Neuroprotective Effects of Cornus officinalis on Stress-Induced Hippocampal Deficits in Rats and H2O2-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CC and FCC Extract
2.2. Loganin, Total Phenolic, and Flavonoid Contents Detection
2.3. Animal Housing
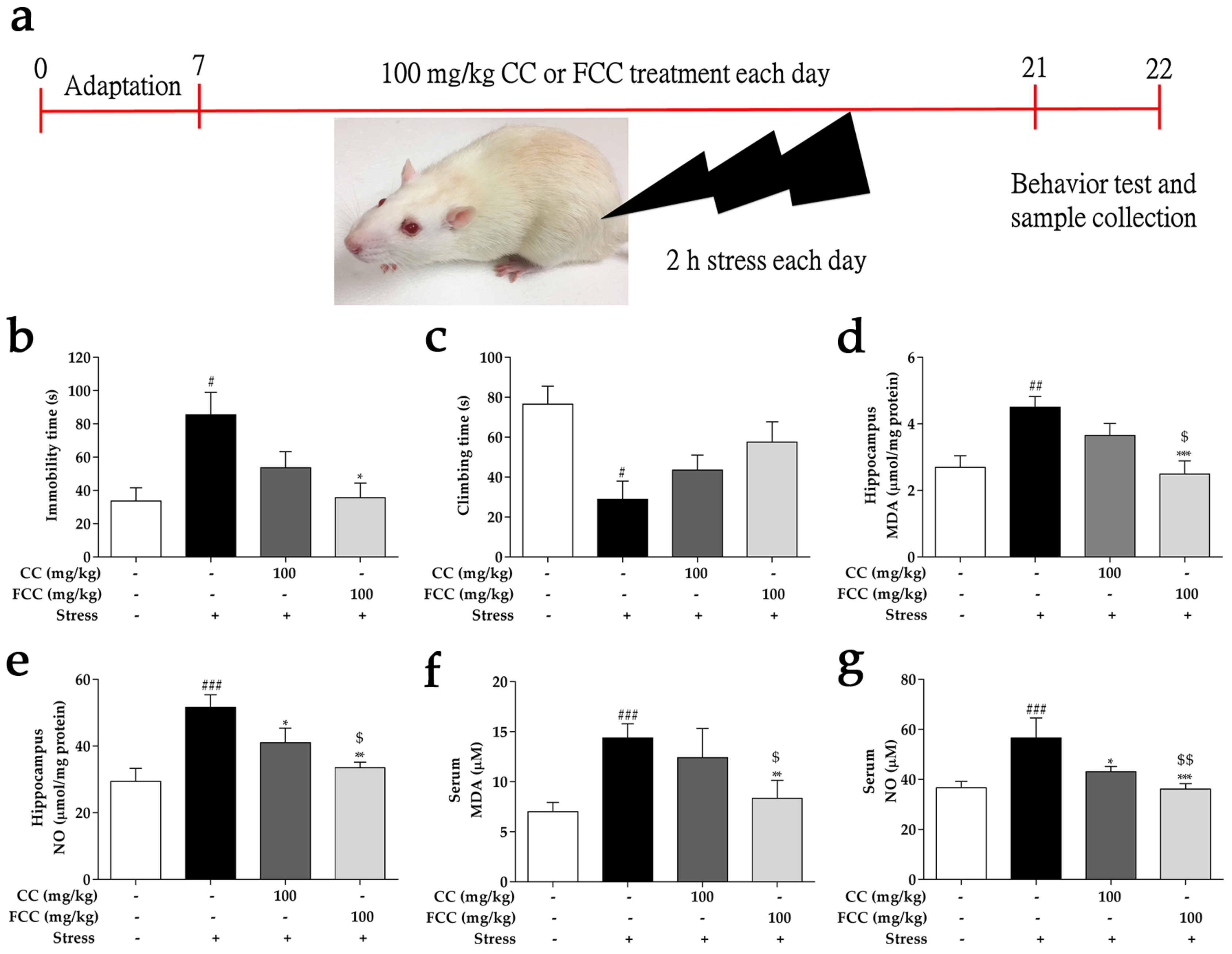
2.4. Experimental Design
2.5. Forced Swim Test (FST)
2.6. Oxidative Stress Markers Malondialdehyde (MDA) and Nitric Oxide (NO) Estimation in Hippocampus and Serum
2.7. Corticosterone, β-Endorphin, and Serotonin Evaluation
2.8. Western Blot
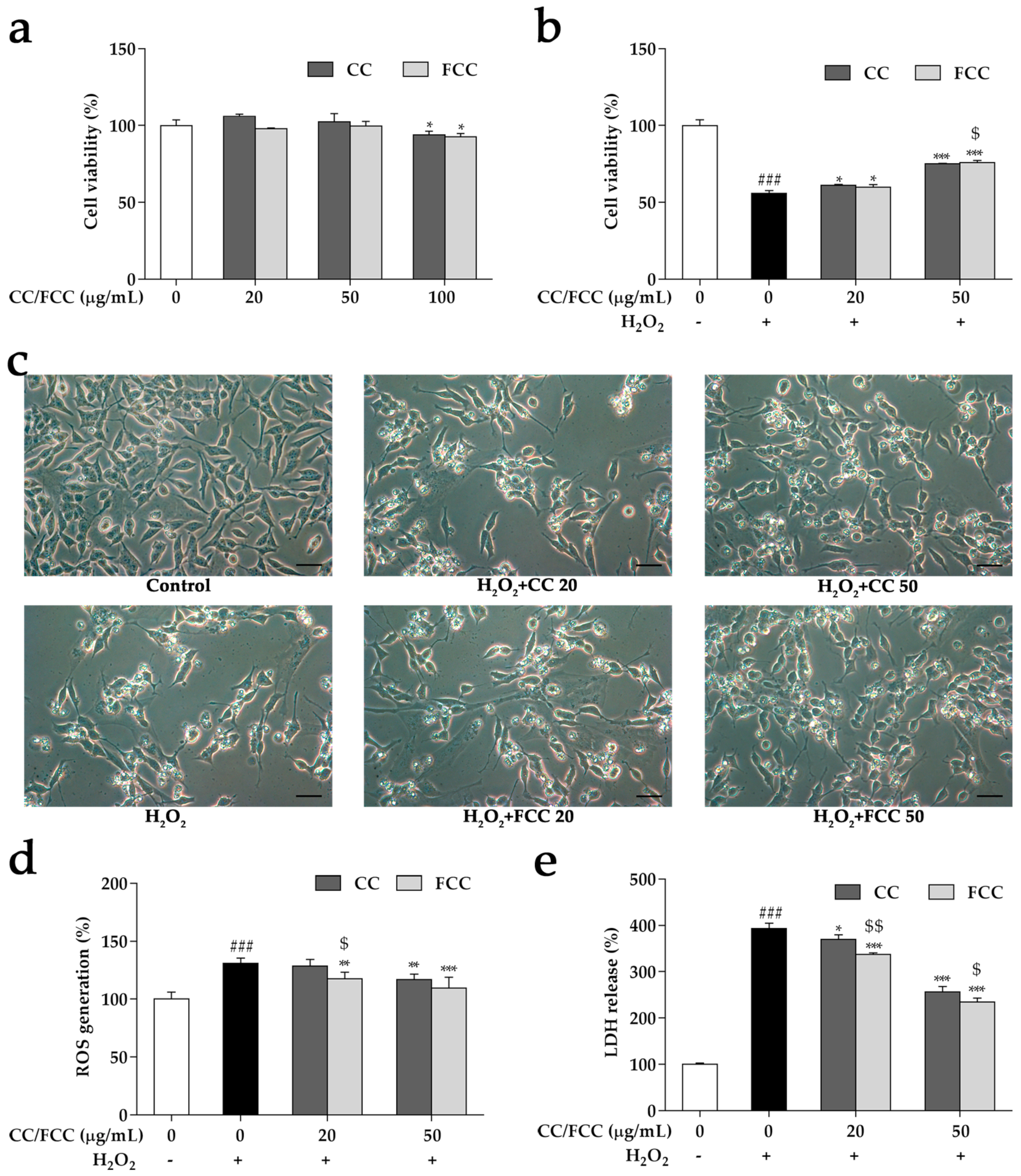
2.9. Cell Culture and MTT Assay
2.10. Estimation of ROS Generation in Cells
2.11. LDH Release Detection in Cells
2.12. RNA Extraction and Quantitative Real-Time PCR (qPCR)
2.13. Statistical Analysis
3. Results
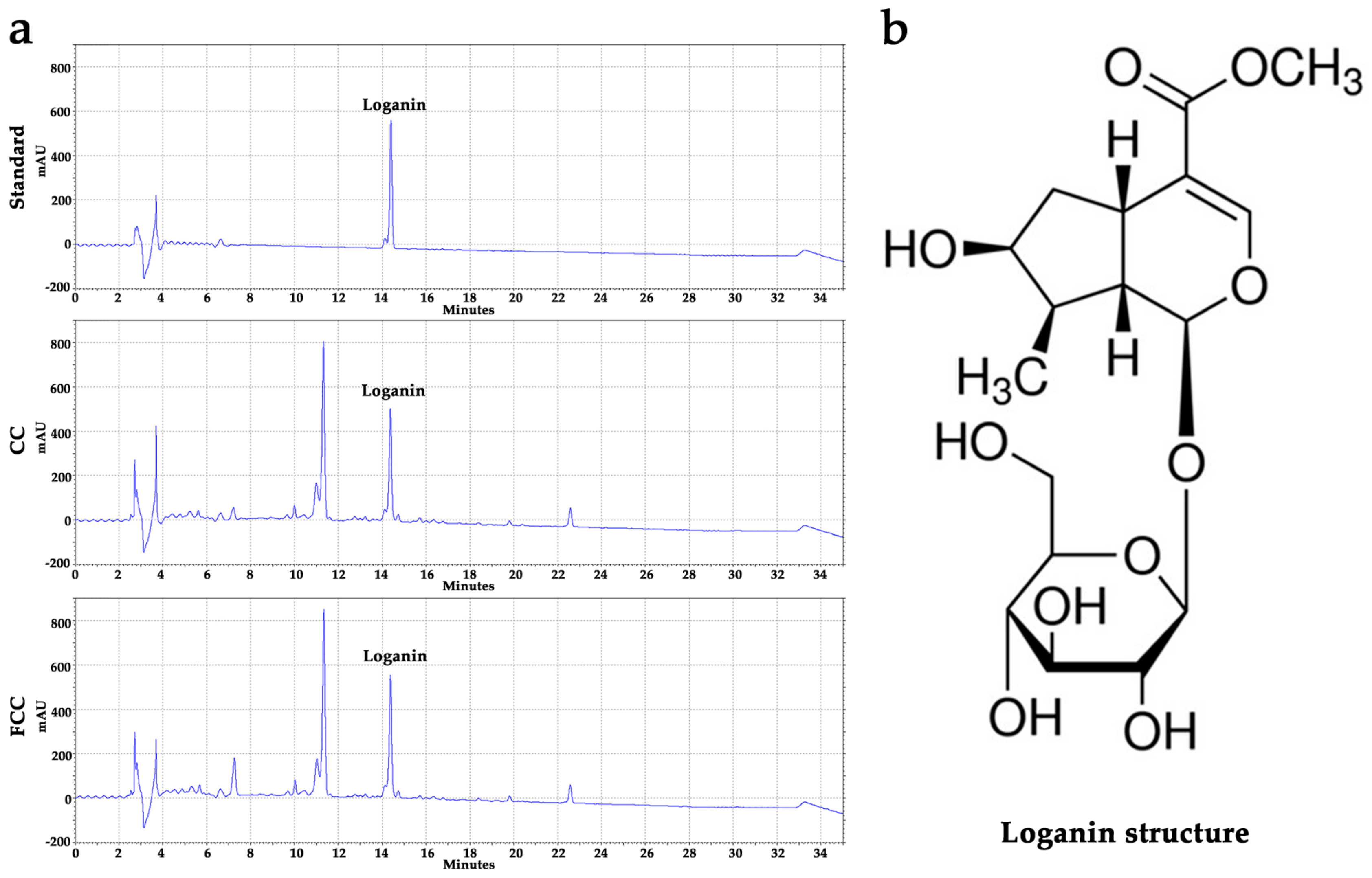
3.1. Evaluation of Active Compounds and the Contents in CC and FCC Extract
3.2. CC and FCC Improved Depression-Like Behavior in Rats
3.3. CC and FCC Attenuated Oxidative Stress in Rats
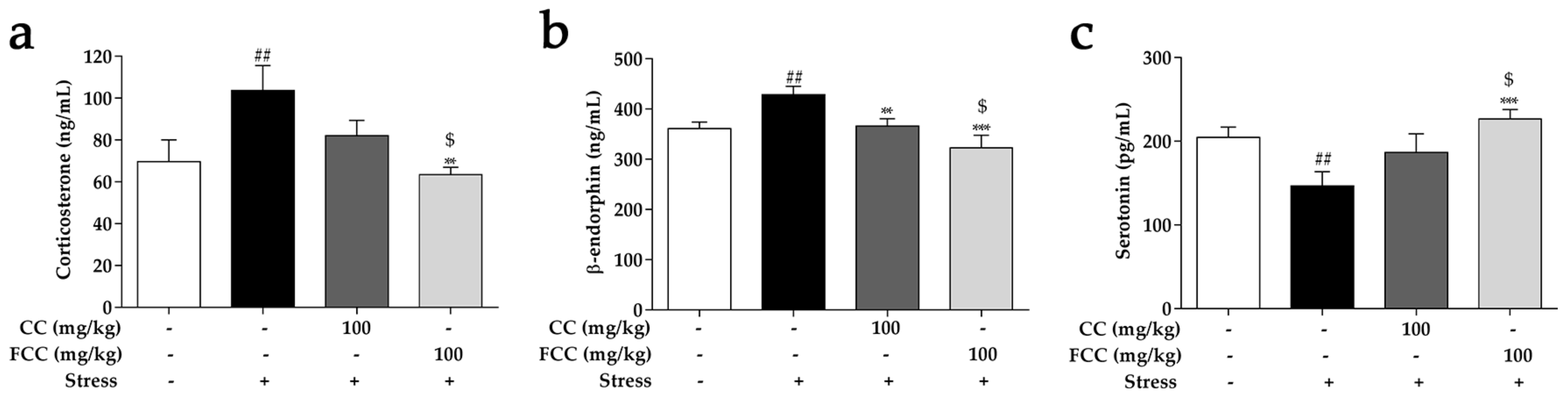
3.4. CC and FCC Regulated Stress-Related Hormones in Stress-Induced Hippocampal Deficits in Rats
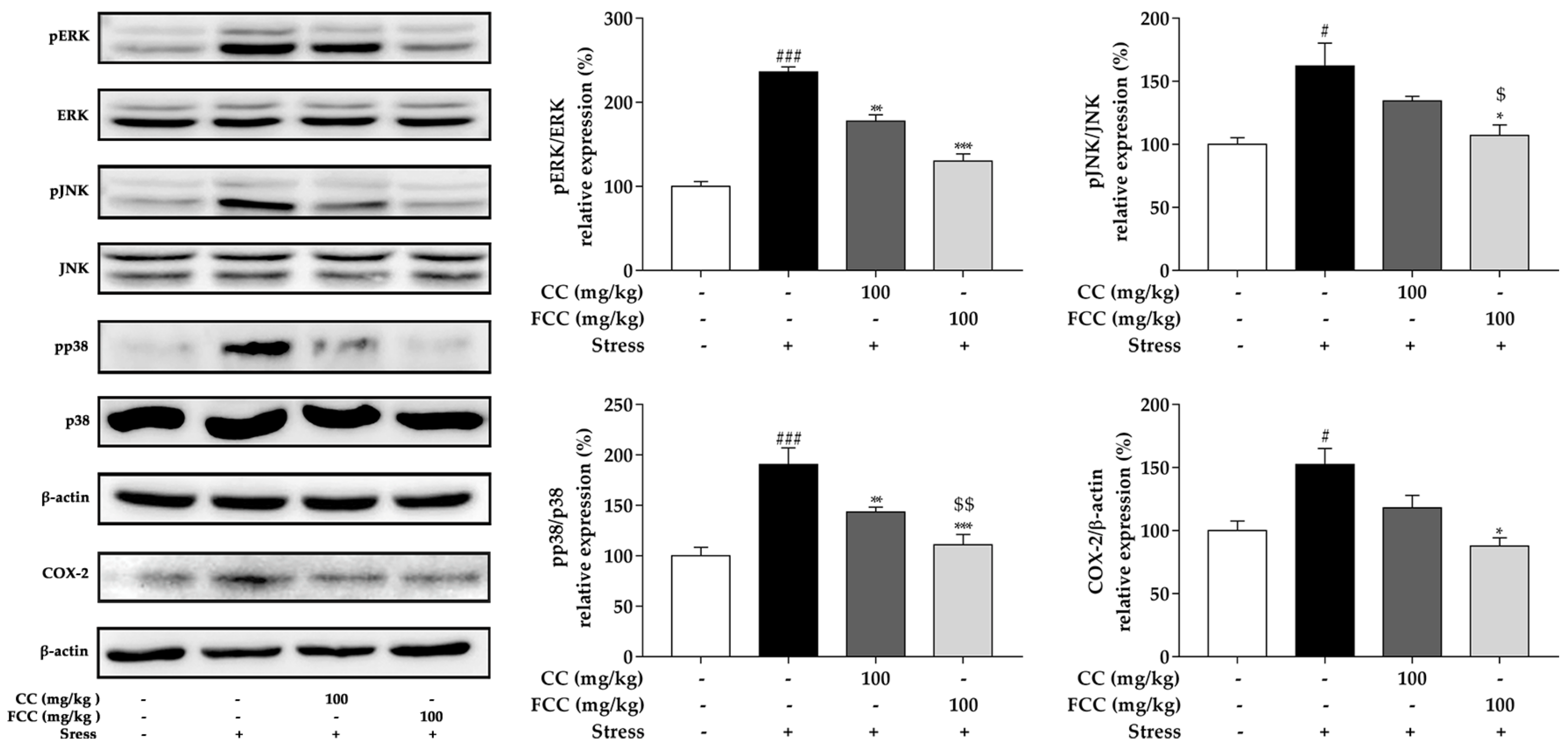
3.5. CC and FCC Blocked the MAPK/COX-2 Signaling Pathways in Rat Hippocampus
3.6. CC and FCC Alleviated H2O2-Induced Apoptosis in Cells
3.7. CC and FCC Attenuated H2O2-Induced ROS Generation and LDH Release in Cells
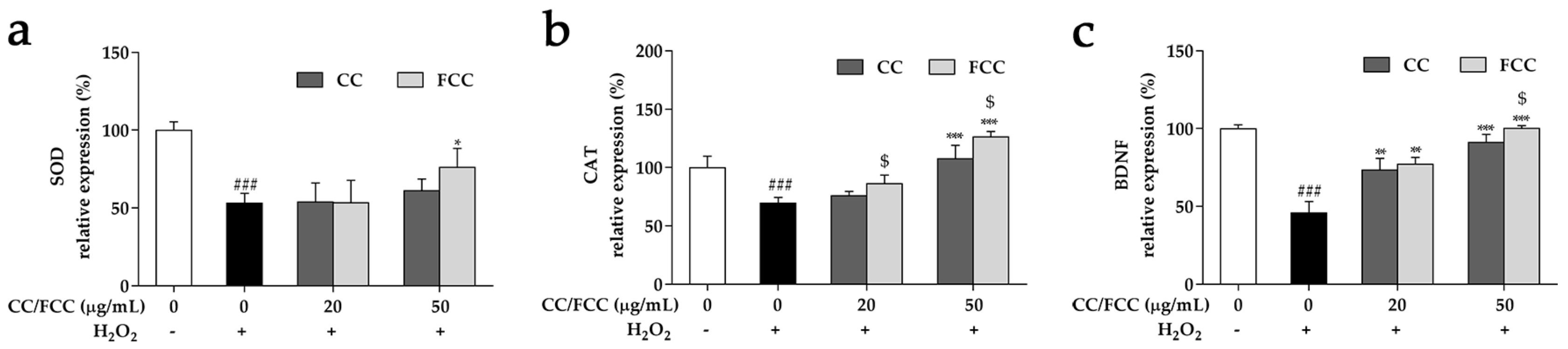
3.8. CC and FCC Enhanced the Antioxidant Enzymes and Neuronal Stress Biomarkers in Cells
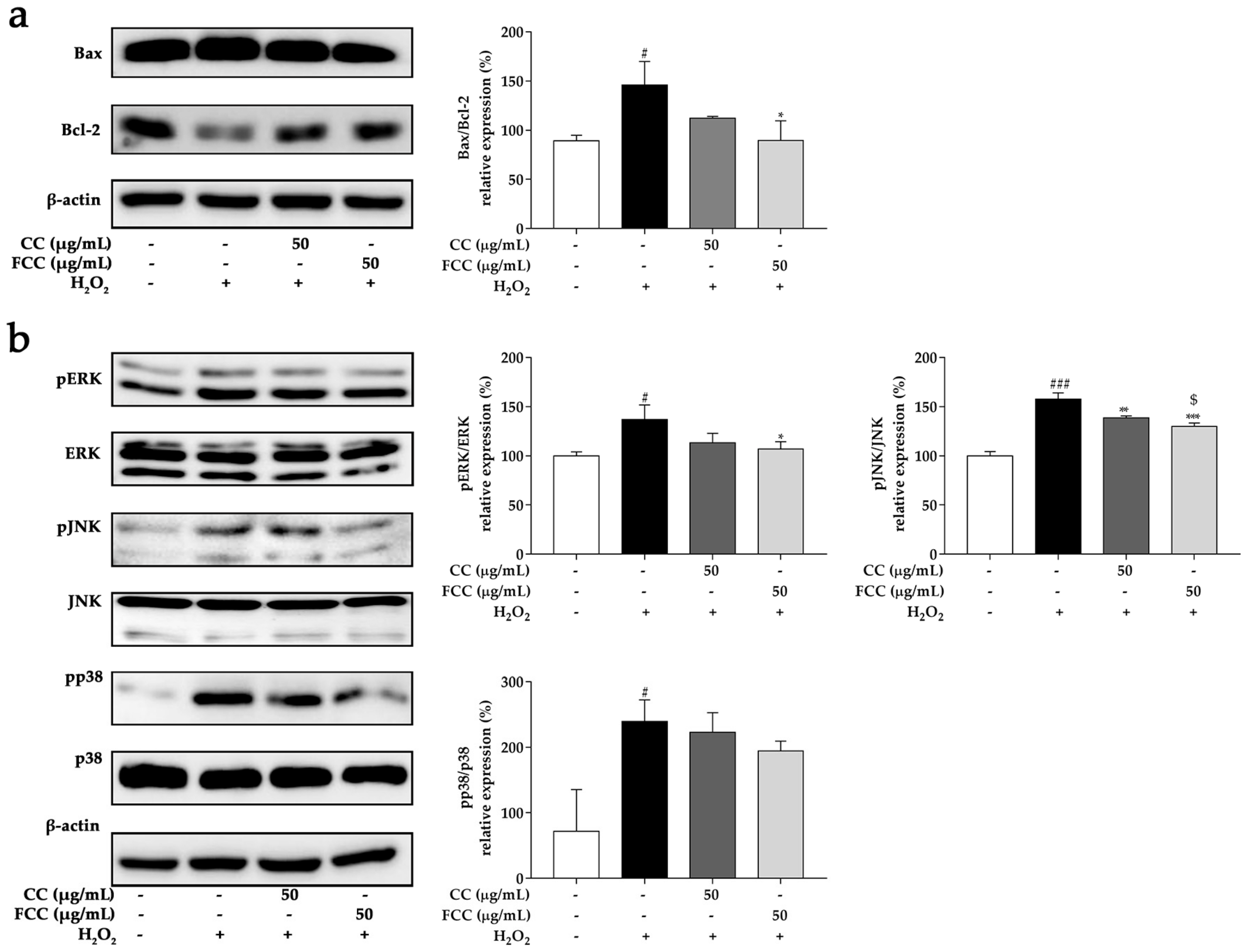
3.9. CC and FCC Inhibited the H2O2-Induced Apoptosis in Cells
3.10. CC and FCC Suppressed MAPK Signaling Pathways in Cells
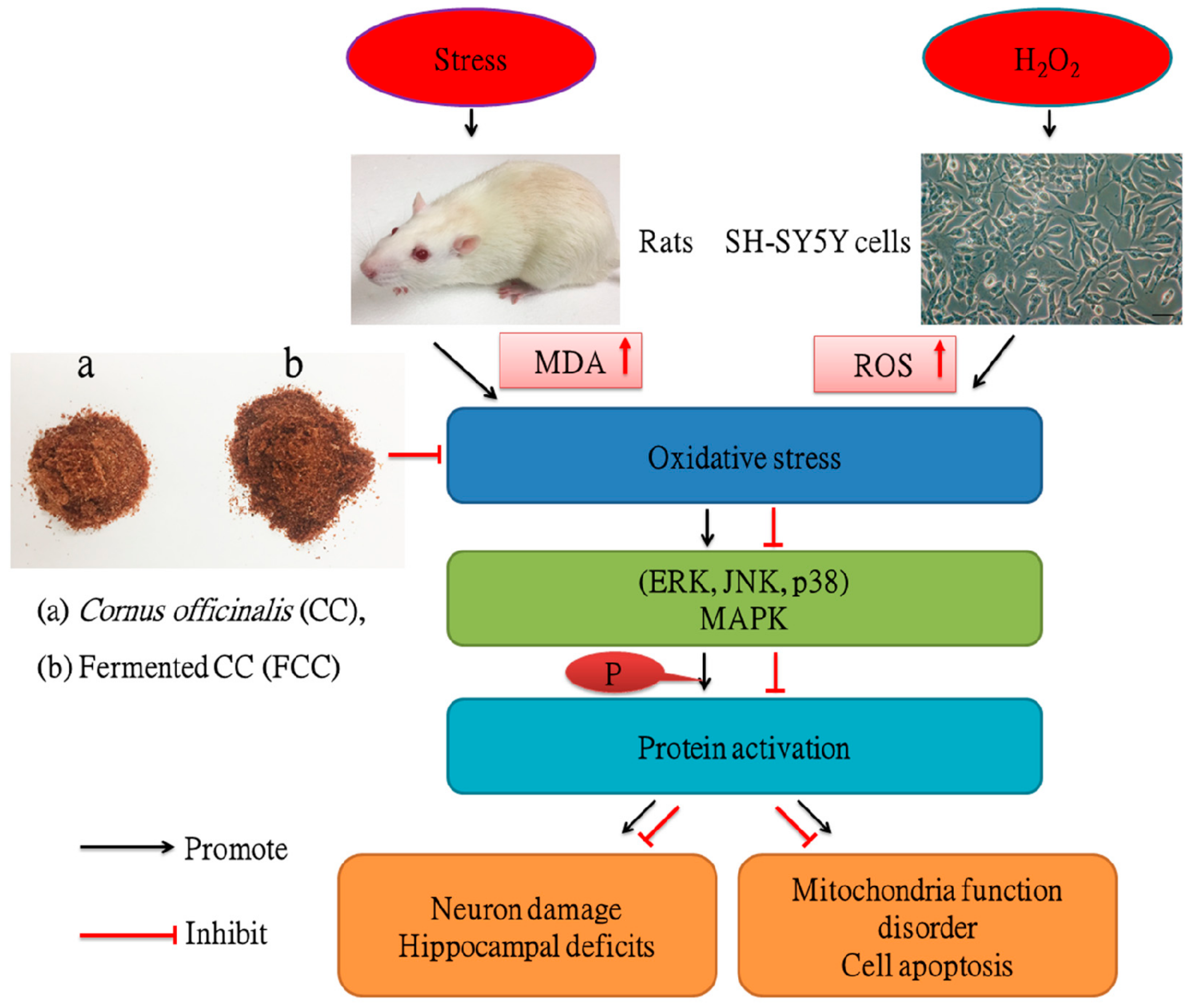
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. Animal models of depression: Molecular perspectives. Curr. Top. Behav. Neurosci. 2011, 7, 121–147. [Google Scholar]
- Gass, N.; Becker, R.; Schwarz, A.J.; Weber-Fahr, W.; Clemm von Hohenberg, C.; Vollmayr, B.; Sartorius, A. Brain network reorganization differs in response to stress in rats genetically predisposed to depression and stress-resilient rats. Transl. Psychiatry 2016, 6, e970. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A. Hippocampal vulnerability to stress and aging: Possible role of neurotrophic factors. Behav. Brain Res. 1996, 78, 25–36. [Google Scholar] [CrossRef]
- Uno, H.; Tarara, R.; Else, J.G.; Suleman, M.A.; Sapolsky, R.M. Hippocampal damage associated with prolonged and fatal stress in primates. J. Neurosci. 1989, 9, 1705–1711. [Google Scholar] [CrossRef]
- Egeland, M.; Zunszain, P.A.; Pariante, C.M. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat. Rev. Neurosci. 2015, 16, 189–200. [Google Scholar] [CrossRef]
- Ryu, J.R.; Hong, C.J.; Kim, J.Y.; Kim, E.K.; Sun, W.; Yu, S.W. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol. Brain 2016, 9, 43. [Google Scholar] [CrossRef]
- Kim, J.J.; Diamond, D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002, 3, 453–462. [Google Scholar] [CrossRef]
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.H. Severe life stress and oxidative stress in the brain: From animal models to human pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef]
- Sahin, E.; Gumuslu, S. Immobilization stress in rat tissues: Alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 144, 342–347. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Kim, K.Y.; Kang, P.; Lee, H.S.; Seol, G.H. Effects of salvia sclarea on chronic immobilization stress induced endothelial dysfunction in rats. BMC Complement. Altern. Med. 2014, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Seo, C.S.; Lee, H.Y.; Jung, D.Y.; Lee, J.K.; Lee, J.A.; Song, K.Y.; Shin, H.K.; Lee, M.Y.; Seo, Y.B.; et al. Hepatoprotective and antioxidative activities of cornus officinalis against acetaminophen-induced hepatotoxicity in mice. Evid. Based Complement. Altern. Med. (eCAM) 2012, 2012, 804924. [Google Scholar]
- Kim, S.H.; Kim, B.K.; Lee, Y.C. Effects of corni fructus on ovalbumin-induced airway inflammation and airway hyper-responsiveness in a mouse model of allergic asthma. J. Inflamm. 2012, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Kim, H.B.; Lee, S.; Kim, M.J.; Lee, S.O.; Han, S.M.; Maeng, S.; Park, J.H. Loganin enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Physiol. Behav. 2017, 171, 243–248. [Google Scholar] [CrossRef]
- Sozanski, T.; Kucharska, A.Z.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piorecki, N.; Szelag, A.; Trocha, M. Loganic acid and anthocyanins from cornelian cherry (Cornus mas l.) fruits modulate diet-induced atherosclerosis and redox status in rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513. [Google Scholar] [CrossRef]
- Jiang, W.L.; Zhang, S.P.; Zhu, H.B.; Jian, H.; Tian, J.W. Cornin ameliorates cerebral infarction in rats by antioxidant action and stabilization of mitochondrial function. Phytother. Res. 2010, 24, 547–552. [Google Scholar] [CrossRef]
- Ma, L.Q.; Yu, Y.; Chen, H.; Li, M.; Ihsan, A.; Tong, H.Y.; Huang, X.J.; Gao, Y. Sweroside alleviated aconitine-induced cardiac toxicity in h9c2 cardiomyoblast cell line. Front. Pharmacol. 2018, 9, 1138. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa b activation in raw 264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef]
- Czerwinska, M.E.; Melzig, M.F. Cornus mas and cornus officinalis-analogies and differences of two medicinal plants traditionally used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Y.; Lu, Z.; Wang, Y. Solid-state fermentation of corn-soybean meal mixed feed with bacillus subtilis and enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. J. Anim. Sci. Biotechnol. 2017, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented fruits and vegetables of asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Akanda, M.R.; Islam, A.; Yang, H.D.; Lee, S.C.; Lee, J.H.; Kim, S.K.; Choi, Y.J.; Im, S.Y.; Park, B.Y. The anti-stress effect of mentha arvensis in immobilized rats. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in pc12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef]
- Shukla, R.K.; Gupta, R.; Srivastava, P.; Dhuriya, Y.K.; Singh, A.; Chandravanshi, L.P.; Kumar, A.; Siddiqui, M.H.; Parmar, D.; Pant, A.B.; et al. Brain cholinergic alterations in rats subjected to repeated immobilization or forced swim stress on lambda-cyhalothrin exposure. Neurochem. Int. 2016, 93, 51–63. [Google Scholar] [CrossRef]
- Gao, D.; Li, Q.; Gao, Z.; Wang, L. Antidiabetic effects of corni fructus extract in streptozotocin-induced diabetic rats. Yonsei Med. J. 2012, 53, 691–700. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Park, J.; Kim, S.H.; Kwon, S.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. Korean J. Physiol. Pharmacol. 2013, 17, 393–403. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gandhi, J.; Khera, L.; Gaur, N.; Paul, C.; Kaul, R. Role of modulator of inflammation cyclooxygenase-2 in gammaherpesvirus mediated tumorigenesis. Front. Microbiol. 2017, 8, 538. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, A.S.; Choi, I. Melandrii herba extract attenuates h(2)o(2)-induced neurotoxicity in human neuroblastoma sh-sy5y cells and scopolamine-induced memory impairment in mice. Molecules 2017, 22, 1646. [Google Scholar] [CrossRef] [PubMed]
- Ruffels, J.; Griffin, M.; Dickenson, J.M. Activation of erk1/2, jnk and pkb by hydrogen peroxide in human sh-sy5y neuroblastoma cells: Role of erk1/2 in h2o2-induced cell death. Eur. J. Pharmacol. 2004, 483, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Wang, S.; Qiu, J.; Yu, C. Astragaloside iv attenuates the h2o2-induced apoptosis of neuronal cells by inhibiting alpha-synuclein expression via the p38 mapk pathway. Int. J. Mol. Med. 2017, 40, 1772–1780. [Google Scholar]
- Lee, C.Y.; Cheng, H.M.; Sim, S.M. Mulberry leaves protect rat tissues from immobilization stress-induced inflammation. BioFactors 2007, 31, 25–33. [Google Scholar] [CrossRef]
- Ozbeyli, D.; Gokalp, A.G.; Koral, T.; Ocal, O.Y.; Dogan, B.; Akakin, D.; Yuksel, M.; Kasimay, O. Protective effect of exercise and sildenafil on acute stress and cognitive function. Physiol. Behav. 2015, 151, 230–237. [Google Scholar] [CrossRef]
- Perez-Nievas, B.G.; Garcia-Bueno, B.; Caso, J.R.; Menchen, L.; Leza, J.C. Corticosterone as a marker of susceptibility to oxidative/nitrosative cerebral damage after stress exposure in rats. Psychoneuroendocrinology 2007, 32, 703–711. [Google Scholar] [CrossRef]
- Mayer, J.L.; Klumpers, L.; Maslam, S.; de Kloet, E.R.; Joels, M.; Lucassen, P.J. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J. Neuroendocrinol. 2006, 18, 629–631. [Google Scholar] [CrossRef]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid generates ros to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef]
- Johnson, S.A.; Fournier, N.M.; Kalynchuk, L.E. Effect of different doses of corticosterone on depression-like behavior and hpa axis responses to a novel stressor. Behav. Brain Res. 2006, 168, 280–288. [Google Scholar] [CrossRef]
- Fontana, F.; Bernardi, P.; Pich, E.M.; Boschi, S.; De Iasio, R.; Spampinato, S.; Grossi, G. Opioid peptide modulation of circulatory and endocrine response to mental stress in humans. Peptides 1997, 18, 169–175. [Google Scholar] [CrossRef]
- Golynski, M.; Lutnicki, K. Influence of beta-endorphin on oxygen activity of neutrophils and total antioxidant status in rats after chronic administration of methimazole. Bull. Vet. Inst. Pulawy 2013, 57, 97–101. [Google Scholar] [CrossRef]
- Wong, M.L.; Licinio, J. From monoamines to genomic targets: A paradigm shift for drug discovery in depression. Nat. Rev. Drug Discov. 2004, 3, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Carneiro, A.M.; Dostmann, W.R.; Hewlett, W.A.; Blakely, R.D. P38 mapk activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2a-dependent process. J. Biol. Chem. 2005, 280, 15649–15658. [Google Scholar] [CrossRef] [PubMed]
- Chetsawang, B.; Putthaprasart, C.; Phansuwan-Pujito, P.; Govitrapong, P. Melatonin protects against hydrogen peroxide-induced cell death signaling in sh-sy5y cultured cells: Involvement of nuclear factor kappa b, bax and bcl-2. J. Pineal Res. 2006, 41, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Lee, J.Y.; Choi, Y.K.; Kim, G.S.; Han, B.H. Neuroprotective effects of flavones on hydrogen peroxide-induced apoptosis in sh-sy5y neuroblostoma cells. Bioorgan. Med. Chem. Lett. 2004, 14, 2261–2264. [Google Scholar] [CrossRef]
- Cavallucci, V.; D’Amelio, M.; Cecconi, F. Abeta toxicity in alzheimer’s disease. Mol. Neurobiol. 2012, 45, 366–378. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Nirmaladevi, D.; Venkataramana, M.; Chandranayaka, S.; Ramesha, A.; Jameel, N.M.; Srinivas, C. Neuroprotective effects of bikaverin on h2o2-induced oxidative stress mediated neuronal damage in sh-sy5y cell line. Cell. Mol. Neurobiol. 2014, 34, 973–985. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, H.; Zhang, B.; Wu, X.; Zhang, Y.; Ge, P.; Chi, G.; Liang, J. Trehalose inhibits h2o2-induced autophagic death in dopaminergic sh-sy5y cells via mitigation of ros-dependent endoplasmic reticulum stress and ampk activation. Int. J. Med. Sci. 2018, 15, 1014–1024. [Google Scholar] [CrossRef]
- Fletcher, J.L.; Murray, S.S.; Xiao, J. Brain-derived neurotrophic factor in central nervous system myelination: A new mechanism to promote myelin plasticity and repair. Int. J. Mol. Sci. 2018, 19, 4131. [Google Scholar] [CrossRef] [PubMed]
- Studer, L.; Spenger, C.; Seiler, R.W.; Othberg, A.; Lindvall, O.; Odin, P. Effects of brain-derived neurotrophic factor on neuronal structure of dopaminergic neurons in dissociated cultures of human fetal mesencephalon. Exp. Brain Res. 1996, 108, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ros activate mapk pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Qiu, J.; Li, Q.; Bell, K.A.; Du, Y.; Jung, D.W.; Lee, J.Y.; Hao, J.; Jiang, J. Cyclooxygenase-2 contributes to oxidopamine-mediated neuronal inflammation and injury via the prostaglandin e2 receptor ep2 subtype. Sci. Rep. 2017, 7, 9459. [Google Scholar] [CrossRef]
| Antibody | Host | Manufacturer | Cat. No. | Dilute Ratio |
|---|---|---|---|---|
| Bcl-2 | Rabbit | Cell Signaling, USA | 3498 | 1:1000 |
| Bax | Rabbit | Santa Cruz Biotechnology, USA | sc-493 | 1:500 |
| COX-2 | Rabbit | Cell Signaling, USA | 12282 | 1:1000 |
| p-JNK | Rabbit | Cell Signaling, USA | 9251 | 1:1000 |
| JNK | Rabbit | Cell Signaling, USA | 9252 | 1:1000 |
| p-ERK | Rabbit | Cell Signaling, USA | 9101 | 1:1000 |
| ERK | Rabbit | Cell Signaling, USA | 9102 | 1:1000 |
| p-p38 | Rabbit | Cell Signaling, USA | 9211 | 1:1000 |
| p38 | Rabbit | Cell Signaling, USA | 9212 | 1:1000 |
| β-actin | Rabbit | Cell Signaling, USA | 4970 | 1:1000 |
| Gene Target | Primer Name | Primer Sequence (5′-3′) | GenBank Accession No. |
|---|---|---|---|
| Catalase | CAT F | CCTTTCTGTTGAAGATGCGGCG | NM_001752 |
| CAT R | GGCGGTGAGTGTCAGGATAG | ||
| Superoxide dismutase | SOD F | AGGCCGTGTGCGTGCTGAAG | NM_000454 |
| SOD R | CACCTTTGCCCAAGTCATCTGC | ||
| BDNF | BDNF F | ATGACCATCCTTTTCCTTACT | NM_170731 |
| BDNF R | GCCACCTTGTCCTCGGAT | ||
| GAPDH | GAPDH F | TTCACCACCATGGAGAAGGC | NM_001357943 |
| GAPDH R | GGCATGGACTGTGGTCATGA |
| Sample | Logainin (mg/g) | Total Phenolic (mg GAE/g) | Total Flavonoid (mg RU/g) |
|---|---|---|---|
| CC | 9.05 ± 0.05 | 112.95 ± 1.94 | 12.45 ± 0.33 |
| FCC | 9.83 ± 0.04 * | 120.98 ± 0.16 * | 15.79 ± 0.15 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Zhao, J.; Lee, J.-H.; Akanda, M.R.; Cho, J.-H.; Kim, S.-K.; Choi, Y.-J.; Park, B.-Y. Neuroprotective Effects of Cornus officinalis on Stress-Induced Hippocampal Deficits in Rats and H2O2-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Antioxidants 2020, 9, 27. https://doi.org/10.3390/antiox9010027
Tian W, Zhao J, Lee J-H, Akanda MR, Cho J-H, Kim S-K, Choi Y-J, Park B-Y. Neuroprotective Effects of Cornus officinalis on Stress-Induced Hippocampal Deficits in Rats and H2O2-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Antioxidants. 2020; 9(1):27. https://doi.org/10.3390/antiox9010027
Chicago/Turabian StyleTian, Weishun, Jing Zhao, Jeong-Ho Lee, Md Rashedunnabi Akanda, Jeong-Hwi Cho, Sang-Ki Kim, Yu-Jin Choi, and Byung-Yong Park. 2020. "Neuroprotective Effects of Cornus officinalis on Stress-Induced Hippocampal Deficits in Rats and H2O2-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells" Antioxidants 9, no. 1: 27. https://doi.org/10.3390/antiox9010027
APA StyleTian, W., Zhao, J., Lee, J.-H., Akanda, M. R., Cho, J.-H., Kim, S.-K., Choi, Y.-J., & Park, B.-Y. (2020). Neuroprotective Effects of Cornus officinalis on Stress-Induced Hippocampal Deficits in Rats and H2O2-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Antioxidants, 9(1), 27. https://doi.org/10.3390/antiox9010027





