Energy Restriction and Colorectal Cancer: A Call for Additional Research
Abstract
1. Colorectal Cancer Overview
2. Energy Restriction Overview
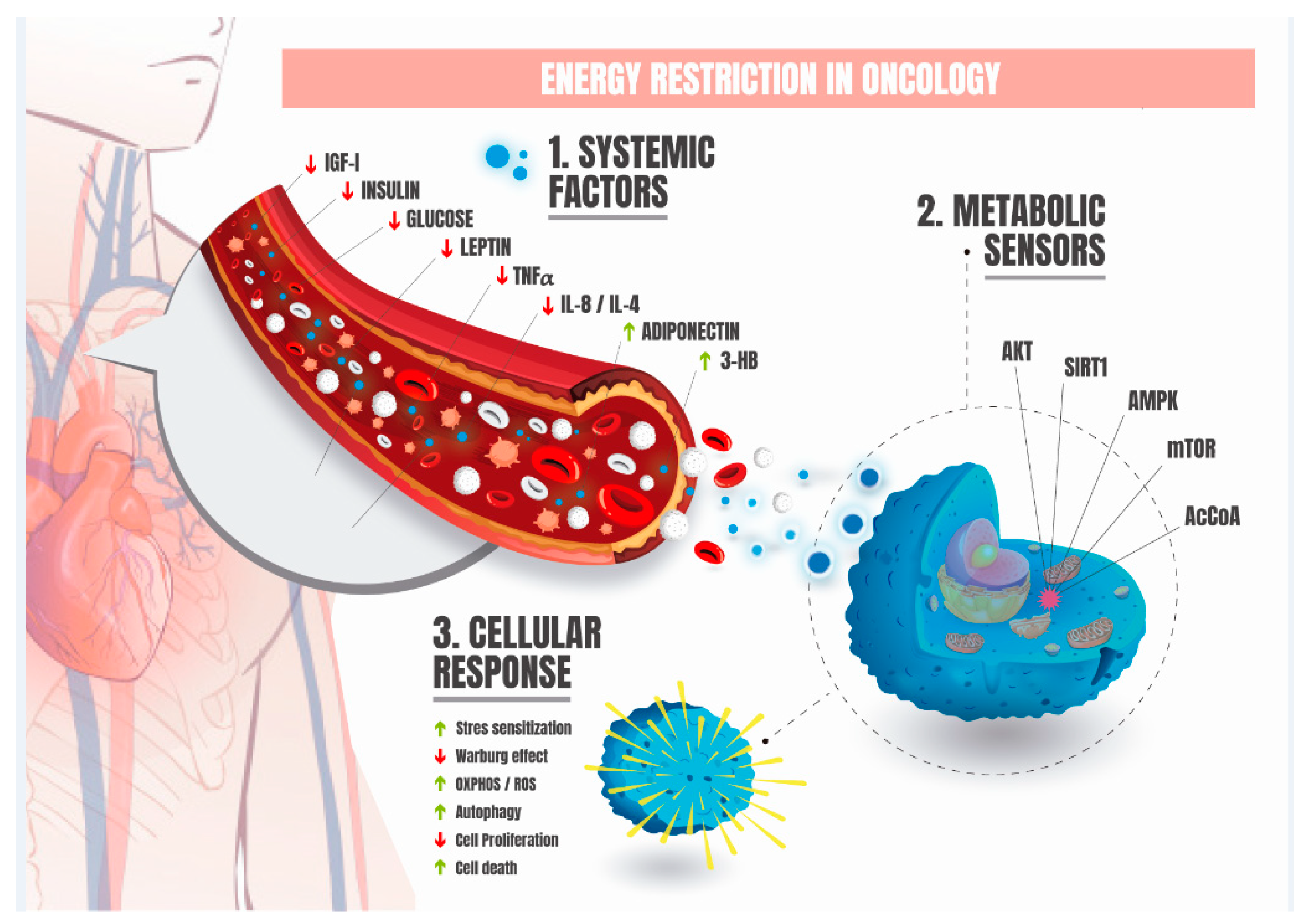
3. Energy Restriction in Oncology
4. Fundamental Metabolic and Systemic Adaptations Induced by Energy Restriction in Oncology
5. Fundamental Cellular and Molecular Adaptations Induced by Energy Restriction in Oncology
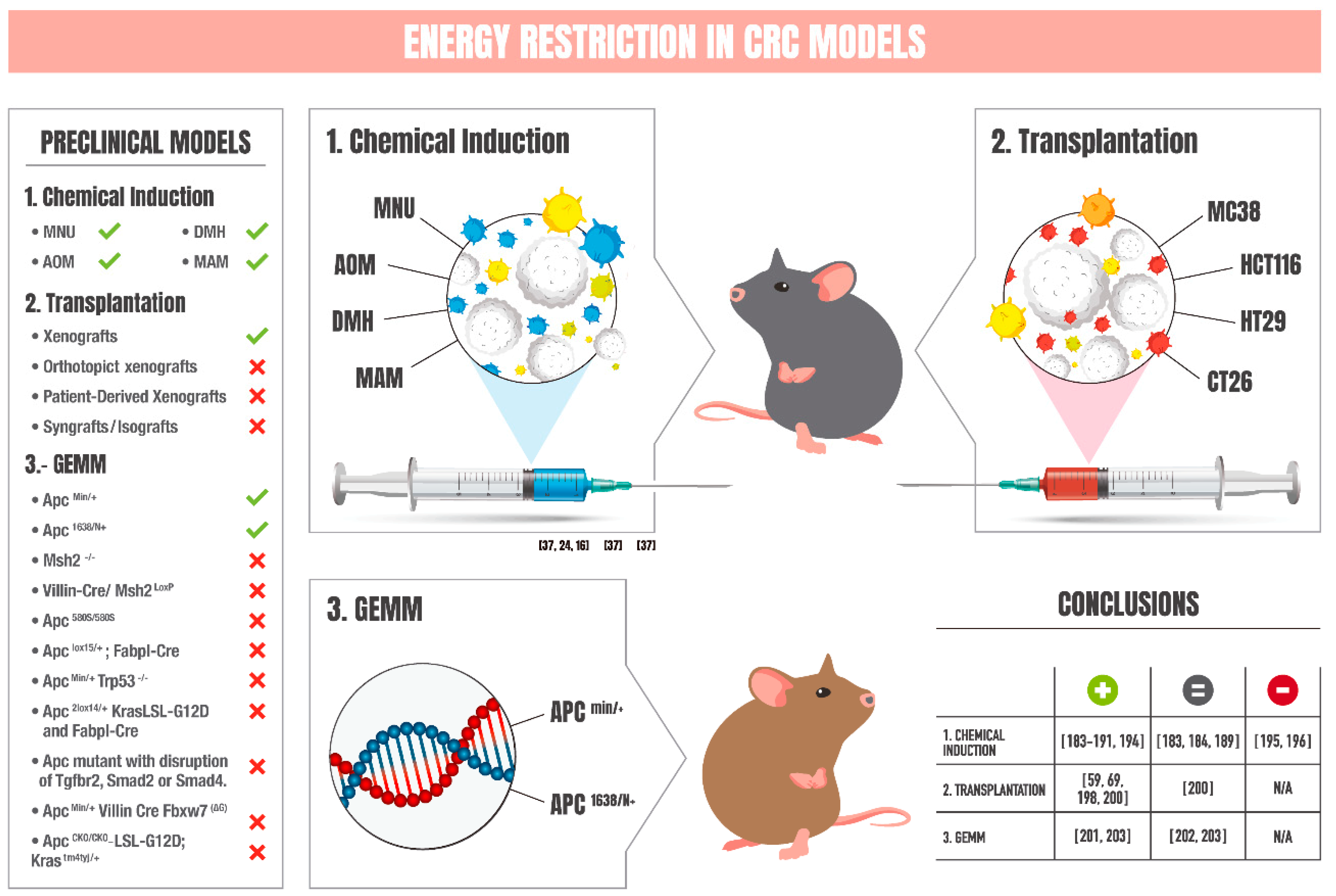
6. Chemical-Induced Models of CRC: Impact of Energy Restriction
7. Transplantation Models of CRC: Influence of Energy Restriction
8. Genetically Engineered Mouse Models of CRC: Effects of Energy Restriction
9. Energy Restriction and Clinical Trials in CRC
10. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, W.; Wang, X.; Zhou, W.; Zhang, Y.; Liu, J.; Zhang, H.; Zhao, Q.; Hong, L.; Fan, D. Advances in prognostic markers for colorectal cancer. Expert Rev. Mol. Diagn. 2019, 19, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. * Stat Database: Incidence-SEER 18 Regs Research Data <Katrina/Rita Population Adjustment>, N. 2017 S. (2000–2015) SEER. Available online: www.seer.cancer.gov (accessed on 17 September 2019).
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- SEER. Surveillance, Epidemiology, and End Results (SEER) Program. * Stat Database: Incidence-SEER 21 Regs Research Data <Katrina/Rita Population Adjustment>, N. 2018 S. (2000–2016), SEER. Available online: www.seer.cancer.gov (accessed on 21 September 2019).
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dauriz, M.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Bonora, E.; Targher, G. Association between nonalcoholic fatty liver disease and colorectal tumours in asymptomatic adults undergoing screening colonoscopy: A systematic review and meta-analysis. Metabolism 2018, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Colorectal Cancer Stages. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html ACS2018 (accessed on 12 October 2019).
- DeSantis, C.E.; Miller, K.D.; Dale, W.; Mohile, S.G.; Cohen, H.J.; Leach, C.R.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J. Clin. 2019, 69, 452–467. [Google Scholar] [CrossRef]
- Tariq, K.; Ghias, K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol. Med. 2016, 13, 120–135. [Google Scholar] [CrossRef]
- Vacante, M.; Borzi, A.M.; Basile, F.; Biondi, A. Biomarkers in colorectal cancer: Current clinical utility and future perspectives. World J. Clin. Cases 2018, 6, 869–881. [Google Scholar] [CrossRef]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The Influence of Meal Frequency and Timing on Health in Humans: The Role of Fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Intermittent v continuous energy restriction: Differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br. J. Nutr. 2018, 119, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef] [PubMed]
- Peos, J.J.; Helms, E.R.; Fournier, P.A.; Sainsbury, A. Continuous versus intermittent moderate energy restriction for increased fat mass loss and fat free mass retention in adult athletes: Protocol for a randomised controlled trial—The ICECAP trial (Intermittent versus Continuous Energy restriction Compared in an Athlete Population). BMJ Open Sport Exerc. Med. 2018, 4, e000423. [Google Scholar] [CrossRef]
- Keogh, J.B.; Pedersen, E.; Petersen, K.S.; Clifton, P.M. Effects of intermittent compared to continuous energy restriction on short-term weight loss and long-term weight loss maintenance. Clin. Obes. 2014, 4, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. 2014, 164, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Seimon, R.V.; Roekenes, J.A.; Zibellini, J.; Zhu, B.; Gibson, A.A.; Hills, A.P.; Wood, R.E.; King, N.A.; Byrne, N.M.; Sainsbury, A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol. Cell. Endocrinol. 2015, 418, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef]
- Harvie, M.; Howell, A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects—A Narrative Review of Human and Animal Evidence. Behav. Sci. 2017, 7, 4. [Google Scholar] [CrossRef]
- Davoodi, S.H.; Ajami, M.; Ayatollahi, S.A.; Dowlatshahi, K.; Javedan, G.; Pazoki-Toroudi, H.R. Calorie Shifting Diet Versus Calorie Restriction Diet: A Comparative Clinical Trial Study. Int. J. Prev. Med. 2014, 5, 447–456. [Google Scholar]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; de Cabo, R.; Anderson, R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815. [Google Scholar] [CrossRef]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Krupa Das, S.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019, 30, 462–476. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Weindruch, R.; Walford, R.L.; Fligiel, S.; Guthrie, D. The Retardation of Aging in Mice by Dietary Restriction: Longevity, Cancer, Immunity and Lifetime Energy Intake. J. Nutr. 1986, 116, 641–654. [Google Scholar] [CrossRef]
- Hursting, S.D.; Lavigne, J.A.; Berrigan, D.; Perkins, S.N.; Barrett, J.C. Calorie restriction, aging, and cancer prevention: Mechanisms of Action and Applicability to Humans. Annu. Rev. Med. 2002, 54, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Hursting, S.D.; Perkins, S.N.; Phang, J.M. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc. Natl. Acad. Sci. USA 1994, 91, 7036–7040. [Google Scholar] [CrossRef] [PubMed]
- Boissonneault, G.A.; Elson, C.E.; Pariza, M.W. Net energy effects of dietary fat on chemically induced mammary carcinogenesis in F344 rats. J. Natl. Cancer Inst. 1986, 76, 335–338. [Google Scholar] [PubMed]
- Lagopoulos, L.; Stalder, R. The influence of food intake on the development of diethylnitrosamine-induced liver tumours in mice. Carcinogenesis 1987, 8, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Kari, F.W.; French, J.; Leininger, J.R.; Travlos, G.; Wilson, R.; Barrett, J.C. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997, 57, 4667–4672. [Google Scholar] [PubMed]
- Gross, L.; Dreyfuss, Y. Prevention of spontaneous and radiation-induced tumors in rats by reduction of food intake. Proc. Natl. Acad. Sci. USA 1990, 87, 6795–6797. [Google Scholar] [CrossRef]
- Harvie, M.N.; Howell, T. Could Intermittent Energy Restriction and Intermittent Fasting Reduce Rates of Cancer in Obese, Overweight, and Normal-Weight Subjects? A Summary of Evidence. Adv. Nutr. 2016, 7, 690–705. [Google Scholar] [CrossRef]
- Dogan, S.; Rogozina, O.P.; Lokshin, A.E.; Grande, J.P.; Cleary, M.P. Effects of chronic vs. intermittent calorie restriction on mammary tumor incidence and serum adiponectin and leptin levels in MMTV-TGF-α mice at different ages. Oncol. Lett. 2010, 1, 167–176. [Google Scholar] [CrossRef]
- Rogozina, O.P.; Bonorden, M.J.L.; Grande, J.P.; Cleary, M.P. Serum Insulin-like Growth Factor-I and Mammary Tumor Development in Ad libitum-Fed, Chronic Calorie-Restricted, and Intermittent Calorie-Restricted MMTV-TGF-α Mice. Cancer Prev. Res. 2009, 2, 712–719. [Google Scholar] [CrossRef]
- Cleary, M.P.; Jacobson, M.K.; Phillips, F.C.; Getzin, S.C.; Grande, J.P.; Maihle, N.J. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-alpha female mice. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 836–843. [Google Scholar]
- Chen, R.F.; Good, R.A.; Engelman, R.W.; Hamada, N.; Tanaka, A.; Nonoyama, M.; Day, N.K. Suppression of mouse mammary tumor proviral DNA and protooncogene expression: Association with nutritional regulation of mammary tumor development. Proc. Natl. Acad. Sci. USA 1990, 87, 2385–2389. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.K.; Rogozina, O.P.; Seppanen, C.M.; Liao, D.J.; Cleary, M.P.; Grossmann, M.E. Combination of Intermittent Calorie Restriction and Eicosapentaenoic Acid for Inhibition of Mammary Tumors. Cancer Prev. Res. 2013, 6, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Berrigan, D.; Perkins, S.N.; Haines, D.C.; Hursting, S.D. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 2002, 23, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, X.; Li, M. Comprehensive Modulation of Tumor Progression and Regression with Periodic Fasting and Refeeding Circles via Boosting IGFBP-3 Loops and NK Responses. Endocrinology 2012, 153, 4622–4632. [Google Scholar] [CrossRef] [PubMed]
- Lanza-Jacoby, S.; Yan, G.; Radice, G.; LePhong, C.; Baliff, J.; Hess, R. Calorie restriction delays the progression of lesions to pancreatic cancer in the LSL-KrasG12D; Pdx-1/Cre mouse model of pancreatic cancer. Exp. Biol. Med. 2013, 238, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Bonorden, M.J.L.; Rogozina, O.P.; Kluczny, C.M.; Grossmann, M.E.; Grande, J.P.; Lokshin, A.; Cleary, M.P. Cross-sectional analysis of intermittent versus chronic caloric restriction in the TRAMP mouse. Prostate 2009, 69, 317–326. [Google Scholar] [CrossRef]
- Tannenbaum, A.; Silverstone, H. Failure to inhibit the formation of mammary carcinoma in mice by intermittent fasting. Cancer Res. 1950, 10, 577–579. [Google Scholar]
- Pape-Ansorge, K.A.; Grande, J.P.; Christensen, T.A.; Maihle, N.J.; Cleary, M.P. Effect of Moderate Caloric Restriction and/or Weight Cycling on Mammary Tumor Incidence and Latency in MMTV-Neu Female Mice. Nutr. Cancer 2002, 44, 162–168. [Google Scholar] [CrossRef]
- Mehta, R.S.; Harris, S.R.; Gunnett, C.A.; Bunce, O.R.; Hartle, D.K. Short communication: The effects of patterned calorie-restricted diets on mammary tumor incidence and plasma endothelin levels in DMBA-treated rats. Carcinogenesis 1993, 14, 1693–1696. [Google Scholar] [CrossRef]
- Thomas, J.A.; Antonelli, J.A.; Lloyd, J.C.; Masko, E.M.; Poulton, S.H.; Phillips, T.E.; Pollak, M.; Freedland, S.J. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis. 2010, 13, 350–355. [Google Scholar] [CrossRef]
- Buschemeyer, W.C.; Klink, J.C.; Mavropoulos, J.C.; Poulton, S.H.; Demark-Wahnefried, W.; Hursting, S.D.; Cohen, P.; Hwang, D.; Johnson, T.L.; Freedland, S.J. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate 2010, 70, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Caderni, G.; Perrelli, M.-G.; Cecchini, F.; Tessitore, L. Enhanced growth of colorectal aberrant crypt foci in fasted/refed rats involves changes in TGFβ1 and p21CIP expressions. Carcinogenesis 2002, 23, 323–327. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomasi, C.; Laconi, E.; Laconi, S.; Greco, M.; Sarma, D.S.R.; Pani, P. Effect of fasting/refeeding on the incidence of chemically induced hepatocellular carcinoma in the rat. Carcinogenesis 1999, 20, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhu, X.; Wang, H.; Wang, F.; Guan, W. Roles of Caloric Restriction, Ketogenic Diet and Intermittent Fasting during Initiation, Progression and Metastasis of Cancer in Animal Models: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e115147. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Cereda, E.; De Lorenzo, F.; Farina, G.; Pedrazzoli, P.; On Behalf of the AIOM-SINPE-FAVO Working Group. To fast, or not to fast before chemotherapy, that is the question. BMC Cancer 2018, 18, 337. [Google Scholar] [CrossRef]
- Caffa, I.; D’Agostino, V.; Damonte, P.; Soncini, D.; Cea, M.; Monacelli, F.; Odetti, P.; Ballestrero, A.; Provenzani, A.; Longo, V.D.; et al. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget 2015, 6, 11820–11832. [Google Scholar] [CrossRef]
- Lee, C.; Raffaghello, L.; Brandhorst, S.; Safdie, F.M.; Bianchi, G.; Martin-Montalvo, A.; Pistoia, V.; Wei, M.; Hwang, S.; Merlino, A.; et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012, 4, 124ra27. [Google Scholar] [CrossRef]
- Safdie, F.; Brandhorst, S.; Wei, M.; Wang, W.; Lee, C.; Hwang, S.; Conti, P.S.; Chen, T.C.; Longo, V.D. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS ONE 2012, 7, e44603. [Google Scholar] [CrossRef]
- D’Aronzo, M.; Vinciguerra, M.; Mazza, T.; Panebianco, C.; Saracino, C.; Pereira, S.P.; Graziano, P.; Pazienza, V. Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget 2015, 6, 18545. [Google Scholar] [CrossRef]
- Brandhorst, S.; Wei, M.; Hwang, S.; Morgan, T.E.; Longo, V.D. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp. Gerontol. 2013, 48, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Pol, J.; Vacchelli, E.; Rao, S.; Enot, D.P.; Baracco, E.E.; Levesque, S.; Castoldi, F.; Jacquelot, N.; Yamazaki, T.; et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 2016, 30, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, S.; Lee, C.; Brandhorst, S.; Manes, B.; Buono, R.; Cheng, C.-W.; Cacciottolo, M.; Martin-Montalvo, A.; de Cabo, R.; Wei, M.; et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016, 30, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Lee, C.; Safdie, F.M.; Wei, M.; Madia, F.; Bianchi, G.; Longo, V.D. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. USA 2008, 105, 8215–8220. [Google Scholar] [CrossRef] [PubMed]
- Tinkum, K.L.; Stemler, K.M.; White, L.S.; Loza, A.J.; Jeter-Jones, S.; Michalski, B.M.; Kuzmicki, C.; Pless, R.; Stappenbeck, T.S.; Piwnica-Worms, D.; et al. Fasting protects mice from lethal DNA damage by promoting small intestinal epithelial stem cell survival. Proc. Natl. Acad. Sci. USA 2015, 112, 201509249. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Martella, R.; Ravera, S.; Marini, C.; Capitanio, S.; Orengo, A.; Emionite, L.; Lavarello, C.; Amaro, A.; Petretto, A.; et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget 2015, 14, 11806. [Google Scholar] [CrossRef]
- Di Biase, S.; Longo, V.D. Fasting-induced differential stress sensitization in cancer treatment. Mol. Cell. Oncol. 2016, 3, e1117701. [Google Scholar] [CrossRef]
- Di Biase, S.; Shim, H.S.; Kim, K.H.; Vinciguerra, M.; Rappa, F.; Wei, M.; Brandhorst, S.; Cappello, F.; Mirzaei, H.; Lee, C.; et al. Fasting regulates EGR1 and protects from glucose- and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol. 2017, 15, e2001951. [Google Scholar] [CrossRef]
- Lee, C.; Safdie, F.M.; Raffaghello, L.; Wei, M.; Madia, F.; Parrella, E.; Hwang, D.; Cohen, P.; Bianchi, G.; Longo, V.D. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010, 70, 1564–1572. [Google Scholar] [CrossRef]
- De Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W.; et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef]
- Bauersfeld, S.P.; Kessler, C.S.; Wischnewsky, M.; Jaensch, A.; Steckhan, N.; Stange, R.; Kunz, B.; Brückner, B.; Sehouli, J.; Michalsen, A. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: A randomized cross-over pilot study. BMC Cancer 2018, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.-W.; Brandhorst, S.; Cohen, P.; Wei, M.; et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer 2016, 16, 360. [Google Scholar] [CrossRef] [PubMed]
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and cancer treatment in humans: A case series report. Aging 2009, 1, 988–1007. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Nix, J.W.; Hunter, G.R.; Rais-Bahrami, S.; Desmond, R.A.; Chacko, B.; Morrow, C.D.; Azrad, M.; Frugé, A.D.; Tsuruta, Y.; et al. Feasibility outcomes of a presurgical randomized controlled trial exploring the impact of caloric restriction and increased physical activity versus a wait-list control on tumor characteristics and circulating biomarkers in men electing prostatectomy for prostate cancer. BMC Cancer 2016, 16, 61. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wust, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Kaiser, D.L. Effects of calorie restriction and weight loss on glucose and insulin levels in obese humans. J. Am. Coll. Nutr. 1985, 4, 411–419. [Google Scholar] [CrossRef]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef]
- Wong, M.H.T.; Holst, C.; Astrup, A.; Handjieva-Darlenska, T.; Jebb, S.A.; Kafatos, A.; Kunesova, M.; Larsen, T.M.; Martinez, J.A.; Pfeiffer, A.F.H.; et al. Caloric Restriction Induces Changes in Insulin and Body Weight Measurements That Are Inversely Associated with Subsequent Weight Regain. PLoS ONE 2012, 7, e42858. [Google Scholar] [CrossRef]
- Rahmani, J.; Kord Varkaneh, H.; Clark, C.; Zand, H.; Bawadi, H.; Ryan, P.M.; Fatahi, S.; Zhang, Y. The influence of fasting and energy restricting diets on IGF-1 levels in humans: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 53, 100910. [Google Scholar] [CrossRef]
- Ghanavati, M.; Rahmani, J.; Rinaldi, G.; Zand, H. Fasting insulin and risk of cancer related mortality in non-diabetic adults: A dose-response meta-analysis of cohort studies. Curr. Diabetes Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Riondino, S.; Laudisi, A.; Portarena, I.; Formica, V.; Alessandroni, J.; DAlessandro, R.; Orlandi, A.; Costarelli, L.; Cavaliere, F.; et al. Pretreatment Insulin Levels as a Prognostic Factor for Breast Cancer Progression. Oncologist 2016, 21, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.M.Y.; Magrill, J.; de Winter, T.J.J.; Hu, X.; Skovsø, S.; Schaeffer, D.F.; Kopp, J.L.; Johnson, J.D. Endogenous Hyperinsulinemia Contributes to Pancreatic Cancer Development. Cell Metab. 2019, 30, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, D.R.; Mittal, A.; Sathian, B.; Bhatta, B. Role of hyperinsulinemia in increased risk of prostate cancer: A case control study from Kathmandu Valley. Asian Pac. J. Cancer Prev. 2014, 15, 1031–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saboori, S.; Rad, E.Y.; Birjandi, M.; Mohiti, S.; Falahi, E. Serum insulin level, HOMA-IR and prostate cancer risk: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ye, Y.; Wu, H.; Duerksen-Hughes, P.; Zhang, H.; Li, P.; Huang, J.; Yang, J.; Wu, Y.; Xia, D. Association between markers of glucose metabolism and risk of colorectal cancer. BMJ Open 2016, 6, e011430. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Kim, M.Y.; Strickler, H.D.; Shikany, J.M.; Lane, D.; Luo, J.; Ning, Y.; Gunter, M.J.; Rohan, T.E. A longitudinal study of serum insulin and glucose levels in relation to colorectal cancer risk among postmenopausal women. Br. J. Cancer 2012, 106, 227–232. [Google Scholar] [CrossRef]
- Limburg, P.J.; Stolzenberg-Solomon, R.Z.; Vierkant, R.A.; Roberts, K.; Sellers, T.A.; Taylor, P.R.; Virtamo, J.; Cerhan, J.R.; Albanes, D. Insulin, glucose, insulin resistance, and incident colorectal cancer in male smokers. Clin. Gastroenterol. Hepatol. 2006, 4, 1514–1521. [Google Scholar] [CrossRef]
- Giovannucci, E.; Pollak, M.N.; Platz, E.A.; Willett, W.C.; Stampfer, M.J.; Majeed, N.; Colditz, G.A.; Speizer, F.E.; Hankinson, S.E. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 345–349. [Google Scholar]
- Renehan, A.G.; Painter, J.E.; O’Halloran, D.; Atkin, W.S.; Potten, C.S.; O’Dwyer, S.T.; Shalet, S.M. Circulating Insulin-Like Growth Factor II and Colorectal Adenomas. J. Clin. Endocrinol. Metab. 2000, 85, 3402–3408. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Frajese, V.; Jones, A.-M.; Camacho-Hubner, C.; Lowe, D.G.; Fairclough, P.D.; Chew, S.L.; Grossman, A.B.; Monson, J.P.; Besser, G.M. Insulin-Like Growth Factor I and the Development of Colorectal Neoplasia in Acromegaly 1. J. Clin. Endocrinol. Metab. 2000, 85, 3218–3221. [Google Scholar] [CrossRef] [PubMed]
- Endogenous Hormones and Breast Cancer Collaborative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar] [CrossRef]
- Lukanova, A.; Lundin, E.; Toniolo, P.; Micheli, A.; Akhmedkhanov, A.; Rinaldi, S.; Muti, P.; Lenner, P.; Biessy, C.; Krogh, V.; et al. Circulating levels of insulin-like growth factor-I and risk of ovarian cancer. Int. J. Cancer 2002, 101, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Weroha, S.J.; Haluska, P. The insulin-like growth factor system in cancer. Endocrinol. Metab. Clin. N. Am. 2012, 41, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kojima, M.; Tokudome, S.; Suzuki, K.; Ozasa, K.; Ito, Y.; Inaba, Y.; Tajima, K.; Nakachi, K.; Watanabe, Y.; et al. Insulin-like growth factor (IGF)-I, IGF-II, IGF binding protein-3, and risk of colorectal cancer: A nested case-control study in the Japan Collaborative Cohort study. Asian Pac. J. Cancer Prev. 2009, 10, 45–49. [Google Scholar] [PubMed]
- Spitz, M.R.; Barnett, M.J.; Goodman, G.E.; Thornquist, M.D.; Wu, X.; Pollak, M. Serum insulin-like growth factor (IGF) and IGF-binding protein levels and risk of lung cancer: A case-control study nested in the beta-Carotene and Retinol Efficacy Trial Cohort. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1413–1418. [Google Scholar]
- Pham, T.-M.; Fujino, Y.; Kikuchi, S.; Tamakoshi, A.; Yatsuya, H.; Matsuda, S.; Yoshimura, T.; JACC Study Group. A nested case-control study of stomach cancer and serum insulin-like growth factor (IGF)-1, IGF-2 and IGF-binding protein (IGFBP)-3. Eur. J. Cancer 2007, 43, 1611–1616. [Google Scholar] [CrossRef]
- Mikami, K.; Ozasa, K.; Nakao, M.; Miki, T.; Hayashi, K.; Watanabe, Y.; Mori, M.; Sakauchi, F.; Washio, M.; Kubo, T.; et al. Prostate cancer risk in relation to insulin-like growth factor (IGF)-I and IGF-binding protein-3: A nested case-control study in large scale cohort study in Japan. Asian Pac. J. Cancer Prev. 2009, 10 (Suppl. S1), 57–61. [Google Scholar]
- Finne, P.; Auvinen, A.; Koistinen, H.; Zhang, W.-M.; Määttänen, L.; Rannikko, S.; Tammela, T.; Seppälä, M.; Hakama, M.; Stenman, U.-H. Insulin-Like Growth Factor I Is Not a Useful Marker of Prostate Cancer in Men with Elevated Levels of Prostate-Specific Antigen. J. Clin. Endocrinol. Metab. 2000, 85, 2744–2747. [Google Scholar]
- Lukanova, A.; Zeleniuch-Jacquotte, A.; Lundin, E.; Micheli, A.; Arslan, A.A.; Rinaldi, S.; Muti, P.; Lenner, P.; Koenig, K.L.; Biessy, C.; et al. Prediagnostic levels of C-peptide, IGF-I, IGFBP -1, -2 and -3 and risk of endometrial cancer. Int. J. Cancer 2004, 108, 262–268. [Google Scholar] [CrossRef]
- Allen, N.E.; Key, T.J.; Appleby, P.N.; Travis, R.C.; Roddam, A.W.; Rinaldi, S.; Egevad, L.; Rohrmann, S.; Linseisen, J.; Pischon, T.; et al. Serum Insulin-like Growth Factor (IGF)-I and IGF-Binding Protein-3 Concentrations and Prostate Cancer Risk: Results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Murtola, T.J.; Vihervuori, V.J.; Lahtela, J.; Talala, K.; Taari, K.; Tammela, T.L.; Auvinen, A. Fasting blood glucose, glycaemic control and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Br. J. Cancer 2018, 118, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Ha, K.H.; Bao, Y.; Chung, M.J.; Kim, H.C.; Giovannucci, E.L. Long-term patterns of fasting blood glucose levels and pancreatic cancer incidence. Cancer Causes Control. 2018, 29, 135–142. [Google Scholar] [CrossRef]
- Bergamino, M.; Rullan, A.J.; Saigí, M.; Peiró, I.; Montanya, E.; Palmero, R.; Ruffinelli, J.C.; Navarro, A.; Arnaiz, M.D.; Brao, I.; et al. Fasting plasma glucose is an independent predictor of survival in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. BMC Cancer 2019, 19, 165. [Google Scholar] [CrossRef]
- Yamagata, H.; Kiyohara, Y.; Nakamura, S.; Kubo, M.; Tanizaki, Y.; Matsumoto, T.; Tanaka, K.; Kato, I.; Shirota, T.; Iida, M. Impact of fasting plasma glucose levels on gastric cancer incidence in a general Japanese population: The Hisayama study. Diabetes Care 2005, 28, 789–794. [Google Scholar] [CrossRef]
- Park, H.; Cho, S.; Woo, H.; Park, S.K.; Shin, H.-R.; Chang, S.-H.; Yoo, K.-Y.; Shin, A. Fasting glucose and risk of colorectal cancer in the Korean Multi-center Cancer Cohort. PLoS ONE 2017, 12, e0188465. [Google Scholar] [CrossRef]
- Pang, Y.; Kartsonaki, C.; Guo, Y.; Chen, Y.; Yang, L.; Bian, Z.; Bragg, F.; Millwood, I.Y.; Shen, L.; Zhou, S.; et al. Diabetes, plasma glucose and incidence of colorectal cancer in Chinese adults: A prospective study of 0.5 million people. J. Epidemiol. Community Health 2018, 72, 919–925. [Google Scholar] [CrossRef]
- Vulcan, A.; Manjer, J.; Ohlsson, B. High blood glucose levels are associated with higher risk of colon cancer in men: A cohort study. BMC Cancer 2017, 17, 842. [Google Scholar] [CrossRef]
- Lecoultre, V.; Ravussin, E.; Redman, L.M. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J. Clin. Endocrinol. Metab. 2011, 96, E1512–E1516. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.; Cho, S.; Lee, M.; Lee, Y.; Lee, Y.; Kang, E.; Cha, B.-S.; Lee, B.-W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A.; Pandi-Perumal, S.R.; Sharif, M.M.; BaHammam, A.S. Diurnal intermittent fasting during Ramadan: The effects on leptin and ghrelin levels. PLoS ONE 2014, 9, e92214. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W.; Schwartz, M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. 2010, 21, 643–651. [Google Scholar] [CrossRef]
- Gu, L.; Wang, C.-D.; Cao, C.; Cai, L.-R.; Li, D.-H.; Zheng, Y.-Z. Association of serum leptin with breast cancer. Medicine (Baltimore) 2019, 98, e14094. [Google Scholar] [CrossRef] [PubMed]
- Akinci, M.; Kosova, F.; Cetin, B.; Aslan, S.; Ari, Z.; Cetin, A. Leptin Levels in Thyroid Cancer. Asian J. Surg. 2009, 32, 216–223. [Google Scholar] [CrossRef]
- Chun, K.A.; Kocarnik, J.M.; Hardikar, S.S.; Robinson, J.R.; Berndt, S.I.; Chan, A.T.; Figueiredo, J.C.; Lindor, N.M.; Song, M.; Schoen, R.E.; et al. Leptin gene variants and colorectal cancer risk: Sex-specific associations. PLoS ONE 2018, 13, e0206519. [Google Scholar] [CrossRef]
- Nakajima, A.; Takahashi, H.; Endo, H.; Sugiyama, M.; Sakai, E.; Hosono, K.; Nagashima, Y.; Inayama, Y.; Wada, K.; Hippo, Y.; et al. Role of the long form leptin receptor and of the STAT3 signaling pathway in colorectal cancer progression. Int. J. Oncol. 2011, 39, 935–940. [Google Scholar] [CrossRef]
- Paik, S.S.; Jang, S.-M.; Jang, K.-S.; Lee, K.H.; Choi, D.; Jang, S.J. Leptin Expression Correlates with Favorable Clinicopathologic Phenotype and Better Prognosis in Colorectal Adenocarcinoma. Ann. Surg. Oncol. 2009, 16, 297–303. [Google Scholar] [CrossRef]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than Just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef]
- Kelesidis, I.; Kelesidis, T.; Mantzoros, C.S. Adiponectin and cancer: A systematic review. Br. J. Cancer 2006, 94, 1221–1225. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Funahashi, T.; Kihara, S.; Taguchi, T.; Tamaki, Y.; Matsuzawa, Y.; Noguchi, S. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 2003, 9, 5699–5704. [Google Scholar] [PubMed]
- Mantzoros, C.; Petridou, E.; Dessypris, N.; Chavelas, C.; Dalamaga, M.; Alexe, D.M.; Papadiamantis, Y.; Markopoulos, C.; Spanos, E.; Chrousos, G.; et al. Adiponectin and Breast Cancer Risk. J. Clin. Endocrinol. Metab. 2004, 89, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.; Mantzoros, C.; Dessypris, N.; Koukoulomatis, P.; Addy, C.; Voulgaris, Z.; Chrousos, G.; Trichopoulos, D. Plasma Adiponectin Concentrations in Relation to Endometrial Cancer: A Case-Control Study in Greece. J. Clin. Endocrinol. Metab. 2003, 88, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kitayama, J.; Kazama, S.; Hiramatsu, T.; Hatano, K.; Nagawa, H. Plasma adiponectin and gastric cancer. Clin. Cancer Res. 2005, 11, 466–472. [Google Scholar]
- Goktas, S.; Yilmaz, M.I.; Caglar, K.; Sonmez, A.; Kilic, S.; Bedir, S. Prostate cancer and adiponectin. Urology 2005, 65, 1168–1172. [Google Scholar] [CrossRef]
- Petridou, E.; Mantzoros, C.S.; Dessypris, N.; Dikalioti, S.K.; Trichopoulos, D. Adiponectin in relation to childhood myeloblastic leukaemia. Br. J. Cancer 2006, 94, 156–160. [Google Scholar] [CrossRef]
- Wei, E.K.; Giovannucci, E.; Fuchs, C.S.; Willett, W.C.; Mantzoros, C.S. Low Plasma Adiponectin Levels and Risk of Colorectal Cancer in Men: A Prospective Study. JNCI J. Natl. Cancer Inst. 2005, 97, 1688–1694. [Google Scholar] [CrossRef]
- Tae, C.H.; Kim, S.-E.; Jung, S.-A.; Joo, Y.-H.; Shim, K.-N.; Jung, H.-K.; Kim, T.H.; Cho, M.-S.; Kim, K.H.; Kim, J.S. Involvement of adiponectin in early stage of colorectal carcinogenesis. BMC Cancer 2014, 14, 811. [Google Scholar] [CrossRef]
- Chandler, P.D.; Buring, J.E.; Manson, J.E.; Moorthy, M.V.; Zhang, S.; Lee, I.-M.; Lin, J.H. Association between plasma adiponectin levels and colorectal cancer risk in women. Cancer Causes Control 2015, 26, 1047–1052. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Lun, M.; Kim, S.M.; Bredella, M.A.; Wright, S.; Zhang, Y.; Lee, H.; Catana, C.; Klibanski, A.; Patwari, P.; et al. FGF21 and the late adaptive response to starvation in humans. J. Clin. Investig. 2015, 125, 4601–4611. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? In Molecular Metabolism; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yamaguchi, M.; Okamura, S.; Yamaji, T.; Iwasaki, M.; Tsugane, S.; Shetty, V.; Koizumi, T. Plasma cytokine levels and the presence of colorectal cancer. PLoS ONE 2019, 14, e0213602. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Ray, A.; Cleary, M.P. The influence of different calorie restriction protocols on serum pro-inflammatory cytokines, adipokines and IGF-I levels in female C57BL6 mice: Short term and long term diet effects. Meta Gene 2017, 12, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, N.H.; Figgers, C.L. Caloric restriction inhibits up-regulation of inflammatory cytokines and TNF-α, and activates IL-10 and haptoglobin in the plasma of streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2007, 18, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Imayama, I.; Ulrich, C.M.; Alfano, C.M.; Wang, C.; Xiao, L.; Wener, M.H.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; Kong, A.; et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: A randomized controlled trial. Cancer Res. 2012, 72, 2314–2326. [Google Scholar] [CrossRef]
- Lu, Y.; Tao, F.; Zhou, M.-T.; Tang, K.-F. The signaling pathways that mediate the anti-cancer effects of calorie restriction. In Pharmacological Research; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Kopeina, G.S.; Senichkin, V.V.; Zhivotovsky, B. Caloric restriction-A promising anti-cancer approach: From molecular mechanisms to clinical trials. Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 29–41. [Google Scholar] [CrossRef]
- Tamada, M.; Nagano, O.; Tateyama, S.; Ohmura, M.; Yae, T.; Ishimoto, T.; Sugihara, E.; Onishi, N.; Yamamoto, T.; Yanagawa, H.; et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012, 72, 1438–1448. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, Y.; Ding, Y.; Liu, H.; Liu, Z.; Fodstad, O.; Riker, A.I.; Kamarajugadda, S.; Lu, J.; Owen, L.B.; et al. Warburg effect in chemosensitivity: Targeting lactate dehydrogenase-A re-sensitizes Taxol-resistant cancer cells to Taxol. Mol. Cancer 2010, 9, 33. [Google Scholar] [CrossRef]
- Diaz-Ruiz, A.; Di Francesco, A.; Carboneau, B.A.; Levan, S.R.; Pearson, K.J.; Price, N.L.; Ward, T.M.; Bernier, M.; De Cabo, R.; Mercken, E.M. Benefits of Caloric Restriction in Longevity and Chemical-Induced Tumorigenesis Are Transmitted Independent of NQO1. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 155–162. [Google Scholar] [CrossRef]
- Pearson, K.J.; Lewis, K.N.; Price, N.L.; Chang, J.W.; Perez, E.; Cascajo, M.V.; Tamashiro, K.L.; Poosala, S.; Csiszar, A.; Ungvari, Z.; et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc. Natl. Acad. Sci. USA 2008, 105, 2325–2330. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef]
- Rubio-Patiño, C.; Bossowski, J.P.; De Donatis, G.M.; Mondragón, L.; Villa, E.; Aira, L.E.; Chiche, J.; Mhaidly, R.; Lebeaupin, C.; Marchetti, S.; et al. Low-Protein Diet Induces IRE1α-Dependent Anticancer Immunosurveillance. Cell Metab. 2018, 27, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Y.; Zamyatnin, A.A.J.; Werner, J.; Bazhin, A.V. Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef]
- De Sa Junior, P.L.; Camara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The Roles of ROS in Cancer Heterogeneity and Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.; Gradzik, K.; Kabzinski, J.; Przybylowska-Sygut, K.; Majsterek, I. The role of the ER-induced UPR pathway and the efficacy of its inhibitors and inducers in the inhibition of tumor progression. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013. [Google Scholar] [CrossRef] [PubMed]
- Spaan, C.N.; Smit, W.L.; van Lidth de Jeude, J.F.; Meijer, B.J.; Muncan, V.; van den Brink, G.R.; Heijmans, J. Expression of UPR effector proteins ATF6 and XBP1 reduce colorectal cancer cell proliferation and stemness by activating PERK signaling. Cell Death Dis. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.V.; Allard, J.; Ingram, D.K.; Navas, P.; et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef]
- Grau, R.; Punzon, C.; Fresno, M.; Iniguez, M.A. Peroxisome-proliferator-activated receptor alpha agonists inhibit cyclo-oxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein-1. Biochem. J. 2006, 395, 81–88. [Google Scholar] [CrossRef]
- Brahmkhatri, V.P.; Prasanna, C.; Atreya, H.S. Insulin-like growth factor system in cancer: Novel targeted therapies. Biomed. Res. Int. 2015, 2015, 538019. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagaron, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Ding, J.; Huang, Y.; Liu, J.; Liu, N.; Ao, Y.; Hong, Y.; Wang, L.; Zhang, L.; et al. Targeting mTOR suppressed colon cancer growth through 4EBP1/eIF4E/PUMA pathway. In Cancer Gene Therapy; Nature Publishing Group: Berlin, Germany, 2019; pp. 1–13. [Google Scholar]
- Johnson, S.M.; Gulhati, P.; Rampy, B.A.; Han, Y.; Rychahou, P.G.; Doan, H.Q.; Weiss, H.L.; Evers, B.M. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J. Am. Coll. Surg. 2010, 210, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Morán, A. Differential colorectal carcinogenesis: Molecular basis and clinical relevance. World J. Gastrointest. Oncol. 2010, 2, 151. [Google Scholar] [CrossRef] [PubMed]
- Kalaany, N.Y.; Sabatini, D.M. Tumours with PI3K activation are resistant to dietary restriction. Nature 2009, 458, 725–731. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: Maintaining energy homeostasis at the cellular and whole-body levels. Annu. Rev. Nutr. 2014, 34, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Cedron, M.; Acin Perez, R.; Sánchez-Martínez, R.; Molina, S.; Herranz, J.; Feliu, J.; Reglero, G.; Enríquez, J.A.; Ramírez de Molina, A. Micro RNA-661 modulates redox and metabolic homeostasis in colon cancer. Mol. Oncol. 2017, 11, 1768–1787. [Google Scholar] [CrossRef]
- Kottakis, F.; Bardeesy, N. LKB1-AMPK axis revisited. Cell Res. 2012, 22, 1617. [Google Scholar] [CrossRef]
- Cantó, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for Metabolic Adaptation to Fasting and Exercise in Skeletal Muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef]
- Luo, Z.; Zang, M.; Guo, W. AMPK as a metabolic tumor suppressor: Control of metabolism and cell growth. Futur. Oncol. 2010, 6, 457–470. [Google Scholar] [CrossRef]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365. [Google Scholar] [CrossRef]
- Meynet, O.; Ricci, J.-E. Caloric restriction and cancer: Molecular mechanisms and clinical implications. Trends Mol. Med. 2014, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, P.; Chen, Q.; Deng, S.; Liu, X.; Situ, H.; Zhong, S.; Hann, S.; Lin, Y. Targeting AMPK Signaling Pathway to Overcome Drug Resistance for Cancer Therapy. Curr. Drug Targets 2016, 17, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Al-Maghrabi, J.; Al-Sakkaf, K.; Qureshi, I.A.; Butt, N.S.; Damnhory, L.; Elshal, M.; Al-Maghrabi, B.; Aldahlawi, A.; Ashoor, S.; Brown, B.; et al. AMPK expression patterns are significantly associated with poor prognosis in breast cancer patients. Ann. Diagn. Pathol. 2017, 29, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Chandel, N.S.; Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012, 485, 661. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Nosho, K.; Shima, K.; Meyerhardt, J.A.; Chan, A.T.; Engelman, J.A.; Cantley, L.C.; Loda, M.; Giovannucci, E.; Fuchs, C.S.; et al. Prognostic significance of AMP-activated protein kinase expression and modifying effect of MAPK3/1 in colorectal cancer. Br. J. Cancer 2010, 103, 1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Lu, Y.-X.; Liu, J.; Jin, Y.; Bi, H.-C.; Zhao, Q.; Liu, Z.-X.; Li, Y.-Q.; Hu, J.-J.; Sheng, H.; et al. AMPKalpha1 confers survival advantage of colorectal cancer cells under metabolic stress by promoting redox balance through the regulation of glutathione reductase phosphorylation. Oncogene 2019. [Google Scholar] [CrossRef]
- Guo, B.; Han, X.; Tkach, D.; Huang, S.-G.; Zhang, D. AMPK promotes the survival of colorectal cancer stem cells. Anim. Model. Exp. Med. 2018, 1, 134–142. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.B.; Hursting, S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Wan, W.; Shou, X.; Qian, J.; You, Z.; Liu, B.; Chang, C.; Zhou, T.; Lippincott-Schwartz, J.; et al. Deacetylation of Nuclear LC3 Drives Autophagy Initiation under Starvation. Mol. Cell 2015, 57, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Burada, F.; Nicoli, E.R.; Ciurea, M.E.; Uscatu, D.C.; Ioana, M.; Gheonea, D.I. Autophagy in colorectal cancer: An important switch from physiology to pathology. World J. Gastrointest. Oncol. 2015, 7, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Lauzier, A.; Normandeau-Guimond, J.; Vaillancourt-Lavigueur, V.; Boivin, V.; Charbonneau, M.; Rivard, N.; Scott, M.S.; Dubois, C.M.; Jean, S. Colorectal cancer cells respond differentially to autophagy inhibition in vivo. Sci. Rep. 2019, 9, 11316. [Google Scholar] [CrossRef] [PubMed]
- Costa-Machado, L.F.; Fernandez-Marcos, P.J. The sirtuin family in cancer. Cell Cycle 2019, 18, 2164–2196. [Google Scholar] [CrossRef]
- Rifaï, K.; Judes, G.; Idrissou, M.; Daures, M.; Bignon, Y.-J.; Penault-Llorca, F.; Bernard-Gallon, D. Dual SIRT1 expression patterns strongly suggests its bivalent role in human breast cancer. Oncotarget 2017, 8, 110922–110930. [Google Scholar] [CrossRef]
- Zu, G.; Ji, A.; Zhou, T.; Che, N. Clinicopathological significance of SIRT1 expression in colorectal cancer: A systematic review and meta analysis. Int. J. Surg. 2016, 26, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Luckert, P.H.; Pan, G.Y. Inhibition of intestinal tumorigenesis in methylazoxymethanol-treated rats by dietary restriction. Cancer Treat. Rep. 1984, 68, 405–408. [Google Scholar]
- Pollard, M.; Luckert, P.H. Tumorigenic effects of direct- and indirect-acting chemical carcinogens in rats on a restricted diet. J. Natl. Cancer Inst. 1985, 74, 1347–1349. [Google Scholar]
- Reddy, B.S.; Wang, C.X.; Maruyama, H. Effect of restricted caloric intake on azoxymethane-induced colon tumor incidence in male F344 rats. Cancer Res. 1987, 47, 1226–1228. [Google Scholar]
- Olivo-Marston, S.E.; Hursting, S.D.; Perkins, S.N.; Schetter, A.; Khan, M.; Croce, C.; Harris, C.C.; Lavigne, J. Effects of calorie restriction and diet-induced obesity on murine colon carcinogenesis, growth and inflammatory factors, and MicroRNA expression. PLoS ONE 2014, 9, e94765. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sakamoto, Y.; Mizowaki, Y.; Iwagaki, Y.; Kimura, T.; Nakagawa, K.; Miyazawa, T.; Tsuduki, T. Intake of mulberry 1-deoxynojirimycin prevents colorectal cancer in mice. J. Clin. Biochem. Nutr. 2017, 61, 47–52. [Google Scholar]
- Tomita, M. Caloric restriction reduced 1, 2-dimethylhydrazine-induced aberrant crypt foci and induces the expression of Sirtuins in colonic mucosa of F344 rats. J. Carcinog. 2012, 11, 10. [Google Scholar] [CrossRef]
- Mcintyre, R.E.; Buczacki, S.J.A.; Arends, M.J.; Adams, D.J. Mouse models of colorectal cancer as preclinical models. BioEssays 2015, 37, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Roy, S.J.; Tokumo, K.; Reddy, B.S. Effect of different levels of calorie restriction on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 1990, 50, 5761–5766. [Google Scholar] [PubMed]
- Lasko, C.M.; Bird, R.P. Modulation of aberrant crypt foci by dietary fat and caloric restriction: The effects of delayed intervention. Cancer Epidemiol. Biomarkers Prev. 1995, 4, 49–55. [Google Scholar] [PubMed]
- Lasko, C.M.; Good, C.K.; Adam, J.; Bird, R.P. Energy restriction modulates the development of advanced preneoplastic lesions depending on the level of fat in the diet. Nutr. Cancer 1999, 33, 69–75. [Google Scholar] [CrossRef]
- Weber, R.V.; Stein, D.E.; Scholes, J.; Kral, J.G. Obesity potentiates AOM-induced colon cancer. Dig. Dis. Sci. 2000, 45, 890–895. [Google Scholar] [CrossRef]
- Kritchevsky, D. Colorectal cancer: The role of dietary fat and caloric restriction. Mutat. Res. 1993, 290, 63–70. [Google Scholar] [CrossRef]
- Klurfeld, D.M.; Weber, M.M.; Kritchevsky, D. Inhibition of Chemically Induced Mammary and Colon Tumor Promotion by Caloric Restriction in Rats Fed Increased Dietary Fat. Cancer Res. 1987, 47, 2759–2762. [Google Scholar]
- Kannen, V.; Fernandes, C.R.; Stopper, H.; Zanette, D.L.; Ferreira, F.R.; Frajacomo, F.T.; Carvalho, M.C.; Brandão, M.L.; Elias Junior, J.; Jordão Junior, A.A.; et al. Colon preneoplasia after carcinogen exposure is enhanced and colonic serotonergic system is suppressed by food deprivation. Toxicology 2013, 312, 123–131. [Google Scholar] [CrossRef]
- Premoselli, F.; Sesca, E.; Binasco, V.; Caderni, G.; Tessitore, L. Fasting/re-feeding before initiation enhances the growth of aberrant crypt foci induced by azoxymethane in rat colon and rectum. Int. J. Cancer 1998, 77, 286–294. [Google Scholar] [CrossRef]
- Harvey, A.E.; Lashinger, L.M.; Otto, G.; Nunez, N.P.; Hursting, S.D. Decreased systemic IGF-1 in response to calorie restriction modulates murine tumor cell growth, nuclear factor-κB activation, and inflammation-related gene expression. Mol. Carcinog. 2013, 52, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, H.; He, Z.; Chen, X.; Wu, Q.; Chen, W.; Sun, Z.; Weng, M.; Zhu, M.; Ma, D.; et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget 2017, 8, 74649–74660. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, K.E.; Williams, E.A.; Smith, N.C.P.; Dillard, A.; Eun, Y.P.; Nunez, N.P.; Hursting, S.D.; Lane, M.A. Low-carbohydrate diet versus caloric restriction: Effects on weight loss, hormones, and colon tumor growth in obese mice. Nutr. Cancer 2008, 60, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Colbert, L.H.; Berrigan, D.; Perkins, S.N.; Pfeiffer, R.; Lavigne, J.A.; Lanza, E.; Haines, D.C.; Schatzkin, A.; Hursting, S.D. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(Min) mice through different mechanisms. Cancer Res. 2003, 63, 1752–1755. [Google Scholar]
- Huffman, D.M.; Augenlicht, L.H.; Zhang, X.; Lofrese, J.J.; Atzmon, G.; Chamberland, J.P.; Mantzoros, C.S. Abdominal obesity, independent from caloric intake, accounts for the development of intestinal tumors in Apc(1638N/+) female mice. Cancer Prev. Res. 2013, 6, 177–187. [Google Scholar] [CrossRef]
- Kadaveru, K.; Protiva, P.; Greenspan, E.J.; Kim, Y.I.; Rosenberg, D.W. Dietary methyl donor depletion protects against intestinal tumorigenesis in ApcMin/+ mice. Cancer Prev. Res. 2012, 5, 911–920. [Google Scholar] [CrossRef]
- Schubel, R.; Nattenmuller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Caccialanza, R.; Aprile, G.; Cereda, E.; Pedrazzoli, P. Fasting in oncology: A word of caution. Nat. Rev. Cancer 2019, 19, 177. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Reply to “Fasting in oncology: A word of caution”. Nat. Rev. Cancer 2019, 19, 178. [Google Scholar] [CrossRef]
- Ershler, W.B.; Berman, E.; Moore, A.L. Slower B16 melanoma growth but greater pulmonary colonization in calorie-restricted mice. J. Natl. Cancer Inst. 1986, 76, 81–85. [Google Scholar] [PubMed]
- Garcia-Jimenez, C.; Goding, C.R. Starvation and Pseudo-Starvation as Drivers of Cancer Metastasis through Translation Reprogramming. Cell Metab. 2019, 29, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.G.; Peeters, P.H.M.; Grobbee, D.E.; van Noord, P.A.H. Transient caloric restriction and cancer risk (The Netherlands). Cancer Causes Control. 2007, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; de Cabo, R. Calorie restriction in rodents: Caveats to consider. Ageing Res. Rev. 2017, 39, 15–28. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Madrigal-Matute, J.; Scheibye-Knudsen, M.; Fang, E.; Aon, M.; Gonzalez-Reyes, J.A.; Cortassa, S.; Kaushik, S.; Gonzalez-Freire, M.; Patel, B.; et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016, 23, 1093–1112. [Google Scholar] [CrossRef] [PubMed]
- Lieffers, J.R.; Mourtzakis, M.; Hall, K.D.; McCargar, L.J.; Prado, C.M.M.; Baracos, V.E. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am. J. Clin. Nutr. 2009, 89, 1173–1179. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef]
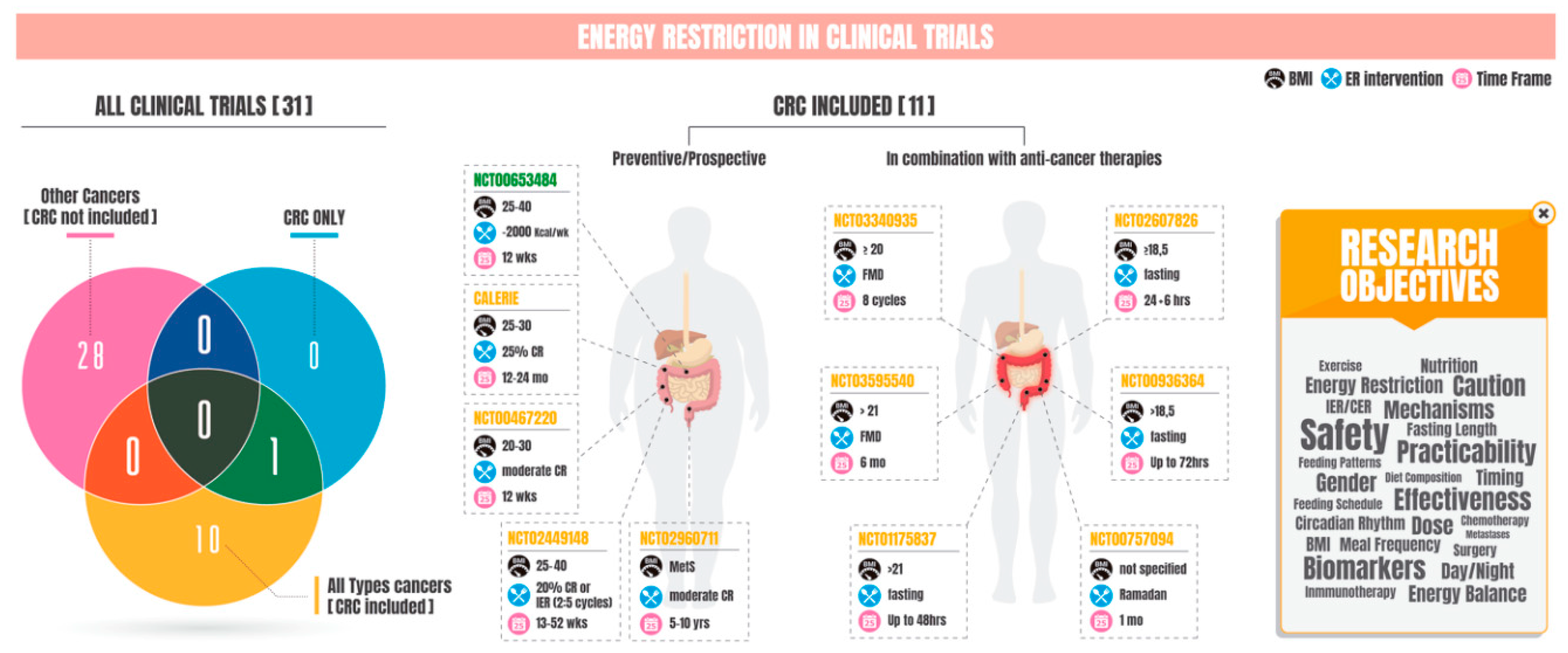
| Clinical Trial Identifier | Clinical Trial Title | Study Objective | Tumor Type | Energy Restriction Type | Primary Outcome | Time Frame | Beginning | Associated Publication |
|---|---|---|---|---|---|---|---|---|
| NCT01535911 | Pilot Study of a Metabolic Nutritional Therapy for the Management of Primary Brain Tumors | In combination with anti-cancer therapy | Glioblastoma | Energy-restricted ketogenic diet (ERKD) (metabolic nutritional therapy). Total calories consumed by each subject will be targeted to 20 to 25 kcal/kg/day. If the tumor has decreased in size or the size has remained the same then subjects will be continued on the ERKD for an additional 6 weeks and a repeat MRI will be obtained. | MRI imaging will be used to measure changes in brain tumor size. (Time Frame: 6 weeks after completion of radiation therapy). Results of the metabolic therapy will be assessed by comparing MRI images obtained at the beginning of the study with those after completion of radiation therapy and after an additional 6 weeks of metabolic therapy. | 6 years | 2012 | doi:10.1186/s40170-015-0129-1 |
| NCT01819233 | A Feasibility Pilot Trial Evaluating Caloric Restriction for Oncology Research in Early-Stage Breast Cancer Patients | In combination with anti-cancer therapy | Stage 0–I breast cancer | Beginning 2–4 weeks after completion of lumpectomy, patients receive food diaries to complete for 7–10 days. Dietary counselors then give patients guidelines for dietary modifications to reduce caloric intake by 25% of their normal diet. Patients follow calorie-restricted diet for 10 weeks (2 weeks prior to radiation therapy, during 6 weeks of radiation therapy, and at least 2 weeks after radiation therapy). Patients undergo radiation therapy QD 5 days a week for 6 weeks. | Proportion of patients who are adherent to the diet restriction. (Time frame: up to week 12). | 4 years | 2013 | |
| NCT03625635 | Effect of a Clinical Nutrition Intervention Program on Body Composition, Metabolism, and Antioxidant Activity Associated With Micronutrients in Breast Cancer Patients During Antineoplastic Treatment | In combination with anti-cancer therapy | Breast cancer | Diet plans and recommendations will be based on the individual’s nutritional status, symptoms, and treatment side-effects; socioeconomic and cultural preferences; as well as the WCRF/AICR guidelines adapting 1.5 g/kg/d of dietary protein, and when required, a caloric restriction (500–1000 kcal/d). Garlic and cruciferous vegetables will be encouraged, as well as 5–9 servings of fruits and vegetables a day. The program will be based on the macronutrient meal equivalent menu method, and standard food servings will be based on the Mexican Food Equivalent System. Breast cancer patients follow-up will be every 2 weeks and a different diet menu will be provided in each session by a specialized dietitian, until 6 months treatment is completed. | Total body weight (time frame: baseline and after the 6 month food-based intervention). | 3 years | 2015 | |
| NCT02983279 | Caloric Restriction Before Surgery in Treating Patients With Endometrial, Prostate, or Breast Cancer: | In combination with anti-cancer therapy | Breast, endometrial, or prostate carcinomas | Dietary counseling, caloric restriction diet. Patients then undergo 25% caloric intake for 3–12 weeks prior to definitive cancer surgery. Patients then undergo 25% caloric intake for 3–12 weeks prior to definitive cancer surgery. | Change in miR-21 expression assessed in serum (time frame: baseline up to 12 weeks). | 5 years | 2016 | |
| NCT03340935 | Safety, Feasibility, and Metabolic Effects of the Fasting-Mimicking Diet (FMD) in Cancer Patients | In combination with anti-cancer therapy | Any malignant neoplasm | Fasting-mimicking diet (or FMD) consisting of a 5-day plant-based, low-calorie (600 Kcal on day 1, followed by 300 KCal/day on days 2 to 5), low-protein, low-carbohydrate diet. | Safety of the fasting-mimicking diet (FMD) in cancer patients. | 2 years | 2017 | |
| NCT00099151, NCT00427193, NCT00099099 | CALERIE: Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy | Preventive/prospective | - | Caloric restriction. Diet: patients will meet with the registered dietitian to discuss calorie, protein, and fluid needs. The dietitian will calculate calorie needs. Calorie needs will then be reduced to 30%. Protein needs will be estimated based on 0.8 g/kg | - | 4 years | 2002 | |
| NCT00653484 | Energy Balance Interventions for Colorectal Cancer Prevention | Preventive/prospective | Colorectal carcinoma-predisposed healthy overweight or mildly obese individuals | for 12-week energy balance interventions, comprising a physical activity intervention (+2000 kcal/week), a dietary energy restriction intervention (DER) (−2000 kcal/week), or a combined physical activity and DER intervention (+1000/−1000 kcal/week). | - Growth factors (i.e., fasting insulin, c-peptide, IGF-1, IGFBPs, and leptin). - Circulating indicators of inflammation (i.e., c-reactive protein) and oxidative stress (i.e., isoprostanes). | 1 year | 2008 | |
| NCT00757094 | Safety and Feasibility of Fasting While Receiving Chemotherapy | In combination with anti-cancer therapy | Malignant neoplasm | Patients planning to observe fasting while receiving chemotherapy during the month of Ramadan. | Safety of fasting while receiving chemotherapy (time frame: two months). | 2 months | 2008 | |
| NCT00936364 | Short-Term Fasting Prior To Platinum-based Chemotherapy: Feasibility and Impact on Toxicity | In combination with anti-cancer therapy | Histologically confirmed malignancy for which platinum-based chemotherapy on a 21 day cycle or 14 day cycle is being recommended. | Stage I: Patients are assigned to 1 of 4 treatment groups. Group I: Patients fast for 24 h on day −1. - Group II: Patients fast for 48 h on days −2 and −1. - Group III: Patients fast for 72 h on days −3, −2, and −1. - Group IV: Patients undergo a modified 48-h fast with minimal caloric intake on days −2 and −1. Stage II: Patients are randomized to 1 of 2 treatment arms. - Arm I: Patients fast for 72 h on days −2 and on day 1. - Arm II: Patients proceed to chemotherapy without fasting. | Identification of the longest duration of fasting that is safe (time frame: up to 5 years). | 11 years | 2009 | |
| NCT01304251 | Effects of Short-term Fasting on Tolerance to Adjuvant Chemotherapy in Breast Cancer Patients | In combination with anti-cancer therapy | Breast cancer patients | - Short-term fasting (i.e., 24 h before and 24 h after administration of chemotherapy). - Control: 20 breast cancer patients eat according to the current guidelines for healthy nutrition, from 24 h before until 24 h after the beginning of administration of chemotherapy. | Chemotherapy-induced neutropenia (time frame: approximately 126 days). | 5 years | 2011 | - Safdiet et al., aging (Albany NY, 2009) - de Groot et al. doi:10.1186/s12885-015-1663-5. |
| NCT01559194 | A Randomized Comparison of a Low-Fat or Low-Carbohydrate Dietary Pattern for Weight Loss and Impact on Biomarkers Associated With Breast Cancer Risk in Overweight and Obese Premenopausal Women: Lifestyle Eating and Fitness | Preventive/prospective | Breast cancer | - Active comparator: Low-fat diet + exercise. Subjects were educated about a low-fat diet plus exercise and then followed for weight loss. They were also asked to monitor their physical activity by wearing a pedometer and recording the total steps walked every day. Intervention: Behavioral: Low-fat diet plus exercise. - Active Comparator: Low-carbohydrate diet + exercise. Subjects were educated about a low-carbohydrate diet plus exercise and then followed this for weight loss. They were also asked to monitor their physical activity by wearing a pedometer and recording the total steps walked every day. Intervention: Behavioral: Low-carbohydrate diet + exercise. | Number of women who lose weight when following 1 of 2 different calorie-restricted diets (time frame: 18 months). | 2 years | 2012 | doi:10.1089/jwh.2013.4638 |
| NCT01511276 | The Effects of Equivalent Weight Loss With or Without Exercise Training on Breast Cancer Risk Biomarkers in Postmenopausal Women: The SHAPE-2 Study | Preventive/prospective | Breast cancer | - Energy-restricted diet according to the national guidelines for healthy nutrition, creating a mean energy deficit of 500 kCal/day. They are asked to keep their habitual sedentary lifestyle. The aim of this group is to lose 5–6 kg of body weight in 14 weeks. - Mainly exercise-induced weight loss. Exercise program consists of 2 h fitness per week, containing endurance and resistance training, as well as 2 h of Nordic walking. Equivalent to an energy expenditure of 350 kCal/day. Along with the exercise program, participants will follow an energy-restricted diet according to the national guidelines of healthy nutrition creating an extra energy deficit of 250 kCal/day. | Serum sex hormone levels (time frame: 21 weeks): estradiol (total, free), estrone, testosterone, sex-hormone-binding globulin. | 5 years | 2012 | doi:10.1186/1471-2407-13-395 |
| NCT01699906 | Diet-Induced Weight Loss Reduces Inflammation and Crown-like Structures and Corrects Immune Dysfunction in Subcutaneous Adipose Tissue In Class 2–3 Obese Women: A Pilot Study | Preventive/prospective | Breast cancer | This study will include nutritional and medical evaluation, a 3 day inpatient hospital stay eating a diet providing 50% of what they were taking before starting the study, and then a nutritionally adequate diet that will allow them to lose about 10% of their initial weight within a 7- to 10-week period. They will have about 4–5 g of fat removed by suction through a syringe and a biopsy of the skin in addition to studies of blood and stool samples. | Adipose tissue inflammation via crown-like structures (time frame: 9 weeks). Diet-induced weight loss of 10% body weight will result in reduction in abdominal subcutaneous fat inflammation, as measured by reduction in adipocyte size determined by microscopy and of CLS number in adipose tissue. Reduction in inflammatory gene expression determined by PCR and selected cytokine protein levels. Increased anti-inflammatory lymphocytes determined by immunohistochemistry or by flowcytometry. | 2 years | 2012 | doi:10.1186/s12967-018-1619-z |
| NCT01754350 | Calorie-restricted, Ketogenic Diet. and Transient Fasting versus Standard Nutrition During Reirradiation for Patients With Recurrent Glioblastoma: The ERGO2 Study | In combination with anti-cancer therapy | Recurrent glioblastoma | Calorie-restricted ketogenic diet and transient fasting. On days 1–3 and days 7–9, restriction of carbohydrates to <60 g and of calories to 21–23 kcal/kg per day; on days 4–6, fasting. On days 1–3 and 7–9, restriction of carbohydrates can be supported by the use of drinks provided by “Tavarlin”. | Progression-free-survival (time frame: 6 months). | 4 years | 2013 | |
| NCT01954836 | Short-Term Fasting During Chemotherapy in Patients With Gynecological Cancer: A Randomized, Controlled Cross-over Trial (FIT) | In combination with anti-cancer therapy | Ovarian or breast cancer | Modified fasting with daily caloric intake of <400 kcal by juices starting 36 to 48 h before beginning chemotherapy, and lasting to 24 h after end of chemotherapy, applied in the first half of scheduled 4 or 6 chemotherapy cycles. | Quality of life, modified FACT-O (time frame: 24 h and 7 days after chemotherapy cycle). | 3 years | 2013 | Bauersfeld et al. doi:10.1186/s12885-018-4353-2. |
| NCT01886677 | Exploring the Impact of Negative Energy Balance in Men With Prostate Cancer | In combination with anti-cancer therapy | Prostate cancer | Both arms will receive the same intervention: a healthful diet plus exercise intervention to promote a weight loss of up to 2 pounds/week. The only difference is the timing of the delivery of the intervention (immediate vs. delayed). | Changes in tumor proliferation rate (Ki-67) over the presurgical study period (minimum of 3.5 weeks, up to 24 weeks) will be explored and compared between the intervention and wait list control arms. | 1 year | 2013 | doi:10.1186/s12885-016-2075-x |
| NCT02126449 | Dietary Restriction as an Adjunct to Neoadjuvant Chemotherapy for HER2-Negative Breast Cancer (DIRECT) | In combination with anti-cancer therapy | Breast cancer patients | FMD | - The percentage of patients with grade III/IV toxicity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03. (time frame: 2 years). - The percentage of pCR. (time frame: 4 years). | 4 years | 2014 | |
| NCT02224807 | Exploring Effects of Weight Loss on Ductal Carcinoma In Situ | In combination with anti-cancer therapy | Breast cancer patients | - Active comparator: Progressive resistance training (PRT) and a healthy diet. PRT will be done with resistance bands; participants will receive instruction on three resistance band exercises (triceps, biceps, and shoulder overhead) from an American College of Sports Medicine (ACSM) certified exercise specialist. Participants will also receive dietary counseling from a registered dietitian on correcting nutrient deficiencies that are detected during analysis of their 2-day dietary recalls. - Experimental: PRT and a healthy diet plus weight loss. This arm will receive all components of the active comparator arm, plus counseling to achieve a weight loss of 1.5–2 pounds/week. Participants will be trained on how to achieve this caloric deficit through both dietary restriction and increased physical activity. Weight loss will be promoted via a healthy, nutritionally adequate diet consistent with American Cancer Society guidelines. Protein levels will be based on 0.8 g/kg body weight. The distribution of food groups will be customized for preferences. An exercise program will be tailored taking into account kcal expenditure for various activities at a specific body weight; expenditures of 200–400 kcal/day will serve as a goal. Aerobic training of large muscles (legs) will be emphasized to achieve a greater kcal deficit; ramping of intensity and volume over time will be pursued as per the ACSM guidelines. Participants will train once weekly while supervised by an exercise physiologist and daily at home. Intervention: Behavioral and experimental: PRT and a healthy diet, plus weight loss. | Tumor proliferation (time frame: baseline to time of surgery): Ki67. Weight (time frame: baseline to time of surgery). Feasibility (time frame: baseline to time of surgery): enroll 40 subjects in 2-year study, retain >80% of the sample and complete > 70% of contact sessions. | 4 years | 2014 | doi:10.1016/j.jand.2018.08.164 |
| NCT02449148 | Healthy Nutrition and Energy Restriction as Cancer Prevention Strategies: A Randomized, Controlled Intervention Trial | Preventive/prospective | - | Three arms: - 2 days per week fasting with 25% energy intake and 5 days per week at 100% energy intake. - Daily energy intake of 80%. - No intervention: general advice on healthy nutrition. | Changes in gene expression in subcutaneous adipose tissue measured by whole genome sequencing (time frame: assessments at baseline (week 0), and after the intervention phase (week 13)). | 2 years | 2015 | doi:10.1093/ajcn/nqy196 |
| NCT02940470 | Weight Loss Pilot Study in Postmenopausal Breast Cancer Survivors | Preventive/prospective | Breast cancer survivors | - Calorie-restricted diet plus exercise. Daily meals plus exercise providing 1000 kcal restriction per day for 12 weeks. | - Change in body weight (time frame: 0, 6, 12, 18 weeks). - The primary objective of this pilot study is to determine the effect of weight loss on a wide range of biomarkers associated with risk of breast cancer recurrence in overweight and obese breast cancer survivors. We hypothesized that weight loss would result in a statistically significant improvement in biomarkers associated with risk of breast cancer recurrence. | 2 years | 2016 | doi:10.1186/s40814-017-0160-9 |
| NCT01175837 | Short-Term Fasting Prior to Systemic Chemotherapy: A Pilot Feasibility Study | In combination with anti-cancer therapy | Malignant neoplasm | Cohort I: Patients fast 24 h before day 1 of course 2 of chemotherapy. If fast is well tolerated, patients may escalate fasting by 12 h for each subsequent course of chemotherapy for up to 3 courses in the absence of unacceptable toxicity. Cohort II: Patients fast at the longest fasting regimen found to be safe and tolerable in cohort I before day 1 of each course of chemotherapy for up to 4 courses in the absence of unacceptable toxicity. | - Number of patients hospitalized during fasting period (for reasons that are not attributed to disease or postoperative complications) (time frame: up to 48 h). - Number of patients experiencing greater than or equal to grade 3 adverse event related to the fasting period (time frame: up to 48 h). - Percentage of patients able to achieve designated fasting regimen (i.e., greater than or equal to 50%) (time frame: up to 48 h). | 3 years | 2010 | |
| NCT02286167 | The Feasibility and Biologic Effect of a Modified Atkins-based Intermittent Fasting Diet in Patients With Glioblastoma (GBM) | In combination with anti-cancer therapy | Glioblastoma multiforme | Modified Atkins diet | Percent of patients able to remain on the diet and achieve nutritional goals as defined by cumulative assessment of diet records collected at weeks 4, 6, and 8 with a 60% completion defined as a positive results | 5 years | 2014 | |
| NCT03795493 | Diet Restriction and Exercise-Induced Adaptations in Metastatic Breast Cancer | In combination with anti-cancer therapy | Breast cancer patients, Stage IV or metastatic | - Short-term diet and exercise intervention. Participants assigned to the intervention group will perform both the diet and acute exercise interventions. The interventions will be applied prior to up to six chemotherapy treatments of a consistent protocol. The total number of treatments of a given protocol received prior to treatment conclusion is dependent on patient condition and oncologic care preferences. | Tumor size (time frame: 0–6 weeks before the first chemotherapy treatment and 1–4 weeks after the last chemotherapy treatment). | 3 years | 2018 | |
| NCT03813381 | The Impact of a Moderate Calorie and Protein Restriction Program (CARE-PRO) as an Efficient and Affordable Therapeutic Strategy in Patients With Barrett’s Esophagus | Preventive/prospective | Barret’s esophagus | Calorie restriction will be up to 600 kcal below patients’ energy requirements and the amount of protein will be 0.8 g of protein/Kg body weight, mostly form plant-origin food. | Body weight change (time frame: baseline and after 24 months). A 7% weight loss. | 1 year | 2019 | |
| NCT02035631 | Prevention of Breast Cancer Recurrence Through Weight Control, Diet, and Physical Activity Intervention | Preventive/prospective | Recurrent breast cancer I, II, IIIA (or T1-3, N0–N2, M0) | - Behavioral: Diet. The dietary component, aimed to reduce calorie intake according to individual requirements, will be structured in 1 h weekly sessions led by trained nutritionists. Sessions will concentrate on teaching participants about food groups, the food pyramid, and the Mediterranean diet, as well as how to choose, prepare, and cook hypo-caloric meals. - Behavioral: Physical activity. The physical activity component will include two sessions per week led by trained physical activity monitors, including aerobic exercise of high/moderate intensity and instruction about the at-home exercise activities (3 more sessions). | Time between recruitment date and local and distant recurrence date or end of the 5-year follow-up (whichever occurs first). | 8 years | 2014 | |
| NCT02792270 | Effects Of Caloric Restriction On Post-Operative Complications In Sarcoma Patients Treated With Pre-Operative Radiation Therapy | In combination with anti-cancer therapy | Sarcoma | Caloric restriction diet: patients will meet with the registered dietitian to discuss calorie, protein, and fluid needs. The dietitian will calculate calorie needs. Calorie needs will then be reduced to 30%. Protein needs will be estimated based on 0.8 g/kg BW and then reduced by 70%. Dietitian will educate participants on electrolytes and fluid intake based on the reduced food intake. | - Change in the rate of physical function (time frame: baseline, 6 week, 3 month, and 6 month visits after surgery): Musculoskeletal Tumor Society rating scale (MSTS, a clinician-rated scale scoring). - Change in the rate of physical function (time frame: baseline, 6 weeks, 3 months, and 6 month visits after surgery): Toronto extremity salvage score (TESS, a patient-reported questionnaire scoring. | 3 years | 2016 | |
| NCT01802346 | A Randomized, Phase II Clinical Trial of a Controlled Diet Prior to Selected Chemotherapy Treatment in Breast and Prostate Cancer to Evaluate the Impact on Toxicity and Efficacy | In combination with anti-cancer therapy | Breast cancer; hormone-resistant or recurrent prostate cancer | Low-calorie diet: patients eat a special low-calorie diet during 3 days prior to chemotherapy, during the 12 weeks of chemotherapy, and 2 days after chemotherapy. Patients are provided with all meals and all food to be consumed and maintain a diary of the food consumed and appropriate amounts. | Rate of chemotherapy-related toxicity (time frame: up to 12 weeks). Occurrence of grade 2+ non-hematologic symptomatic toxicity (fatigue, nausea and vomiting, anorexia, neuropathy, mucositis, cystitis, stomatitis), evaluated according to Common Terminology Criteria for Adverse Events version 4.0. The two arms will be compared, in terms of the proportion of patients with the occurrence of one of these toxicities. | 7 years | 2013 | |
| NCT02710721 | Fasting and Nutritional Therapy in Patients With Advanced Metastatic Prostate Cancer | In combination with anti-cancer therapy | Prostatic neoplasms | Patients realize a 60 h modified fast (36 h before and 24 h after chemotherapy) with a dietary energy supply 350–400 kcal per day with fruit and vegetable juices, or if not feasible, an established fasting-mimicking diet of 600–800 kcal according to Longo et al. Between chemotherapy, a Mediterranean diet will be practiced with nutrition training individually and in small groups by trained nutritionists at the study center. Controls: Mediterranean diet. | FACT-P/Taxane/An sum score (time frame: assessment day 0 (baseline) and 7 days after each of 6 chemotherapies (study weeks 1,4,7,10,13,16)), summarized change of FACT score from baseline to day 8 after each chemotherapy session. | 3 years | 2016 | |
| NCT03162289 | Intermittent Fasting Accompanying Chemotherapy in Gynecological Cancers (FIT2) | In combination with anti-cancer therapy | Ovarian or breast cancer | - Fasting patients follow a modified fasting regime of 60–72 h (36–48 h before and 24 h after chemotherapy (CT) with a dietary energy supply of 350–400 kcal per day with vegetable juices during the first four cycles of CT. During the rest of the CT cycles, they will observe two days of caloric restriction (24 h before and after CT). Between CTs, a mainly vegetarian diet will be performed and the patients are encouraged to follow a pattern of time-restricted feeding with 14 h fasting overnight for at least six days a week. The patients will receive individual nutrition training by trained nutritionists. - Control patients follow a 60–72 h vegan diet with sugar restriction (36–48 h before and 24 h after CT) during the first four cycles of CT. During the rest of the CT cycles, they will observe two days of vegan- and sugar-restricted diet (24 h before and after CT). Between CTs, a mainly vegetarian diet will be performed. The patients will receive individual nutrition training by trained nutritionists. | FACT-G (time frame: date of inclusion (baseline), day −2 and +7 at each chemotherapy (CT) in triweekly cycles/−2 days at each CT in weekly cycles; and +7 after the last weekly CT, 4 months after inclusion, 3 weeks after end of CT, and 1, 2, and 3 years after inclusion). | 4 years | 2017 | |
| NCT03131024 | The Effects of Short-term Exercise or Caloric Restriction on Anthracycline Chemotherapy-Related Treatment Toxicity | In combination with anti-cancer therapy | Breast cancer patients, stage I–III | - Dietary supplement: 50% caloric restriction. Meals mimicking participant dietary preferences and matching North American macronutrient guidelines will be provided, consisting of 50% of total caloric intake for 48 h. - Other: Aerobic exercise. The supervised exercise session will consist of a 10 min warm-up, 30 min performed at 70–75% of heart rate reserve, which corresponds to a vigorous intensity, followed by a 5 min cool down. | Change in left ventricular ejection fraction reserve (peak exercise—rest) (time frame: 3–14 days before first anthracycline treatment, 2–3 weeks after completion of anthracycline treatment, one year after initiation of anthracycline treatment). | 3 years | 2017 | |
| NCT03160599 | Restricted Calorie Ketogenic Diet as a Treatment in Glioblastoma Multiforme: A Clinical Study | In combination with anti-cancer therapy | Glioblastoma multiforme | Ketogenic diet will consist of 4:1–1:1 fat/protein + carbohydrate. Carbohydrate is limited to 10–30 g/day. The diet will be supplemented with vitamins, calcium, phosphorus, zinc, and selenium supplements to meet the requirements of U.S. Dietary Reference Intakes (DRI) standard. The basis of dietary design is 70–85% of individual’s total calories. The total calorie intake is based on patient’s activity level and their basal metabolism values, which is obtained from indirect calorimetry or Harris–Benedict formula. | Adverse events of patients on high-fat diet (time frame: 2 years). | 2 years | 2017 | |
| NCT03700437 | Randomized, Controlled Pilot Study to Evaluate Fasting-Mimicking Diet in Patients Receiving Chemo-immunotherapy for Treatment of Metastatic Non-Small Cell Lung Cancer | In combination with anti-cancer therapy | Non-Small Cell Lung Cancer | Chemolieve®, a plant-based FMD that provides ~300 calories/fasting day and includes all the food to be consumed during the dietary intervention, including supplements. Subjects will start the diet 3 days prior to chemo-immunotherapy and continue on the first day of chemo-immunotherapy for the first 4 cycles of therapy. | To determine the effect of fasting-mimicking diet (FMD) on circulating tumor cells (CTCs) in patients with advanced NSCLC receiving chemo-immunotherapy. | 2018 | ||
| NCT03595540 | Phase II Clinical Study of a Fasting-Mimicking Diet in Patients Undergoing Oncologic Treatment | In combination with anti-cancer therapy | Breast and colorectal cancer | Prolon FMD | - Percentage of prescribed diet consumed and intake of any extra food (time frame: 6 months). - Quantification of FMD-emergent adverse events (time frame: 6 months), according to NCI CTCAE 5.0. | 2 years | 2018 | |
| NCT02379585 | A Pilot Study of Short-Term Fasting on Neoadjuvant Chemotherapy in Patients With Newly Diagnosed Breast Cancer (STEFNE Study) | In combination with anti-cancer therapy | HER2-positive breast cancer | Patients will fast 24 h before and 24 h after the administration of chemotherapy. | Pathological response rate at the time of surgery or at the time of biopsy (time frame: 4–6 cycles (up to 12 weeks)). | 2 years | 2015 | |
| NCT00467220 | Effect of Daily Calorie Restriction or Alternate-Day Reductions in Calorie Intake on Risk for Cardiovascular Disease and Cancer | Preventive/prospective | - | - Alternate day fasting arm: Subjects in this arm will be asked to alternate between one day of eating as they wish versus one day on a calorie-restricted meal plan. Subjects will follow this alternating meal plan for 3 months. - Calorie restriction: subjects in this arm will be asked to follow a calorie-restricted meal plan, daily, for three months. | Adipose tissue dynamics (triglyceride turnover, lipolysis, de novo lipogenesis, adipose cell proliferation), adipose tissue morphology (cell size and number), adipose tissue hormone levels (adiponectin, leptin), skin turnover (keratin dynamics), T-lymphocyte proliferation, as well as plasma lipid and lipoprotein, homocysteine, and C-reactive protein levels. | 10 years | 2007 | |
| NCT02607826 | Short-Term Starvation vs. Normal Diet Before Chemotherapy of Solid Tumors | In combination with anti-cancer therapy | Cholangiocarcinoma pancreatic ductal adenocarcinoma Colorectal cancer Gastric cancer Adenocarcinoma of the esophagogastreal Junction esophagus cancer | Short-term starvation for a timeframe beginning 24 h prior to chemotherapy administration, lasting until 6 h after administration. | Primary endpoint of this study is to assess the improvement in response to therapy for patients undergoing short-term starvation before chemotherapy of solid tumors in comparison to patients without dietary restrictions. Response to therapy on MRI or CT scans will be measured using the RECIST criteria version 1.1. | 4 years | 2015 | |
| NCT02960711 | Randomized, Controlled Trial of Metformin and Dietary Restriction to Prevent Age-Related Morbid Events in People With Metabolic Syndrome | Preventive/prospective | Any malignant neoplasm | - Experimental: Metformin (1700 mg/day) + Lifestyle Metformin: 2 tablets per day, one at breakfast (or lunch) and one at dinner, of either metformin (two 850 mg tablets/day) + participation in the lifestyle intervention activities. Intervention: Drug: Metformin hydrochloride 850 mg oral tablet (Glucophage). Placebo comparator: placebo + lifestyle. Placebo: (two identical tablets) according to the blind assignment + participation in the lifestyle intervention activities. Intervention: Drug: Ludipress, magnesium stearate, micronized hydrated silica, talcum. - Experimental: Metformin (1700 mg/day) alone. Metformin: 2 tablets per day, one at breakfast (or lunch) and one at dinner, of metformin (two 850 mg tablets/day). Intervention: Drug: Metformin hydrochloride 850 mg oral tablet (glucophage). Placebo comparator: placebo alone. Placebo: (two identical tablets) according to the blind assignment. Intervention: Drug: Ludipress, magnesium stearate, micronized hydrated silica, talcum. | Total incidence of age-related chronic diseases (time frame: 5 years). Records for all age-related chronic diseases, but first concentrate the analysis on cancer, coronary heart disease, stroke, and diabetes. | 2 years | 2016 | doi:10.5301/tj.5000599 |
| NCT01784042 | Effect of Dietary Energy Restriction and Omega-3 Fatty Acids on Mammary Tissue and Systemic Biomarkers of Breast Cancer Risk | Preventive/prospective | Breast cancer patients | Lovaza (omega-3-acid ethyl esters) +/– dietary energy restriction. | Ki67 expression by hyperplastic breast lesions. | 4 years | 2013 | |
| NCT00470119 | Effect of a Low-Calorie Diet and/or Exercise Program on Risk Factors for Developing Breast Cancer in Overweight or Obese Postmenopausal Women | Preventive/prospective | Breast cancer | - Caloric restriction. Nutritionist-delivered weight loss intervention though diet modification with an aim of 10% weight loss over a year-long intervention based on the DPP and LookAHEAD interventions. Participants meet with a nutritionist individually and in small groups. Participants receive general information about diet and behavior strategies, such as self-monitoring, goal-setting, stimulus control, problem-solving, and relapse prevention training. Participants learn to set a calorie goal and a fat gram goal, and how to achieve the goal calorie reduction. Meetings are held weekly during the first 6 months of the diet program but taper off over the course of the study. - Exercise intervention participants exercise 3 days per week under the supervision of a physiologist and 2 days per week independently at home, for a total of 5 exercise sessions (at least 45 min of moderate-intensity exercise per session) weekly over 12 months. - Caloric restriction and exercise intervention. Combined caloric restriction and exercise intervention. | Serum estrone concentrations as measured by radioimmunoassay (time frame: at baseline and 12 months timepoint). | 5 years | 2007 | doi:10.1249/MSS.0000000000000480 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castejón, M.; Plaza, A.; Martinez-Romero, J.; Fernandez-Marcos, P.J.; de Cabo, R.; Diaz-Ruiz, A. Energy Restriction and Colorectal Cancer: A Call for Additional Research. Nutrients 2020, 12, 114. https://doi.org/10.3390/nu12010114
Castejón M, Plaza A, Martinez-Romero J, Fernandez-Marcos PJ, de Cabo R, Diaz-Ruiz A. Energy Restriction and Colorectal Cancer: A Call for Additional Research. Nutrients. 2020; 12(1):114. https://doi.org/10.3390/nu12010114
Chicago/Turabian StyleCastejón, Maria, Adrian Plaza, Jorge Martinez-Romero, Pablo Jose Fernandez-Marcos, Rafael de Cabo, and Alberto Diaz-Ruiz. 2020. "Energy Restriction and Colorectal Cancer: A Call for Additional Research" Nutrients 12, no. 1: 114. https://doi.org/10.3390/nu12010114
APA StyleCastejón, M., Plaza, A., Martinez-Romero, J., Fernandez-Marcos, P. J., de Cabo, R., & Diaz-Ruiz, A. (2020). Energy Restriction and Colorectal Cancer: A Call for Additional Research. Nutrients, 12(1), 114. https://doi.org/10.3390/nu12010114





