The Gene and Protein Expression of the Main Components of the Lipolytic System in Human Myocardium and Heart Perivascular Adipose Tissue. Effect of Coronary Atherosclerosis
Abstract
1. Introduction
2. Results
2.1. Patient Clinical Characteristics
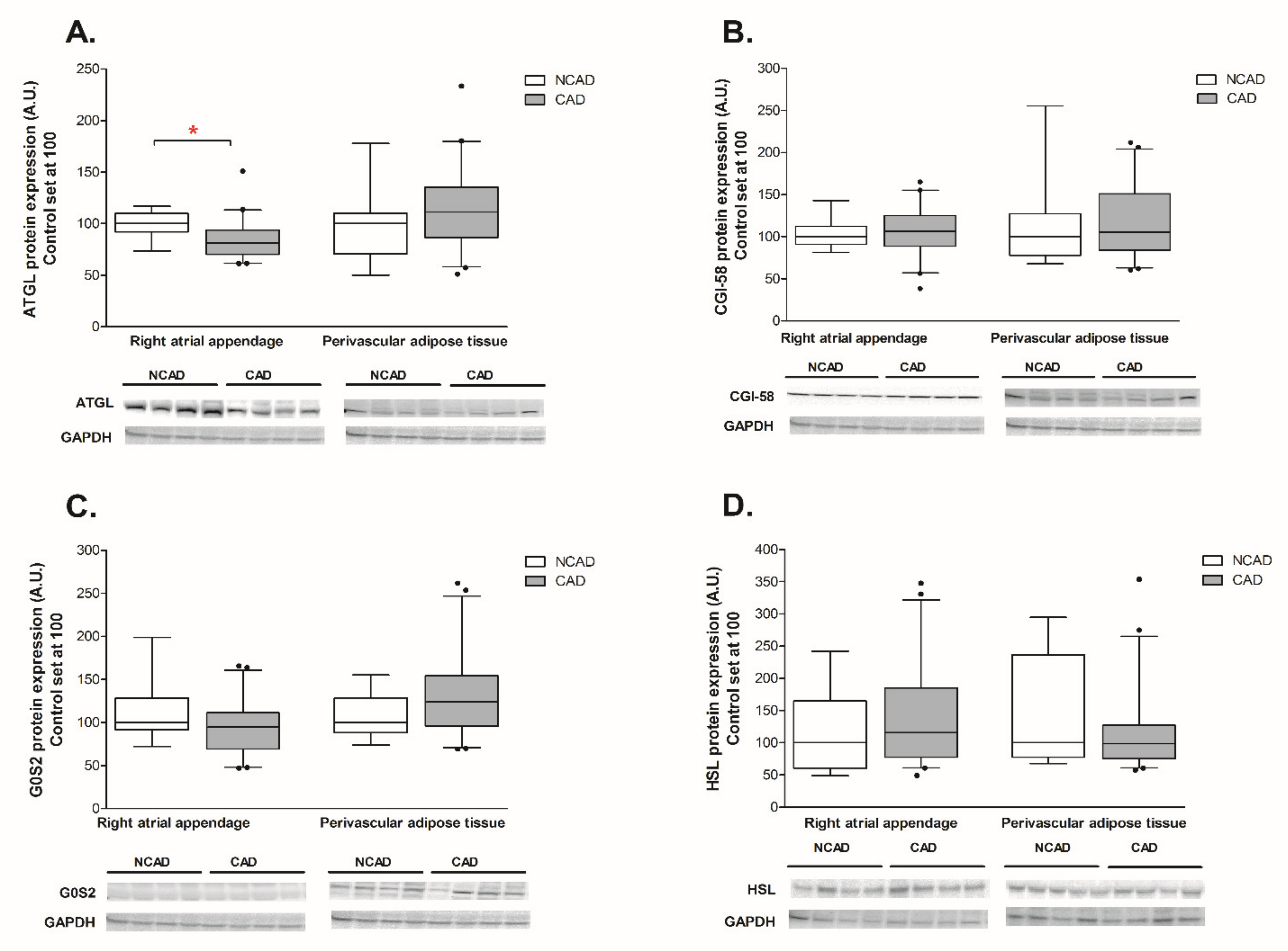
2.2. Myocardial and Perivascular Adipose Tissue Expression of ATGL, CGI-58, G0S2, and HSL at the Transcript (mRNA) and Protein Levels in CAD and NCAD Patients
2.2.1. Myocardium
2.2.2. Perivascular Adipose Tissue (PVAT)
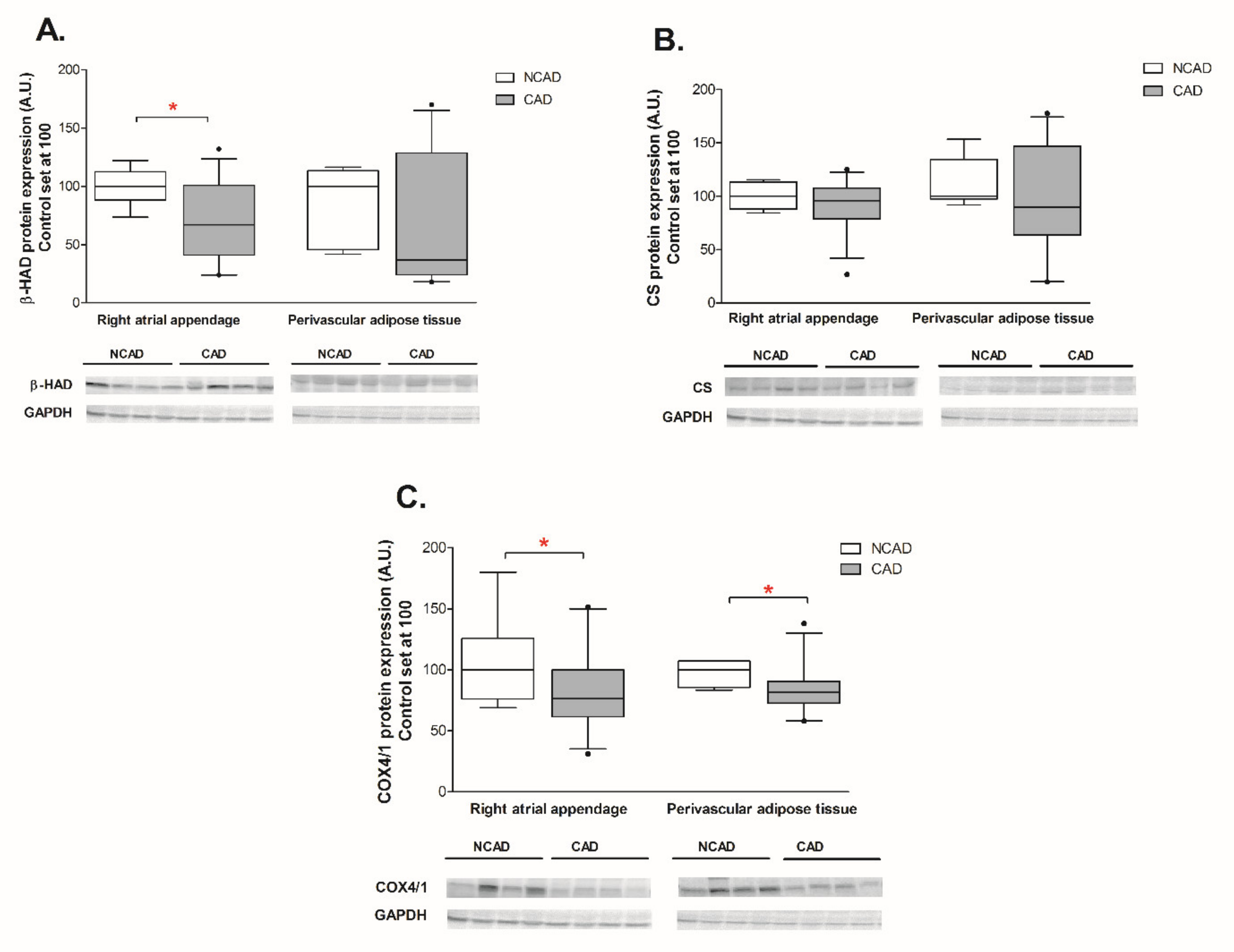
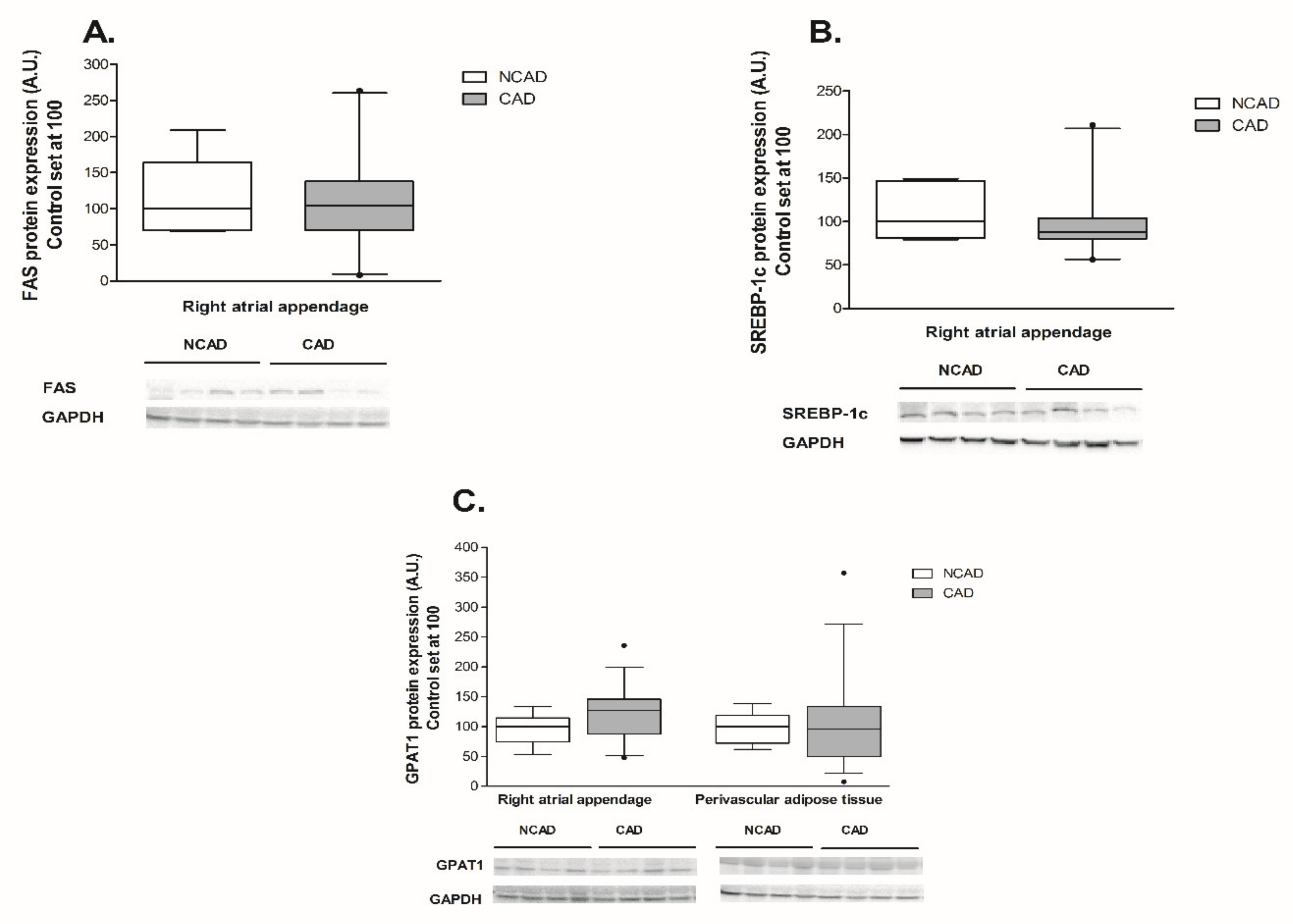
2.3. Compounds Involved in Fatty Acid Metabolism i.e., β-HAD, CS, COX4/1, FAS, SREBP-1c, GPAT1, FAT/CD36, LPL, and FABP4 at the Transcript (mRNA) and Protein Levels in Myocardium and Perivascular Adipose Tissue of CAD and NCAD Patients
2.3.1. Myocardium
2.3.2. Perivascular Adipose Tissue (PVAT)
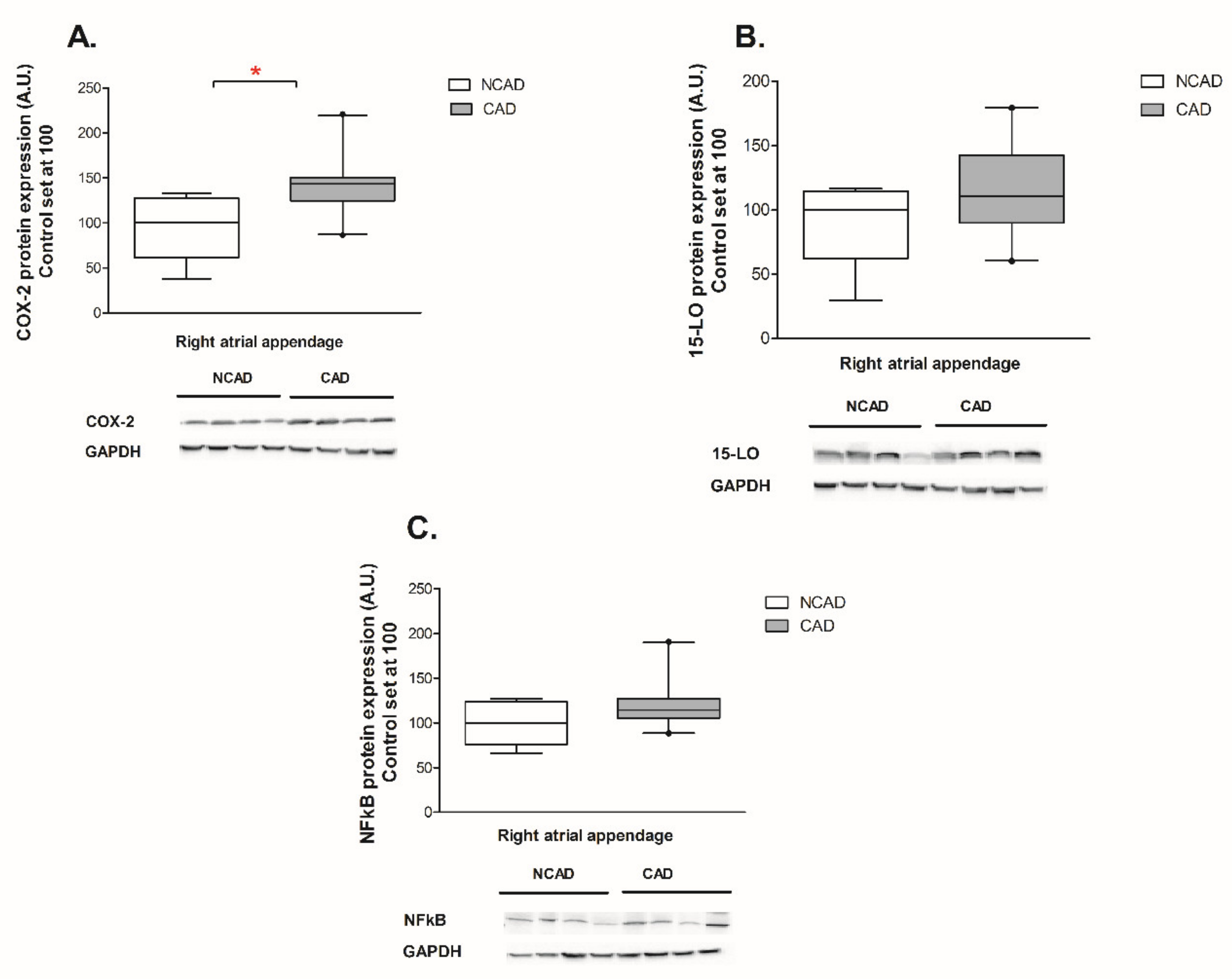
2.4. Myocardial Expression of Proteins Contributing to Inflammation, i.e., COX-2, NFκβ and 15-LO at the Protein Level of CAD and NCAD Patients
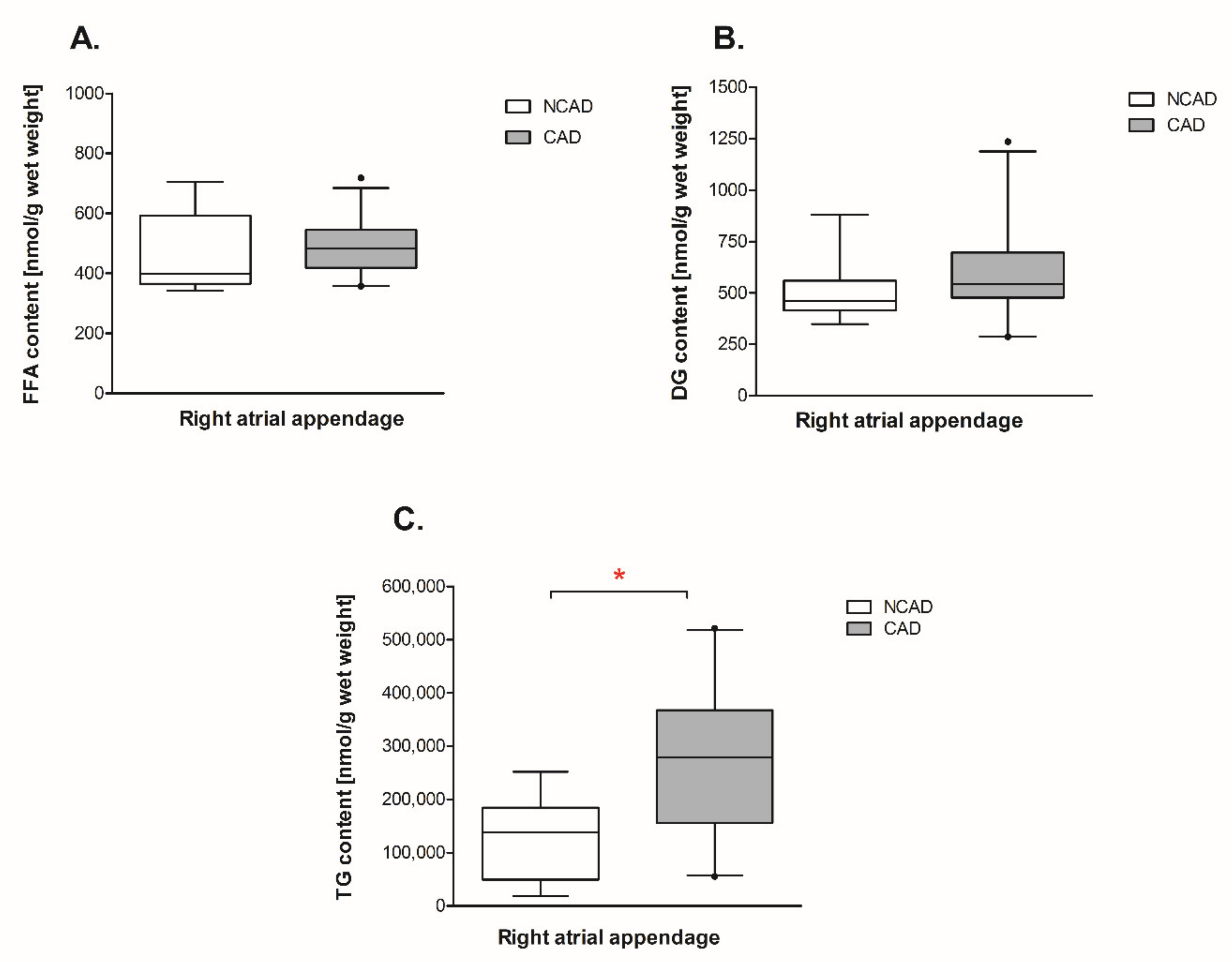
2.5. Myocardial Lipid Content and Fatty Acid Composition in CAD and NCAD Patients
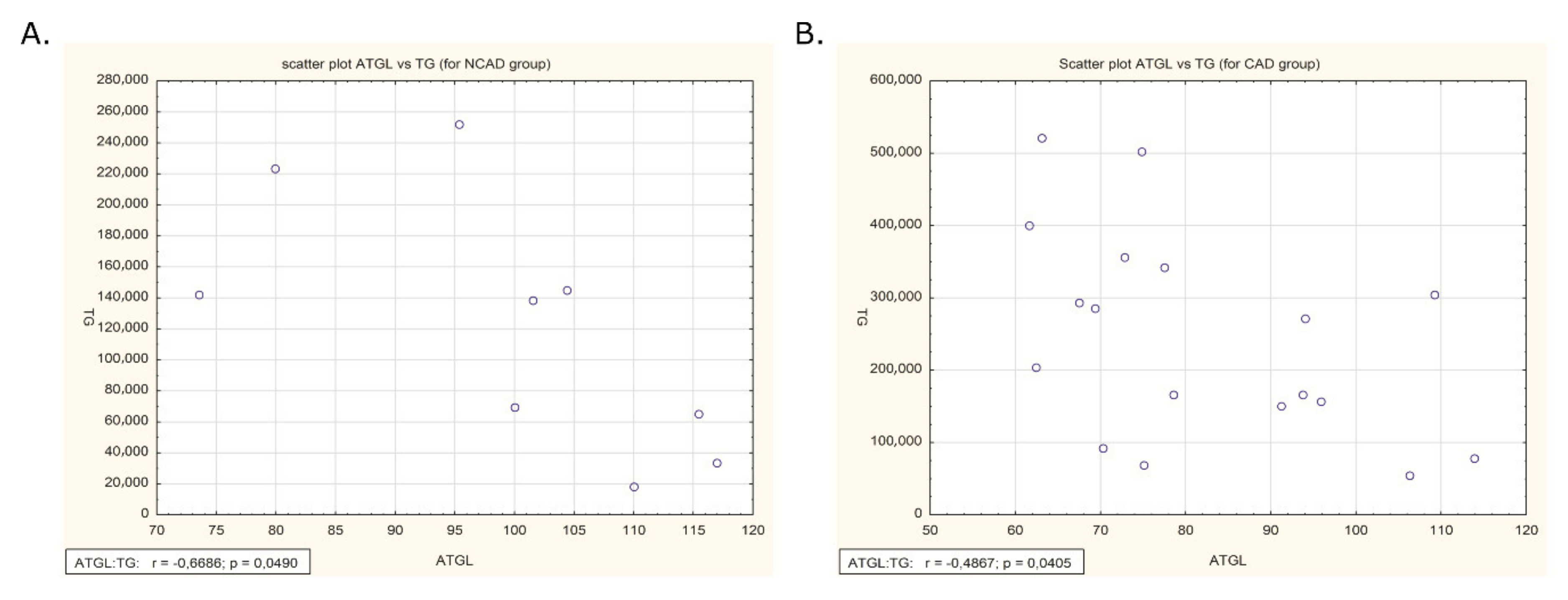
2.6. Correlation of ATGL Expression and TG Content in Human Myocardium
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Quantitative Real-Time PCR
4.3. Protein Extraction and Western Blot
4.4. The Myocardial Lipids
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATGL | Adipose triglyceride lipase |
| β-HAD | Beta-hydroxyacyl CoA dehydrogenase |
| CGI-58 | Comparative gene identification 58 |
| CAD | Coronary artery disease |
| COX-2 | cyclooxygenase-2 |
| DG | Diacylglycerols |
| FABP-4 | Fatty acid-binding protein 4 |
| FASN | Fatty acid synthase |
| FAT/CD36 | Fatty acid translocase (cluster of differentiation 36) |
| FFA | Free fatty acids |
| G0S2 | G0/G1 switch protein 2 |
| GPAT1 | Glycerol-3-phosphate acyltransferase 1 |
| HSL | Hormone sensitive lipase |
| 15-LO | Lipoxygenase |
| COX4/1 | Mitochondrial electron transport chain (cytochrome c oxidase subunit IV isoform 1) |
| MG | Monoacylglycerol |
| NFκβ | Nuclear factor kappa B |
| NCAD | Patients with no atherosclerosis qualified for a valve replacement |
| PVAT | Perivascular adipose tissue |
| SREBP-1c | Sterol regulatory element-binding protein 1 |
| TG | Triacylglycerols |
| CS | Tricarboxylic acid cycle (citrate synthase) |
References
- Granneman, J.G.; Moore, H.-P.H.; Mottillo, E.P.; Zhu, Z. Functional interactions between mldp (lsdp5) and abhd5 in the control of intracellular lipid accumulation. J. Biol. Chem. 2009, 284, 3049–3057. [Google Scholar] [CrossRef]
- Osumi, T.; Kuramoto, K. Heart lipid droplets and lipid droplet-binding proteins: Biochemistry, physiology, and pathology. Exp. Cell Res. 2016, 340, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Zechner, R.; Kienesberger, P.C.; Haemmerle, G.; Zimmermann, R.; Lass, A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 2009, 50, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Banke, N.H.; Wende, A.R.; Leone, T.C.; O’Donnell, J.M.; Abel, E.D.; Kelly, D.P.; Lewandowski, E.D. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor pparalpha. Circ. Res. 2010, 107, 233–241. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.; Nzirorera, C.; Kienesberger, P.C. Lipid metabolism and signaling in cardiac lipotoxicity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J.; Trent, C.M.; Schulze, P.C. Lipid metabolism and toxicity in the heart. Cell Metab. 2012, 15, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Heier, C.; Haemmerle, G. Fat in the heart: The enzymatic machinery regulating cardiac triacylglycerol metabolism. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 1500–1512. [Google Scholar] [CrossRef]
- Saddik, M.; Lopaschuk, G.D. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J. Biol. Chem. 1991, 266, 8162–8170. [Google Scholar]
- Szczepaniak, L.S.; Dobbins, R.L.; Metzger, G.J.; Sartoni-D’Ambrosia, G.; Arbique, D.; Vongpatanasin, W.; Unger, R.; Victor, R.G. Myocardial triglycerides and systolic function in humans: In vivo evaluation by localized proton spectroscopy and cardiac imaging. Magnetic Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2003, 49, 417–423. [Google Scholar] [CrossRef]
- Iozzo, P. Myocardial, perivascular, and epicardial fat. Diabetes Care 2011, 34, S371–S379. [Google Scholar] [CrossRef]
- McGavock, J.M.; Lingvay, I.; Zib, I.; Tillery, T.; Salas, N.; Unger, R.; Levine, B.D.; Raskin, P.; Victor, R.G.; Szczepaniak, L.S. Cardiac steatosis in diabetes mellitus: A 1H-magnetic resonance spectroscopy study. Circulation 2007, 116, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Rijzewijk, L.J.; van der Meer, R.W.; Smit, J.W.A.; Diamant, M.; Bax, J.J.; Hammer, S.; Romijn, J.A.; de Roos, A.; Lamb, H.J. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008, 52, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, R.W.; Rijzewijk, L.J.; Diamant, M.; Hammer, S.; Schär, M.; Bax, J.J.; Smit, J.W.A.; Romijn, J.A.; de Roos, A.; Lamb, H.J. The ageing male heart: Myocardial triglyceride content as independent predictor of diastolic function. Eur. Heart J. 2008, 29, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Jessen, N.; Jørgensen, J.O.L.; Møller, N.; Lund, S. Dissecting adipose tissue lipolysis: Molecular regulation and implications for metabolic disease. J. Mol. Endocrinol. 2014, 52, R199–R222. [Google Scholar] [CrossRef]
- Lass, A.; Zimmermann, R.; Haemmerle, G.; Riederer, M.; Schoiswohl, G.; Schweiger, M.; Kienesberger, P.; Strauss, J.G.; Gorkiewicz, G.; Zechner, R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by cgi-58 and defective in chanarin-dorfman syndrome. Cell Metab. 2006, 3, 309–319. [Google Scholar] [CrossRef]
- Heier, C.; Radner, F.P.W.; Moustafa, T.; Schreiber, R.; Grond, S.; Eichmann, T.O.; Schweiger, M.; Schmidt, A.; Cerk, I.K.; Oberer, M.; et al. G0/g1 switch gene 2 regulates cardiac lipolysis. J. Biol. Chem. 2015, 290, 26141–26150. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Lombès, M.; Rha, G.B.; Chi, Y.-I.; Guerin, T.M.; Smart, E.J.; Liu, J. The g(0)/g(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010, 11, 194–205. [Google Scholar] [CrossRef]
- Coe, N.R.; Bernlohr, D.A. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 1998, 1391, 287–306. [Google Scholar] [CrossRef]
- Hertzel, A.V.; Bernlohr, D.A. The mammalian fatty acid-binding protein multigene family: Molecular and genetic insights into function. Trends Endocrinol. Metab. 2000, 11, 175–180. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, R.; Girona, J.; Alegret, J.M.; Bosquet, A.; Ibarretxe, D.; Masana, L. Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J. Endocrinol. 2017, 233, R173–R184. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Lass, A.; Zimmermann, R.; Gorkiewicz, G.; Meyer, C.; Rozman, J.; Heldmaier, G.; Maier, R.; Theussl, C.; Eder, S.; et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Lefèvre, C.; Morava, E.; Mussini, J.-M.; Laforêt, P.; Negre-Salvayre, A.; Lathrop, M.; Salvayre, R. The gene encoding adipose triglyceride lipase (pnpla2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 2007, 39, 28–30. [Google Scholar] [CrossRef]
- Missaglia, S.; Coleman, R.A.; Mordente, A.; Tavian, D. Neutral Lipid Storage Diseases as Cellular Model to Study Lipid Droplet Function. Cells 2019, 8, 187. [Google Scholar] [CrossRef]
- Missaglia, S.; Maggi, L.; Mora, M.; Gibertini, S.; Blasevich, F.; Agostoni, P.; Moro, L.; Cassandrini, D.; Santorelli, F.M.; Gerevini, S.; et al. Late onset of neutral lipid storage disease due to novel pnpla2 mutations causing total loss of lipase activity in a patient with myopathy and slight cardiac involvement. Neuromuscul. Disord. 2017, 27, 481–486. [Google Scholar] [CrossRef]
- Pasanisi, M.B.; Missaglia, S.; Cassandrini, D.; Salerno, F.; Farina, S.; Andreini, D.; Agostoni, P.; Morandi, L.; Mora, M.; Tavian, D. Severe cardiomyopathy in a young patient with complete deficiency of adipose triglyceride lipase due to a novel mutation in pnpla2 gene. Int. J. Cardiol. 2016, 207, 165–167. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Uchasova, E.; Dyleva, Y.; Borodkina, D.; Akbasheva, O.; Karetnikova, V.; Brel, N.; Alexander, K.; Barbarash, O. Relationship between epicardial and perivascular fatty tissue and adipokine-cytokine level in coronary artery disease patients. PLoS ONE 2019, 14, e0208156. [Google Scholar] [CrossRef]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef]
- Matloch, Z.; Kotulák, T.; Haluzík, M. The role of epicardial adipose tissue in heart disease. Physiol. Res. 2016, 65, 23–32. [Google Scholar]
- Nagy, E.; Jermendy, A.L.; Merkely, B.; Maurovich-Horvat, P. Clinical importance of epicardial adipose tissue. Arch. Med. Sci. 2017, 13, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Fain, J.N. Human epicardial fat: What is new and what is missing? Clin. Exp. Pharmacol. Physiol. 2011, 38, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, I.; Riederer, M.; Shah, P.; Peters, P.; Quehenberger, F.; Wood, A.; Scharnagl, H.; März, W.; Kostner, K.M.; Kostner, G.M. Expression of fat mobilizing genes in human epicardial adipose tissue. Atherosclerosis 2012, 220, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef]
- Hirano, K.-I.; Ikeda, Y.; Zaima, N.; Sakata, Y.; Matsumiya, G. Triglyceride deposit cardiomyovasculopathy. N. Engl. J. Med. 2008, 359, 2396–2398. [Google Scholar] [CrossRef]
- Hannukainen, J.C.; Lautamäki, R.; Mari, A.; Pärkkä, J.P.; Bucci, M.; Guzzardi, M.A.; Kajander, S.; Tuokkola, T.; Knuuti, J.; Iozzo, P. Elevated glucose oxidation, reduced insulin secretion, and a fatty heart may be protective adaptions in ischemic cad. J. Clin. Endocrinol. Metab. 2016, 101, 2701–2710. [Google Scholar] [CrossRef]
- Kolleritsch, S.; Kien, B.; Schoiswohl, G.; Diwoky, C.; Schreiber, R.; Heier, C.; Maresch, L.K.; Schweiger, M.; Eichmann, T.O.; Stryeck, S.; et al. Low cardiac lipolysis reduces mitochondrial fission and prevents lipotoxic heart dysfunction in perilipin 5 mutant mice. Cardiovasc. Res. 2019, cvz119. [Google Scholar] [CrossRef]
- Coassin, S.; Schweiger, M.; Kloss-Brandstätter, A.; Lamina, C.; Haun, M.; Erhart, G.; Paulweber, B.; Rahman, Y.; Olpin, S.; Wolinski, H.; et al. Investigation and functional characterization of rare genetic variants in the adipose triglyceride lipase in a large healthy working population. PLoS Genet. 2010, 6, e1001239. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Mead, J.R.; Ramji, D.P. The pivotal role of lipoprotein lipase in atherosclerosis. Cardiovasc. Res. 2002, 55, 261–269. [Google Scholar] [CrossRef]
- Poeckel, D.; Zemski Berry, K.A.; Murphy, R.C.; Funk, C.D. Dual 12/15- and 5-lipoxygenase deficiency in macrophages alters arachidonic acid metabolism and attenuates peritonitis and atherosclerosis in apoe knock-out mice. J. Biol. Chem. 2009, 284, 21077–21089. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of vegf and a regulator of cell proliferation in endothelial cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Lamounier-Zepter, V.; Look, C.; Alvarez, J.; Christ, T.; Ravens, U.; Schunck, W.-H.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Morano, I. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: A new link between obesity and heart disease. Circ. Res. 2009, 105, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kralisch, S.; Fasshauer, M. Adipocyte fatty acid binding protein: A novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia 2013, 56, 10–21. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; Infusino, F.; Maestrini, V.; Mancone, M.; Fedele, F. Myocardial ischemia and diabetes mellitus: Role of oxidative stress in the connection between cardiac metabolism and coronary blood flow. J. Diabetes Res. 2019, 2019, 9489826. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; De Marchis, M.; Palmirotta, R.; Volterrani, M.; Mancone, M.; Fedele, F. Diabetes mellitus and ischemic heart disease: The role of ion channels. Int. J. Mol. Sci. 2018, 19, 802. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time rt-pcr. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Mikłosz, A.; Łukaszuk, B.; Żendzian-Piotrowska, M.; Brańska-Januszewska, J.; Ostrowska, H.; Chabowski, A. Challenging of as160/tbc1d4 alters intracellular lipid milieu in l6 myotubes incubated with palmitate. J. Cell. Physiol. 2017, 232, 2373–2386. [Google Scholar] [CrossRef]
- Mikłosz, A.; Łukaszuk, B.; Żendzian-Piotrowska, M.; Kurek, K.; Chabowski, A. The effects of as160 modulation on fatty acid transporters expression and lipid profile in l6 myotubes. Cell. Physiol. Biochem. 2016, 38, 267–282. [Google Scholar] [CrossRef]
- Nawrocki, A.; Górski, J. Effect of plasma free fatty acid concentration on the content and composition of the free fatty acid fraction in rat skeletal muscles. Horm. Metab. Res. 2004, 36, 601–606. [Google Scholar] [CrossRef]




| Item | Control (NCAD) (n = 11) | Coronary Atherosclerosis (CAD) (n = 42) | p Value |
|---|---|---|---|
| Age (years) | 61.1 ± 8.3 | 63.6 ± 8.3 | 0.37 |
| Sex (M/F) | 5/6 (45.4/55.6%) | 40/2 (95.3/4.7%) * | 0.008 |
| BMI (kg/m2) | 26.7 ± 5.2 | 27.3 ± 3.2 | 0.68 |
| Hypertension # | 5 (45.4%) | 34 (80.9%) * | 0.02 |
| Hyperlipidemia | 8 (72.7%) | 32 (76.1%) | 1.0 |
| History of infarction | 0 | 17 (40.4%) * | 0.012 |
| Left ventricular ejection fraction (%) | 52.2 ± 6.8 | 53.1 ± 9.5 | 0.76 |
| Fasting blood glucose (mg/dL) | 95.5 ± 10.8 | 96.2 ± 14.1 | 0.94 |
| Creatinine clearance (ml/min) | 86.2 ± 23.6 | 91.9 ± 0.2 | 0.37 |
| Total cholesterol (mg/dL) | 198.1 ± 30.2 | 183.4 ± 41.7 | 0.32 |
| HDL cholesterol (mg/dL) | 53.9 ± 10.5 | 46.6 ± 11.3 | 0.065 |
| LDL cholesterol (mg/dL) | 120.4 ± 17.9 | 109.9 ± 36.6 | 0.45 |
| Triglyceride (mg/dL) | 112.4 ± 22.5 | 152.9 ± 83.7 | 0.14 |
| C-reactive protein (mg/L) | 2.0 ± 2.0 | 4.9 ± 9.5 | 0.25 |
| Hemoglobin (g/dL) | 13.3 ± 1.1 | 13.7 ± 1.4 | 0.42 |
| Platelets (×103/mm3) | 195.8 ± 29.9 | 232.8 ± 49.6 * | 0.03 |
| HbA1C (%) | 5.5 ± 1.1 | 5.9 ± 0.3 | 0.43 |
| Alanine aminotransferase (U/L) | 25.1 ± 12.7 | 52.9 ± 42.6 * | 0.02 |
| Aspartate aminotransferase (U/L) | 30.6 ± 15.7 | 39.9 ± 28.4 | 0.27 |
| Medication: | |||
| ACEI/ ARB | 4 (36.4%) | 40 (95.2%) * | 0.00001 |
| Beta-blockers | 10 (90.9%) | 41 (97.6%) | 0.78 |
| Statins | 6 (54.5%) | 41 (97.6%) * | 0.000001 |
| Proton pump inhibitors | 10 (90.9%) | 39 (92.8%) | 0.83 |
| Spironolaktone | 3 (27.3%) | 7 (16.6%) | 0.42 |
| Furosemide | 4 (36.4%) | 8 (19.0%) | 0.22 |
| Aspirin | None | 42 (100%) | 0.00 |
| Composition | NCAD | CAD |
|---|---|---|
| Myristic acid (14:0) | 4282.14 ± 2613.1 (3.66%) | 9158.87 ± 5719.6 (3.33%) * |
| Palmitic acid (16:0) | 38,589.25 ± 25,242.1 (31.9%) | 84,158.08 ± 45,434.1 (31.31%) * |
| Palmitooleic acid (16:1) | 10,635.3 ± 7981.3 (8.48%) | 27,838.65 ± 16,791.5 (10.22%) * |
| Stearic acid (18:0) | 5371.43 ± 2945.7 (4.95%) | 9332.44 ± 5345.3 (3.64%) * |
| Oleic acid (18:1n9c) | 48,556.93 ± 35,024.1 (39.48%) | 109,021.54 ± 57,447.9 (40.86%) * |
| Linoleic acid (18:2n6c) | 10,304.8 ± 8165.3 (8.69%) | 20,929.94 ± 11,997.4 (7.93%) * |
| Arachidic acid (20:0) | 70.65 ± 35.7 (0.08%) | 122.86 ± 72.2 (0.05%) * |
| Linolenic acid (C18:9n3) | 1855.38 ± 1434.9 (1.6%) | 3744.42 ± 2893.9 (1.4%) |
| Behenic acid (22:0) | 34.93 ± 18.2 (0.04%) | 110.63 ± 57.7 (0.04%) * |
| Arachidonic acid (20:4n6) | 634.53 ± 429.7 (0.59%) | 1469.99 ± 767 (0.58%) * |
| Eicosapentaenoic acid (20:5n3) | 17.01 ± 11.9 (0.02%) | 116.3 ± 100.7 (0.04%) * |
| Nervonic acid (24:1) | 16.52 ± 9.8 (0.02%) | 25.71 ± 14.2 (0.01%) |
| Docosahexaenoic acid (22:6n3) | 498.41 ± 353.5 (0.42%) | 1292.91 ± 805.5 (0.5%) * |
| Lignoceric acid (24:0) | 104.82 ± 71.7 (0.09%) | 272.75 ± 145.9 (0.11%) * |
| Saturated | 48,453.23 ± 30,585.3 (40.71%) | 103,155.63 ± 56,045.7 (38.47%) * |
| Unsaturated | 72,518.89 ± 51,264.1 (59.29%) | 164,439.46 ± 87,040.3 (61.53%) * |
| Total | 120,972.12 ± 81,391.2 (100%) | 267,595.09 ± 140,943.4 (100%) * |
| Composition | NCAD | CAD |
|---|---|---|
| Myristic acid (14:0) | 47.98 ± 8.3 (9.9%) | 58.16 ± 25.1 (9.4%) |
| Palmitic acid (16:0) | 184.72 ± 59.6 (36.3%) | 232.98 ± 97.1 (37.4%) |
| Palmitooleic acid (16:1) | 24.18 ± 12.3 (4.6%) | 40.08 ± 27.1 (6%) |
| Stearic acid (18:0) | 96.22 ± 18.5 (19.8%) | 85.84 ± 19 (15%) |
| Oleic acid (18:1n9c) | 81.82 ± 41.3 (15.5%) | 118.47 ± 61.2 (18.5%) |
| Linoleic acid (18:2n6c) | 29.97 ± 16 (5.7%) | 39.83 ± 17 (6.4%) * |
| Arachidic acid (20:0) | 1.74 ± 0.4 (0.4%) | 2.02 ± 1 (0.3%) |
| Linolenic acid (C18:9n3) | 4.67 ± 2.1 (0.9%) | 4.71 ± 3 (0.7%) |
| Behenic acid (22:0) | 1.28 ± 0.5 (0.3%) | 1.38 ± 0.4 (0.2%) |
| Arachidonic acid (20:4n6) | 26.26 ± 13.2 (5.1%) | 22.09 ± 6.5 (4.1%) |
| Eicosapentaenoic acid (20:5n3) | 1.94 ± 0.8 (0.4%) | 1.86 ± 1 (0.3%) |
| Nervonic acid (24:1) | 0.82 ± 0.3 (0.2%) | 0.89 ± 0.3 (0.2%) |
| Docosahexaenoic acid (22:6n3) | 3.69 ± 2.1 (0.7%) | 4.47 ± 1.7 (0.8%) |
| Lignoceric acid (24:0) | 1.03 ± 0.7 (0.2%) | 2.66 ± 2.3 (0.5%) * |
| Saturated | 332.98 ± 77.1 (66.9%) | 383.04 ± 134.5 (62.9%) |
| Unsaturated | 173.34 ± 82.9 (33.1%) | 232.41 ± 105.2 (37.1%) |
| Total | 506.32 ± 157.5 (100%) | 615.45 ± 234.7 (100%) |
| Composition | NCAD | CAD |
|---|---|---|
| Myristic acid (14:0) | 26.68 ± 5.7 (5.9%) | 26.38 ± 12 (5.3%) |
| Palmitic acid (16:0) | 167.38 ± 42.2 (36.3%) | 173.44 ± 30.1 (35.9%) |
| Palmitooleic acid (16:1) | 13.63 ± 5.9 (2.9%) | 15.16 ± 5.9 (3.1%) |
| Stearic acid (18:0) | 103.15 ± 19.5 (22.9%) | 102.87 ± 18.5 (21.6%) |
| Oleic acid (18:1n9c) | 74.79 ± 35.1 (15.4%) | 88.02 ± 17.5 (18.3%) |
| Linoleic acid (18:2n6c) | 32.77 ± 15.5 (6.7%) | 31.88 ± 11.9 (6.5%) |
| Arachidic acid (20:0) | 1.46 ± 0.3 (0.3%) | 1.74 ± 0.6 (0.4%) |
| Linolenic acid (C18:9n3) | 5.15 ± 2.5 (1.1%) | 3.91 ± 2.4 (0.8%) |
| Behenic acid (22:0) | 0.99 ± 0.3 (0.2%) | 1.14 ± 0.5 (0.2%) |
| Arachidonic acid (20:4n6) | 31.65 ± 19.2 (6.8%) | 29.96 ± 19.6 (6.1%) |
| Eicosapentaenoic acid (20:5n3) | 0.93 ± 0.2 (0.2%) | 1.63 ± 1 (0.3%) * |
| Nervonic acid (24:1) | 0.59 ± 0.2 (0.1%) | 0.68 ± 0.4 (0.1%) |
| Docosahexaenoic acid (22:6n3) | 3.93 ± 3.3 (0.8%) | 2.55 ± 1.2 (0.5%) |
| Lignoceric acid (24:0) | 1.5 ± 0.6 (0.3%) | 3.16 ± 3 (0.7%) |
| Saturated | 301.17 ± 60.8 (66%) | 308.72 ± 47 (64.1%) |
| Unsaturated | 163.43 ± 70.6 (34%) | 173.8 ± 36.9 (35.9%) |
| Total | 463.31 ± 128.8 (100%) | 482.52 ± 75.9 (100%) |
| Gene | Primer Sequence | |
|---|---|---|
| Forward | Reverse | |
| GAPDH | 5′-AAGCCTGCCGGTGACTAAC-3′ | 5′-GTTAAAAGCAGCCCTGGTGAC-3′ |
| ATGL | 5′-GCTTCCTCGGCGTCTACTAC-3′ | 5′-CAATGAACTTGGCACCAGCC-3′ |
| G0S2 | 5′-ACCACAAGCATCCACCAA-3′ | 5′-GCATTTATCCTTCCTCCCTA-3′ |
| CGI-58 | 5′-AGACCCAGGTTTGACAGTGATG-3′ | 5′-AGTAAGCAGCAGCCAAGAATCC-3′ |
| HSL | 5′-CACGATGGGTGGAATGGTGG-3′ | 5′-ACCAGCGACTGTGTCATTGT-3′ |
| FABP4 | 5′-GGGCCAGGAATTTGACGAAG-3′ | 5′-AACTCTCGTGGAAGTGACGC-3′ |
| FAT/CD36 | 5′-AAGTCACTGCGACATGATTAATGG-3′ | 5′-GAACTGCAATACCTGGCTTTTCTC-3′ |
| GPAT1 | 5′-AACCCCAGTATCCCGTCTTT-3′ | 5′-CAGTCACATTGGTGGCAAAC-3′ |
| β-HAD | 5′-CTTGCTCCGAGAGGGAGTC-3′ | 5′-AGCTCGTAGCTGGGAGGAAC-3′ |
| CS | 5′-GATTGTGCCCAATGTCCTCT-3′ | 5′-TTCATCTCCGTCATGCCATA-3′ |
| COX4/1 | 5′-GGTCACGCCGATCCATATAAG-3′ | 5′-TCTGTGTGTGTACGAGCTCATGA-3′ |
| LPL | 5′–GAGATTTCTCTGTATGGCACC-3′ | 5′–CTGCAAATGAGACACTTTCTC-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapp, M.; Górski, J.; Lewkowicz, J.; Lisowska, A.; Gil, M.; Wójcik, B.; Hirnle, T.; Chabowski, A.; Mikłosz, A. The Gene and Protein Expression of the Main Components of the Lipolytic System in Human Myocardium and Heart Perivascular Adipose Tissue. Effect of Coronary Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 737. https://doi.org/10.3390/ijms21030737
Knapp M, Górski J, Lewkowicz J, Lisowska A, Gil M, Wójcik B, Hirnle T, Chabowski A, Mikłosz A. The Gene and Protein Expression of the Main Components of the Lipolytic System in Human Myocardium and Heart Perivascular Adipose Tissue. Effect of Coronary Atherosclerosis. International Journal of Molecular Sciences. 2020; 21(3):737. https://doi.org/10.3390/ijms21030737
Chicago/Turabian StyleKnapp, Małgorzata, Jan Górski, Janina Lewkowicz, Anna Lisowska, Monika Gil, Beata Wójcik, Tomasz Hirnle, Adrian Chabowski, and Agnieszka Mikłosz. 2020. "The Gene and Protein Expression of the Main Components of the Lipolytic System in Human Myocardium and Heart Perivascular Adipose Tissue. Effect of Coronary Atherosclerosis" International Journal of Molecular Sciences 21, no. 3: 737. https://doi.org/10.3390/ijms21030737
APA StyleKnapp, M., Górski, J., Lewkowicz, J., Lisowska, A., Gil, M., Wójcik, B., Hirnle, T., Chabowski, A., & Mikłosz, A. (2020). The Gene and Protein Expression of the Main Components of the Lipolytic System in Human Myocardium and Heart Perivascular Adipose Tissue. Effect of Coronary Atherosclerosis. International Journal of Molecular Sciences, 21(3), 737. https://doi.org/10.3390/ijms21030737







