Preparation and Characterization of Octenyl Succinate β-Cyclodextrin and Vitamin E Inclusion Complex and Its Application in Emulsion
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Simulation and Single-Factor Analysis
2.2. SEM Analysis
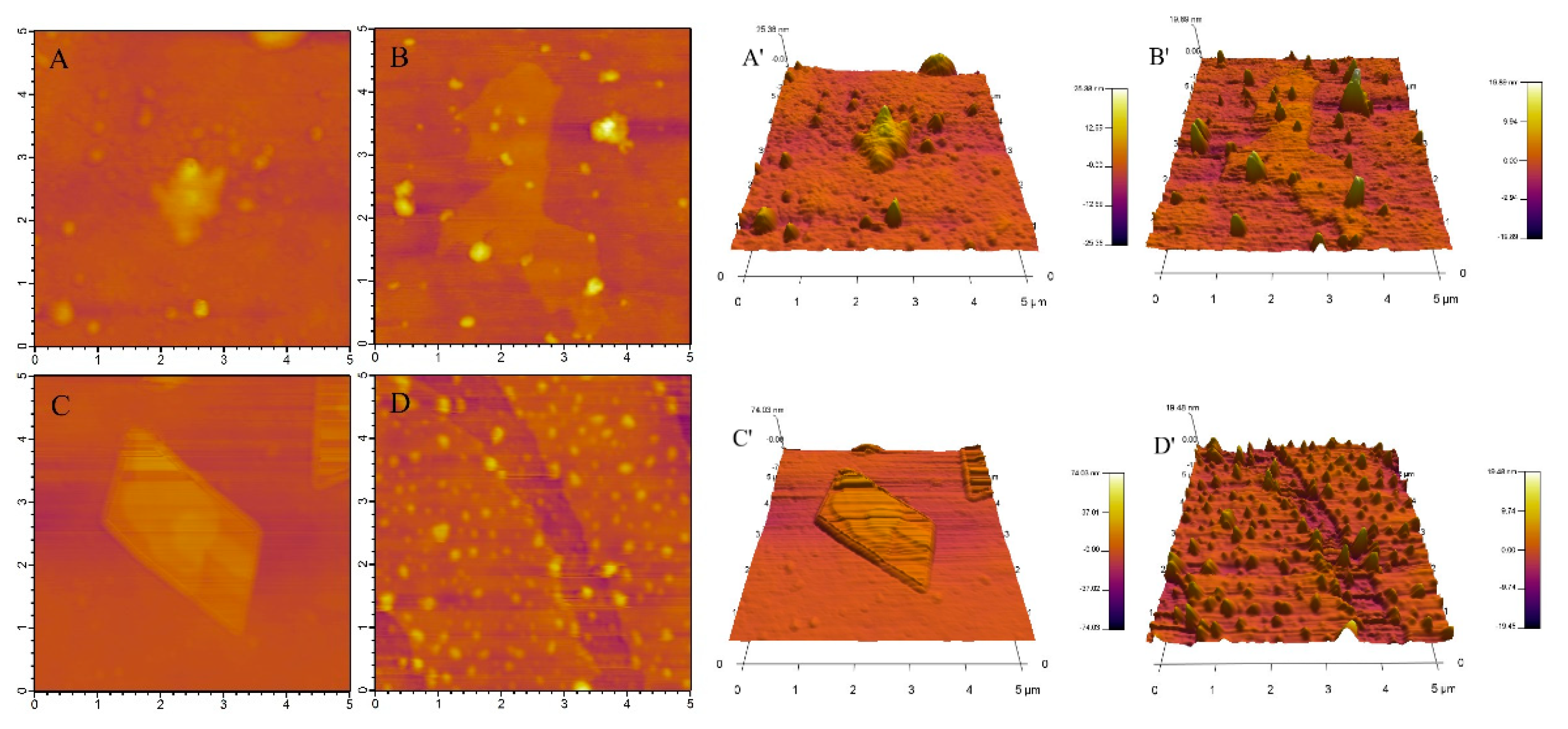
2.3. AFM Analysis
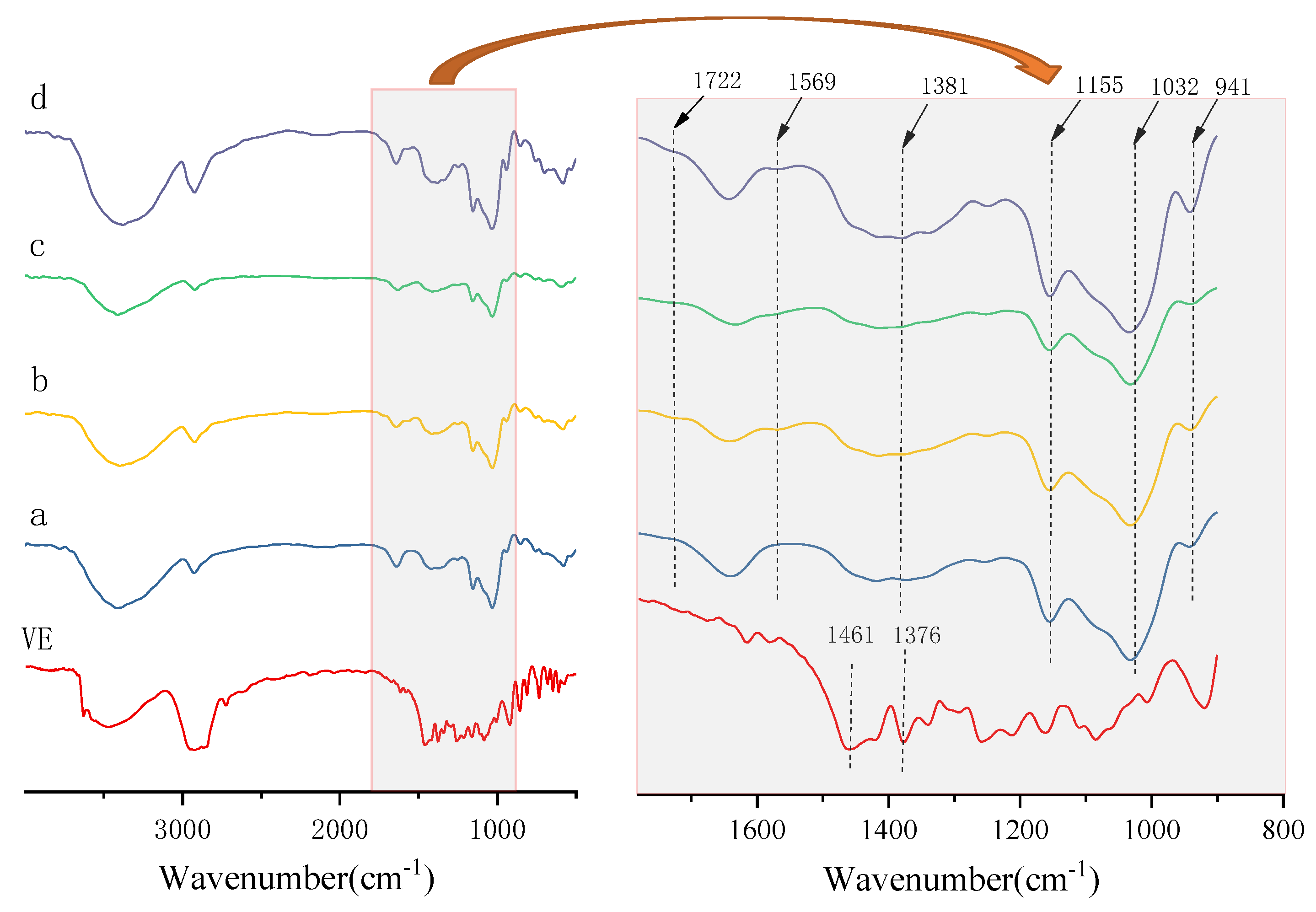
2.4. FT-IR Analysis
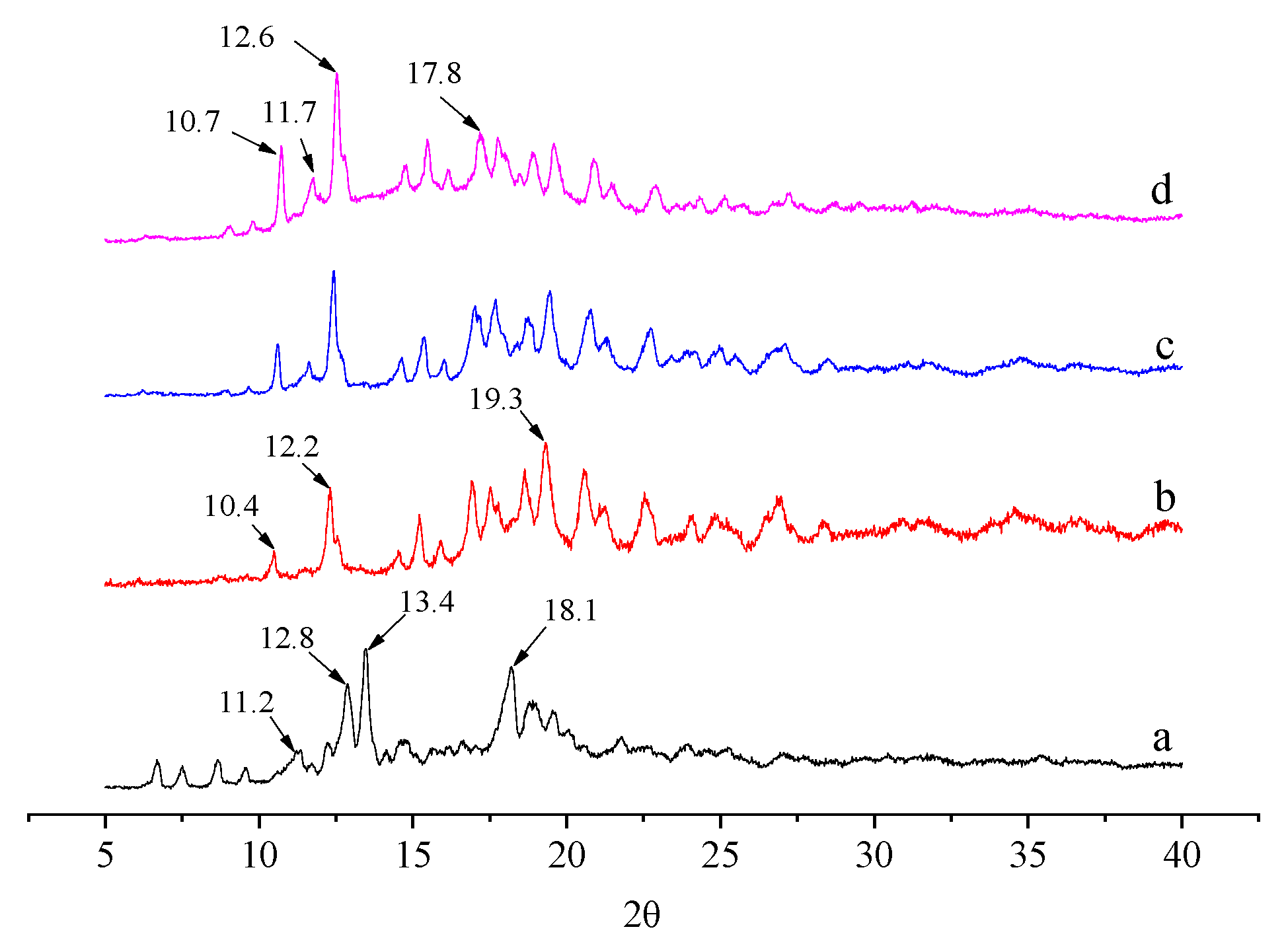
2.5. XRD Analysis
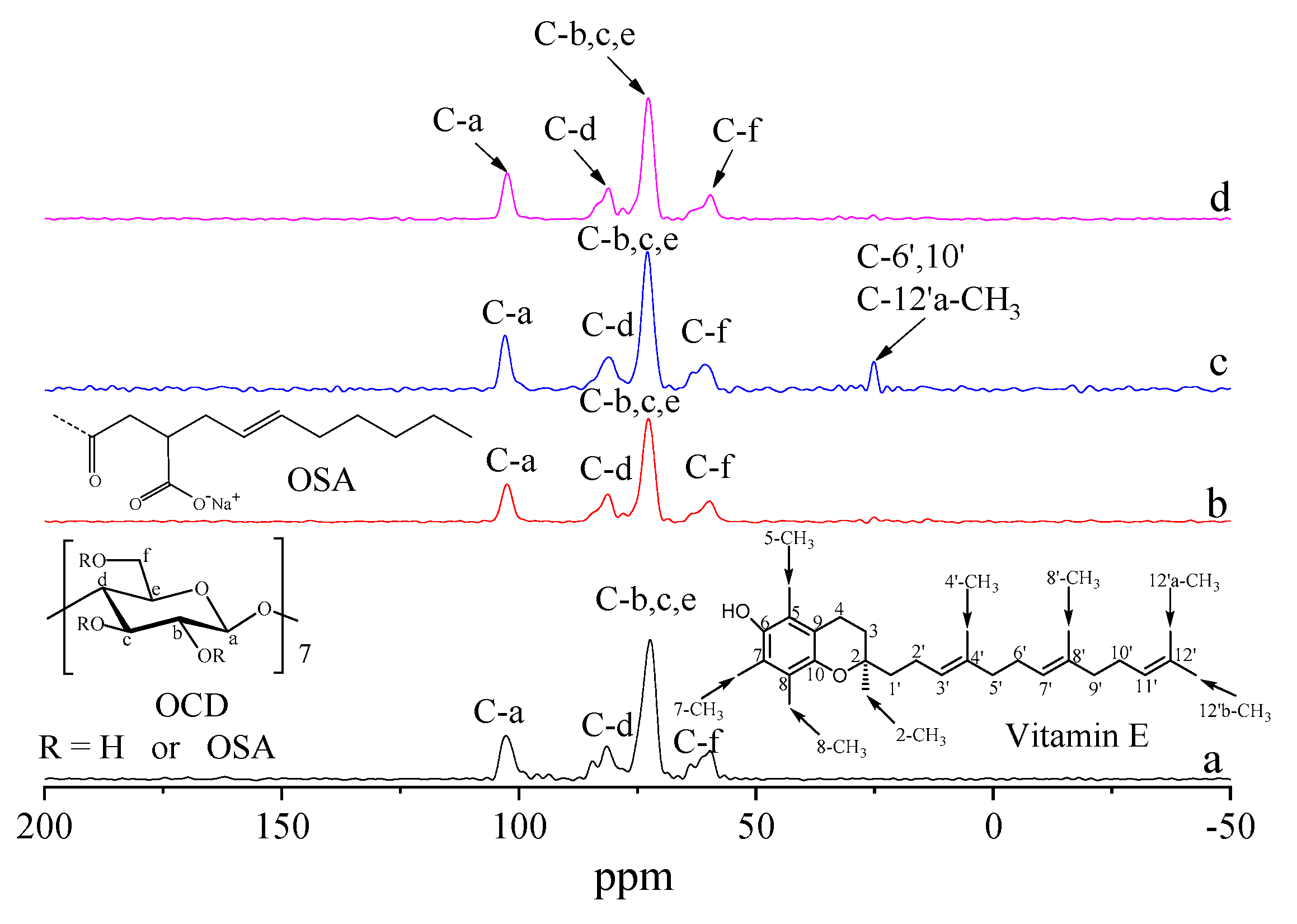
2.6. 13C CP/MAS NMR Analysis
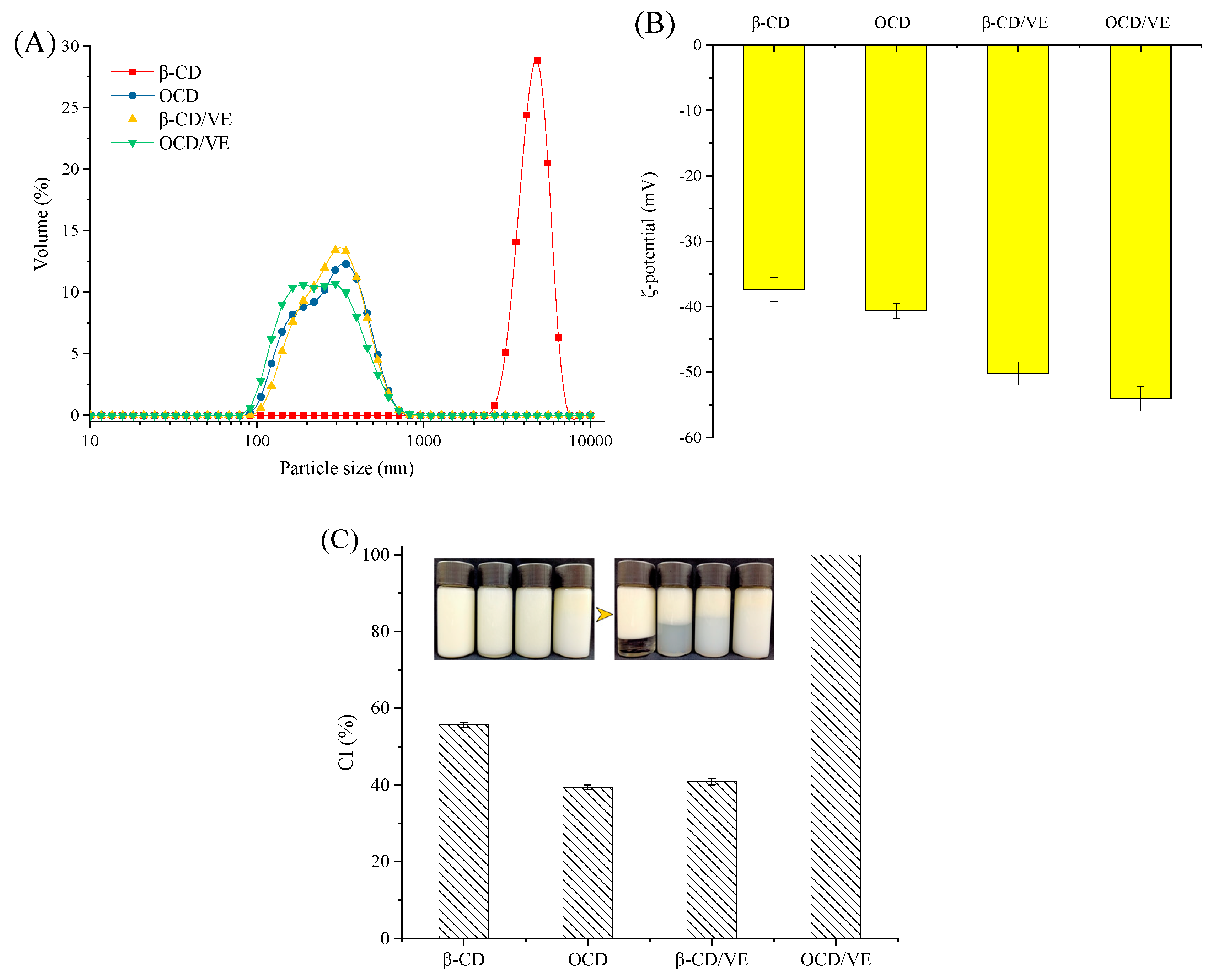
2.7. Emulsions Physical Stabilities
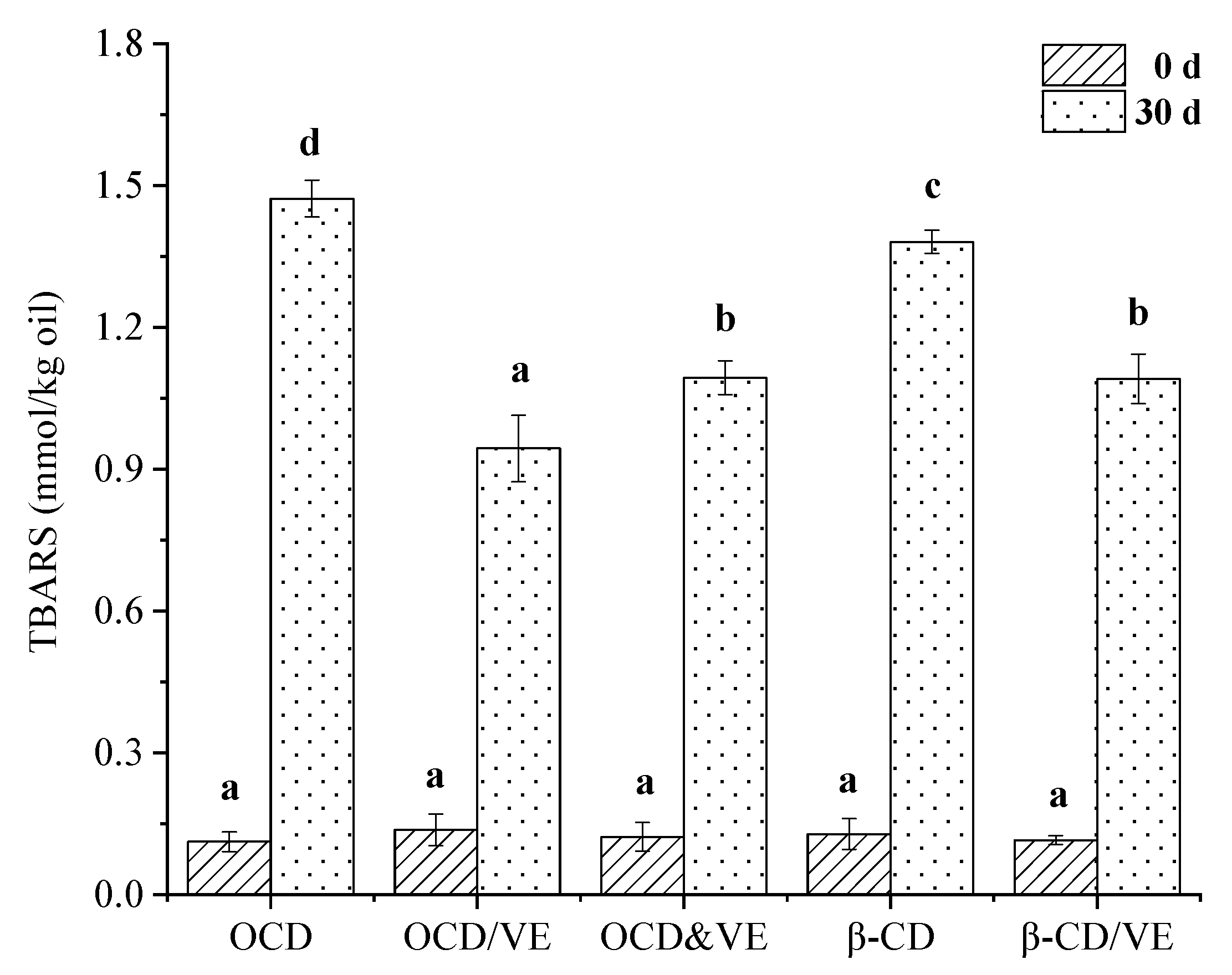
2.8. Oxidative Stability in Oil-in-Water Emulsions
3. Materials and Methods
3.1. Materials
3.2. Molecular Docking
3.3. Preparation of OCD/VE Inclusion Complex
3.4. Scanning Electron Microscopy (SEM)
3.5. Atomic Force Microscopy (AFM)
3.6. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.7. X-Ray Diffraction (XRD)
3.8. 13C CP/MAS NMR
3.9. Emulsion Preparation
3.10. ζ-Potential Measurements
3.11. Particle Size Distribution Measurements
3.12. Creaming Stability Measurement
3.13. Emulsion Oxidative Stability
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perumalla, A.; Hettiarachchy, N.S. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Food emulsions—Their structures and structure-forming properties. Food Hydrocoll. 2006, 20, 415–422. [Google Scholar] [CrossRef]
- Chen, H.; Niu, H.; Zhang, H.; Yun, Y.; Chen, W.; Zhong, Q.; Chen, W.; Fu, X.; Yun, Y. Preparation and properties of ferulic acid-sugar beet pulp pectin ester and its application as a physical and antioxidative stabilizer in a fish oil-water emulsion. Int. J. Boil. Macromol. 2019, 139, 290–297. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 41–54. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.; Decker, E. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Coupland, J.N.; McClements, D. Lipid oxidation in food emulsions. Trends Food Sci. Technol. 1996, 7, 83–91. [Google Scholar] [CrossRef]
- Mei, L.; Decker, E.A.; McClements, D.J. Evidence of Iron Association with Emulsion Droplets and Its Impact on Lipid Oxidation. J. Agric. Food Chem. 1998, 46, 5072–5077. [Google Scholar] [CrossRef]
- Lomova, M.V.; Sukhorukov, G.B.; Antipina, M.N. Antioxidant Coating of Micronsize Droplets for Prevention of Lipid Peroxidation in Oil-in-Water Emulsion. ACS Appl. Mater. Interfaces 2010, 2, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Huang, Q.; Fu, X.; Luo, F.-X. Ultrasonic effect on the octenyl succinate starch synthesis and substitution patterns in starch granules. Food Hydrocoll. 2014, 35, 636–643. [Google Scholar] [CrossRef]
- Cheng, W.; Luo, Z.; Li, L.; Fu, X. Preparation and Characterization of Debranched-Starch/Phosphatidylcholine Inclusion Complexes. J. Agric. Food Chem. 2015, 63, 634–641. [Google Scholar] [CrossRef]
- Eenschooten, C.; Guillaumie, F.; Kontogeorgis, G.M.; Stenby, E.H.; Schwach-Abdellaoui, K. Preparation and structural characterisation of novel and versatile amphiphilic octenyl succinic anhydride–modified hyaluronic acid derivatives. Carbohydr. Polym. 2010, 79, 597–605. [Google Scholar] [CrossRef]
- Sarkar, S.; Singhal, R.S. Esterification of guar gum hydrolysate and gum Arabic with n-octenyl succinic anhydride and oleic acid and its evaluation as wall material in microencapsulation. Carbohydr. Polym. 2011, 86, 1723–1731. [Google Scholar] [CrossRef]
- Chen, H.-M.; Fu, X.; Luo, Z.-G. Esterification of sugar beet pectin using octenyl succinic anhydride and its effect as an emulsion stabilizer. Food Hydrocoll. 2015, 49, 53–60. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Hu, Y.-N.; Luo, Z.-G.; Chen, W.; Chen, H.-M.; Peng, X.-C. Preparation and properties of octenyl succinate β-cyclodextrin and its application as an emulsion stabilizer. Food Chem. 2017, 218, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Chen, W.; Chen, W.; Yun, Y.; Zhong, Q.; Fu, X.; Chen, H.; Liu, G. Preparation and Characterization of a Modified-β-Cyclodextrin/β-Carotene Inclusion Complex and Its Application in Pickering Emulsions. J. Agric. Food Chem. 2019, 67, 12875–12884. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; Ostan, R.; Cevenini, E.; Gonos, E.S.; Franceschi, C. V E–gene interactions in aging and inflammatory age-related diseases: Implications for treatment. A systematic review. Ageing Res. Rev. 2014, 14, 81–101. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Hennekens, C.H.; Manson, J.E.; Colditz, G.A.; Rosner, B.; Willett, W.C. VE consumption and the risk of coronary disease in women. N. Engl. J. Med. 1993, 328, 1444–1449. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Traber, M.G. VE: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Xi, Y.; Zou, Y.; Luo, Z.; Qi, L.; Lu, X. pH-Responsive Emulsions with β-Cyclodextrin/V E Assembled Shells for Controlled Delivery of Polyunsaturated Fatty Acids. J. Agric. Food Chem. 2019, 67, 11931–11941. [Google Scholar] [CrossRef]
- Bulani, V.D.; Kothavade, P.S.; Kundaikar, H.S.; Gawali, N.B.; Chowdhury, A.A.; Degani, M.S.; Juvekar, A.R. Inclusion complex of ellagic acid with β-cyclodextrin: Characterization and in vitro anti-inflammatory evaluation. J. Mol. Struct. 2016, 1105, 308–315. [Google Scholar] [CrossRef]
- Zou, A.; Zhao, X.; Handge, U.A.; Garamus, V.M.; Willumeit-Römer, R.; Yin, P. Folate receptor targeted bufalin/β-cyclodextrin supramolecular inclusion complex for enhanced solubility and anti-tumor efficiency of bufalin. Mater. Sci. Eng. C 2017, 78, 609–618. [Google Scholar] [CrossRef]
- Srinivasan, K.; Kayalvizhi, K.; Sivakumar, K.; Stalin, T. Study of inclusion complex of β-cyclodextrin and diphenylamine: Photophysical and electrochemical behaviors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, R.; Kothainayaki, S.; Rajamohan, R.; Sivakumar, K. Spectral investigation and characterization of host–guest inclusion complex of 4,4′-methylene-bis(2-chloroaniline) with beta-cyclodextrin. Carbohydr. Polym. 2014, 114, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, R.; Kothainayaki, S.; Sivakumar, K. Preparation, physicochemical analysis and molecular modeling investigation of 2,2′-Bipyridine: β-Cyclodextrin inclusion complex in solution and solid state. J. Mol. Struct. 2015, 1100, 59–69. [Google Scholar] [CrossRef]
- Periasamy, R.; Rajamohan, R.; Kothainayaki, S.; Sivakumar, K. Spectral investigation and structural characterization of Dibenzalacetone: β-Cyclodextrin inclusion complex. J. Mol. Struct. 2014, 1068, 155–163. [Google Scholar] [CrossRef]
- Černá, M.; Barros, A.S.; Nunes, A.; Rocha, S.M.; Delgadillo, I.; Čopíková, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch. Food Chem. 2014, 151, 154–160. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. The effect of ultrasound irradiation on the physicochemical properties and α-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocoll. 2019, 96, 568–576. [Google Scholar] [CrossRef]
- Song, X.; He, G.; Ruan, H.; Chen, Q. Preparation and Properties of Octenyl Succinic Anhydride Modified EarlyIndica Rice Starch. Starch Stärke 2006, 58, 109–117. [Google Scholar] [CrossRef]
- Simsek, T.; Simsek, S.; Mayer, C.; Rasulev, B. Combined computational and experimental study on the inclusion complexes of β-cyclodextrin with selected food phenolic compounds. Struct. Chem. 2019, 30, 1395–1406. [Google Scholar] [CrossRef]
- Yuan, L.; Li, S.; Huo, D.; Zhou, W.; Wang, X.; Bai, D.; Hu, J. Studies on the preparation and photostability of avobenzone and (2-hydroxy)propyl-β-cyclodextrin inclusion complex. J. Photochem. Photobiol. A Chem. 2019, 369, 174–180. [Google Scholar] [CrossRef]
- Okumura, H.; Kawaguchi, Y.; Harada, A. Preparation and Characterization of the Inclusion Complexes of Poly(dimethylsilane)s with Cyclodextrins. Macromolecules 2003, 36, 6422–6429. [Google Scholar] [CrossRef]
- Li, J.; Yan, D. Inclusion Complexes Formation between Cyclodextrins and Poly(1,3-dioxolane). Macromolecules 2001, 34, 1542–1544. [Google Scholar] [CrossRef]
- Jiang, L.; Peng, Y.; Yan, Y.; Huang, J. Aqueous self-assembly of SDS@2β-cd complexes: Lamellae and vesicles. Soft Matter 2011, 7, 1726–1731. [Google Scholar] [CrossRef]
- Wang, P.P.; Qin, X.S.; Yang, Q.Y.; Luo, Z.G.; Xiao, Z.G.; Peng, X.C. Comparative structural characterization of spiral dextrin inclusion complexes with VE or soy isoflavone. J. Agric. Food Chem. 2017, 65, 8744–8753. [Google Scholar] [CrossRef]
- Jiao, H.; Goh, S.H.; Valiyaveettil, S.; Zheng, J. Inclusion Complexes of Perfluorinated Oligomers with Cyclodextrins. Macromolecules 2003, 36, 4241–4243. [Google Scholar] [CrossRef]
- Harada, A.; Okada, M.; Li, J.; Kamachi, M. Preparation and Characterization of Inclusion Complexes of Poly(propylene glycol) with Cyclodextrins. Macromolecules 1995, 28, 8406–8411. [Google Scholar] [CrossRef]
- Ohnmacht, S.; West, R.; Simionescu, R.; Atkinson, J. Assignment of the1H and13C NMR of tocotrienols. Magn. Reson. Chem. 2008, 46, 287–294. [Google Scholar] [CrossRef]
- Li, J.; Geng, S.; Wang, Y.; Lv, Y.; Wang, H.; Liu, B.; Liang, G. The interaction mechanism of oligopeptides containing aromatic rings with β-cyclodextrin and its derivatives. Food Chem. 2019, 286, 441–448. [Google Scholar] [CrossRef]
- Zhu, J.; Zhong, L.; Chen, W.; Song, Y.; Qian, Z.; Cao, X.; Huang, Q.; Zhang, B.; Chen, H.; Chen, W. Preparation and characterization of pectin/chitosan beads containing porous starch embedded with doxorubicin hydrochloride: A novel and simple colon targeted drug delivery system. Food Hydrocoll. 2019, 95, 562–570. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Jiang, F. Complexation of bovine serum albumin and sugar beet pectin: Stabilising oil-in-water emulsions. J. Colloid Interface Sci. 2012, 388, 103–111. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the OCD/VE inclusion-complex are available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, D.; Chen, W.; Chen, W.; Yun, Y.-H.; Zhong, Q.; Su, X.; Chen, H. Preparation and Characterization of Octenyl Succinate β-Cyclodextrin and Vitamin E Inclusion Complex and Its Application in Emulsion. Molecules 2020, 25, 654. https://doi.org/10.3390/molecules25030654
Ke D, Chen W, Chen W, Yun Y-H, Zhong Q, Su X, Chen H. Preparation and Characterization of Octenyl Succinate β-Cyclodextrin and Vitamin E Inclusion Complex and Its Application in Emulsion. Molecules. 2020; 25(3):654. https://doi.org/10.3390/molecules25030654
Chicago/Turabian StyleKe, Dongmei, Wenxue Chen, Weijun Chen, Yong-Huan Yun, Qiuping Zhong, Xiaotang Su, and Haiming Chen. 2020. "Preparation and Characterization of Octenyl Succinate β-Cyclodextrin and Vitamin E Inclusion Complex and Its Application in Emulsion" Molecules 25, no. 3: 654. https://doi.org/10.3390/molecules25030654
APA StyleKe, D., Chen, W., Chen, W., Yun, Y.-H., Zhong, Q., Su, X., & Chen, H. (2020). Preparation and Characterization of Octenyl Succinate β-Cyclodextrin and Vitamin E Inclusion Complex and Its Application in Emulsion. Molecules, 25(3), 654. https://doi.org/10.3390/molecules25030654






