0640 | 0.0587 | 0.1162 | 0.2133 | 0.1958 |
| trans-Linalool oxide | 2.15–215 | 2.15–215 | 2.15–215 | 0.9958 | 0.9979 | 0.9993 | 3.7 | 3.7 | 5.2 | 101 | 93 | 90 | 0.0361 | 0.0679 | 0.0627 | 0.1203 | 0.2265 | 0.2091 |
| Linalool | 5–500 | 5–500 | 5–500 | 0.9988 | 0.9987 | 0.9983 | 4.3 | 4.1 | 3.6 | 96 | 94 | 96 | 0.0662 | 0.0279 | 0.0006 | 0.2205 | 0.0931 | 0.0021 |
| Linalyl acetate | 5–500 | 5–500 | 5–500 | 0.9959 | 0.9978 | 0.9992 | 4.7 | 7.0 | 3.4 | 90 | 92 | 100 | 0.0445 | 0.0323 | 0.0369 | 0.1483 | 0.1077 | 0.1229 |
| α-Terpineol | 5–500 | 5–500 | 5–500 | 0.9987 | 0.9983 | 0.9996 | 5.3 | 5.5 | 3.4 | 98 | 96 | 101 | 0.0346 | 0.1404 | 0.0151 | 0.1152 | 0.4679 | 0.0504 |
| β-Citronellol | 5–500 | 5–500 | 5–500 | 0.9987 | 0.9980 | 0.9987 | 6.5 | 3.6 | 7.4 | 90 | 91 | 102 | 0.1458 | 0.1078 | 0.0880 | 0.4859 | 0.3595 | 0.2935 |
| Nerol | 5–500 | 5–500 | 5–500 | 0.9995 | 0.9985 | 0.9995 | 3.7 | 4.1 | 4.6 | 91 | 91 | 96 | 0.2273 | 0.1985 | 0.2093 | 0.7575 | 0.6618 | 0.6977 |
| β-Damascone | 5–500 | 5–500 | 5–500 | 0.9994 | 0.9988 | 0.9995 | 6.9 | 4.2 | 3.5 | 95 | 94 | 102 | 0.0074 | 0.0074 | 0.0073 | 0.0247 | 0.0248 | 0.0242 |
| β-Damascenone | 5–500 | 5–500 | 5–500 | 0.9993 | 0.9987 | 0.9994 | 6.5 | 4.2 | 3.1 | 95 | 94 | 103 | 0.0087 | 0.0088 | 0.0095 | 0.0289 | 0.0294 | 0.0317 |
| Geraniol | 5–500 | 5–500 | 5–500 | 0.9989 | 0.9987 | 0.9998 | 6.0 | 5.6 | 5.7 | 91 | 94 | 100 | 0.3121 | 0.2234 | 0.2554 | 1.0402 | 0.7447 | 0.8515 |
| α-Ionone | 5–500 | 5–500 | 5–500 | 0.9994 | 0.9985 | 0.9996 | 6.8 | 4.0 | 3.6 | 93 | 92 | 100 | 0.0097 | 0.0105 | 0.0122 | 0.0323 | 0.0351 | 0.0407 |
| β-Ionone | 5–500 | 5–500 | 5–500 | 0.9996 | 0.9987 | 0.9995 | 6.6 | 4.1 | 4.8 | 95 | 93 | 103 | 0.0057 | 0.0059 | 0.0061 | 0.0188 | 0.0196 | 0.0202 |
| cis-Pseudoionone | 1.38–138 | 1.38–138 | 1.38–138 | 0.998 | 0.9961 | 0.9983 | 6.2 | 7.6 | 5.1 | 88 | 88 | 97 | 0.0461 | 0.0826 | 0.0714 | 0.1538 | 0.2752 | 0.2381 |
| trans-Pseudoionone | 3.63–363 | 3.63–363 | 3.63–363 | 0.9992 | 0.9981 | 0.9996 | 6.3 | 4.2 | 5.7 | 90 | 89 | 101 | 0.0343 | 0.0594 | 0.0564 | 0.1143 | 0.1980 | 0.1879 |
| Farnesol (Z,E) | 0.88–441 | 0.88–441 | 0.88–441 | 0.9978 | 0.9991 | 0.9990 | 3.1 | 7.6 | 7.9 | 94 | 91 | 96 | 0.1079 | 0.1553 | 0.0665 | 0.3596 | 0.5177 | 0.2218 |
| Farnesol (E,Z) | 1.75–175 | 1.75–175 | 1.75–175 | 0.9971 | 0.9965 | 0.9990 | 7.9 | 1.0 | 6.5 | 85 | 81 | 98 | 0.1320 | 0.1865 | 0.0792 | 0.4400 | 0.6218 | 0.2642 |
| Farnesol (E,E) | 2.8–280 | 2.8–280 | 2.8–280 | 0.9954 | 0.9974 | 0.9978 | 7.0 | 5.2 | 6.0 | 83 | 83 | 101 | 0.1202 | 0.1464 | 0.0513 | 0.4006 | 0.4881 | 0.1709 |
| Online HS–SPME | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FLY16S | ARV16S | ANI16/17S | JCB16CH | FRN17SB | FLK17AL | KZC18CB | KLA16S | ORB17AL | LNC17SB | ARV16S/B | GWZ19C | GWC19E | MRF17SB | |
| Limonene | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.27 | <LOQ | <LOQ | <LOQ | <LOQ | 24.39 | 0.13 | 0.01 | <LOQ |
| 6-Methyl-5-hepten-2-one | <LOQ | <LOQ | 0.06 | <LOQ | <LOQ | 0.8 | 0.46 | <LOQ | <LOQ | <LOQ | 0.31 | 0.87 | 1.06 | <LOQ |
| Fenchone | <LOQ | <LOD | <LOD | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| cis-Linalool oxide | 20.79 | 8.1 | 18.93 | 6.58 | 4.84 | 20.56 | <LOD | 19.08 | 2.84 | 10.33 | 14.01 | 5.53 | 2.34 | 3.69 |
| trans-Linalool oxide | 6.14 | 3.07 | 5.96 | 2.84 | 1.77 | 21.38 | 0.78 | 5.38 | 4.71 | 3.86 | 4.81 | 3.24 | 2.22 | 1.7 |
| Linalool | 0.29 | 0.82 | 1.31 | 1.93 | 2.06 | 37.9 | 2.44 | 2.13 | 15.22 | 3.49 | 0.1 | 22.04 | 14.6 | 3.96 |
| Linalyl acetate | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOQ | <LOD | <LOQ | <LOQ | <LOQ | <LOQ | <LOD |
| α-Terpineol | 2.92 | 4.03 | 4.11 | 4.97 | 3.18 | 56.23 | 0.91 | 4.73 | 11.79 | 12.68 | 5.21 | 10.76 | 6.3 | 3.46 |
| β-Citronellol | 1.35 | 0.76 | 1.73 | 1.81 | 1.75 | 5.27 | 2.13 | 1.61 | 3.13 | 2.24 | 0.52 | 34 | 75.43 | 3.69 |
| Nerol | 0.76 | 2.08 | <LOD | 5.05 | 5.55 | 7.82 | 0.76 | 3.46 | 2.92 | 7.19 | 2.1 | 13.78 | 21.04 | <LOD |
| β-Damascone | 0.01 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| β-Damascenone | 0.64 | 0.75 | 0.66 | 0.74 | 0.27 | 1.31 | 0.35 | 1.69 | 1.68 | <LOQ | 2.45 | 3.24 | 1.75 | 1.45 |
| Geraniol | 3.27 | 64.39 | 9.45 | 5.41 | 6.49 | 20.21 | 3.48 | 3.56 | 8.67 | 7.77 | 78.95 | 60.22 | 63.37 | 13.14 |
| α-Ionone | 0.04 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| β-Ionone | 0.26 | 0.06 | 0.04 | 0.13 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.01 | 0.32 | 0.02 | 0.02 | 0.03 |
| cis-Pseudoionone | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.36 | <LOD | <LOD | <LOD |
| trans-Pseudoionone | 0.27 | 0.34 | 1.02 | 0.35 | 0.23 | 0.18 | 0.32 | 0.2 | 0.16 | 0.19 | 1.11 | 0.29 | 0.29 | 0.28 |
| Farnesol (Z,E) | 0.11 | <LOD | 0.05 | 0.63 | 0.1 | 0.29 | 0.21 | 0.06 | 0.42 | 0.03 | 0.03 | 0.01 | 0.01 | 0.62 |
| Farnesol (E,Z) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.32 | <LOD | <LOD | <LOD |
| Farnesol (E,E) | 0.15 | 0.11 | 0.4 | 1.77 | 0.53 | 0.69 | 3.66 | 0.09 | 1.87 | 0.06 | 0.91 | 3.12 | 3.24 | 1.87 |
| Offline SPE | ||||||||||||||
| FLY16S | ARV16S | ANI16/17S | JCB16CH | FRN17SB | FLK17AL | KZC18CB | KLA16S | ORB17AL | LNC17SB | ARV16S/B | GWZ19C | GWC19E | MRF17SB | |
| Limonene | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 6-Methyl-5-hepten-2-one | <LOD | <LOD | <LOQ | 0.02 | <LOD | 0.91 | 0.53 | <LOD | <LOD | <LOD | 0.07 | 1.68 | 1.63 | <LOD |
| Fenchone | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| cis-Linalool oxide | 24.5 | 11.01 | 19.81 | 11.12 | 8.11 | 30.41 | 0.07 | 16.48 | 4.24 | 18.26 | 12.89 | 1.39 | 0.87 | 5.17 |
| trans-Linalool oxide | 8.23 | 3.48 | 5.84 | 4.1 | 2.7 | 21.62 | 0.19 | 5.15 | 1.32 | 4.65 | 4.33 | 1.45 | 1.18 | 1.19 |
| Linalool | 1.45 | 1.66 | 2.37 | 4.22 | 3.41 | 63.16 | 4.04 | 8.02 | 21.51 | 5.33 | 0.62 | 19.09 | 12.43 | 6.01 |
| Linalyl acetate | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| α-Terpineol | 3.46 | 4.7 | 4.87 | 5.97 | 2.99 | 85.92 | <LOQ | 7.01 | 14.83 | 18.97 | 6.02 | 3.81 | 1.72 | 3.4 |
| β-Citronellol | 2.12 | 1.47 | 2.36 | 2.35 | 2.32 | 5.98 | 2.64 | 2.81 | 3.29 | 2.68 | 1.23 | 48.86 | 92.34 | 3.7 |
| Nerol | <LOD | <LOD | <LOD | <LOD | <LOD | 5.92 | <LOD | <LOD | 2.88 | <LOD | <LOD | 18 | 24.25 | <LOD |
| β-Damascone | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| β-Damascenone | 0.98 | 1.19 | 1.19 | 1.07 | 0.71 | 1.61 | 0.86 | 2.42 | 1.68 | 0.36 | 2.97 | 3.79 | 2.01 | 1.47 |
| Geraniol | <LOD | 9.63 | 0.42 | 1.56 | 1.71 | 14.72 | 2.83 | <LOD | 6.01 | 3.5 | 13.68 | 66.92 | 60.07 | 4.78 |
| α-Ionone | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.79 | 0.75 | <LOD |
| β-Ionone | <LOD | <LOD | <LOD | <LOD | <LOD | 0.42 | <LOD | 0.47 | <LOD | <LOD | 0.51 | <LOD | <LOD | <LOD |
| cis-Pseudoionone | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 1.48 | 1.41 | <LOD |
| trans-Pseudoionone | 0.72 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.74 | <LOD | <LOD |
| Farnesol (Z,E) | 0.59 | <LOD | <LOD | 0.62 | <LOD | 0.58 | <LOD | <LOD | 0.73 | 0.7 | <LOD | 0.68 | 0.71 | 0.85 |
| Farnesol (E,Z) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.77 | <LOD | <LOD |
| Farnesol (E,E) | 0.13 | 0.23 | 0.24 | 0.91 | 0.51 | 0.59 | 2.44 | 0.42 | 1.36 | 0.19 | 0.16 | 12.06 | 12.07 | 1.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, C.; Buica, A. Comparison of an Offline SPE–GC–MS and Online HS–SPME–GC–MS Method for the Analysis of Volatile Terpenoids in Wine. Molecules 2020, 25, 657. https://doi.org/10.3390/molecules25030657
Williams C, Buica A. Comparison of an Offline SPE–GC–MS and Online HS–SPME–GC–MS Method for the Analysis of Volatile Terpenoids in Wine. Molecules. 2020; 25(3):657. https://doi.org/10.3390/molecules25030657
Chicago/Turabian StyleWilliams, Cody, and Astrid Buica. 2020. "Comparison of an Offline SPE–GC–MS and Online HS–SPME–GC–MS Method for the Analysis of Volatile Terpenoids in Wine" Molecules 25, no. 3: 657. https://doi.org/10.3390/molecules25030657
APA StyleWilliams, C., & Buica, A. (2020). Comparison of an Offline SPE–GC–MS and Online HS–SPME–GC–MS Method for the Analysis of Volatile Terpenoids in Wine. Molecules, 25(3), 657. https://doi.org/10.3390/molecules25030657






