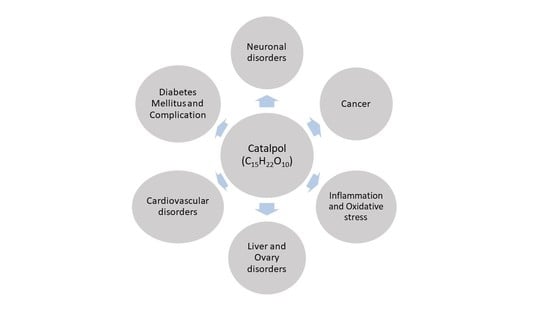
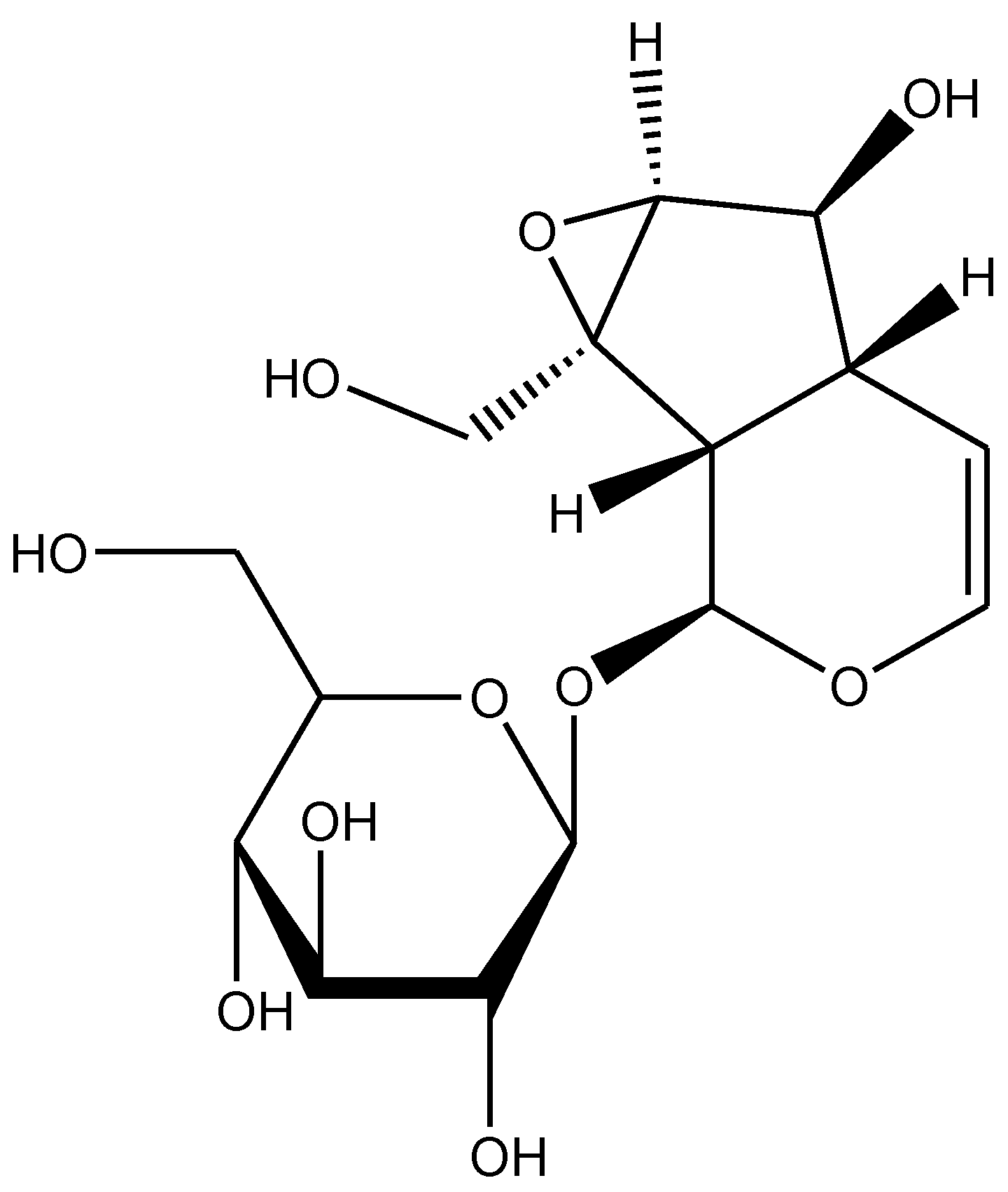
Multiple Biological Effects of an Iridoid Glucoside, Catalpol, and Its Underlying Molecular Mechanisms
Abstract
:1. Introduction
2. Effects of Catalpol in Inflammatory and Oxidative Stress Conditions
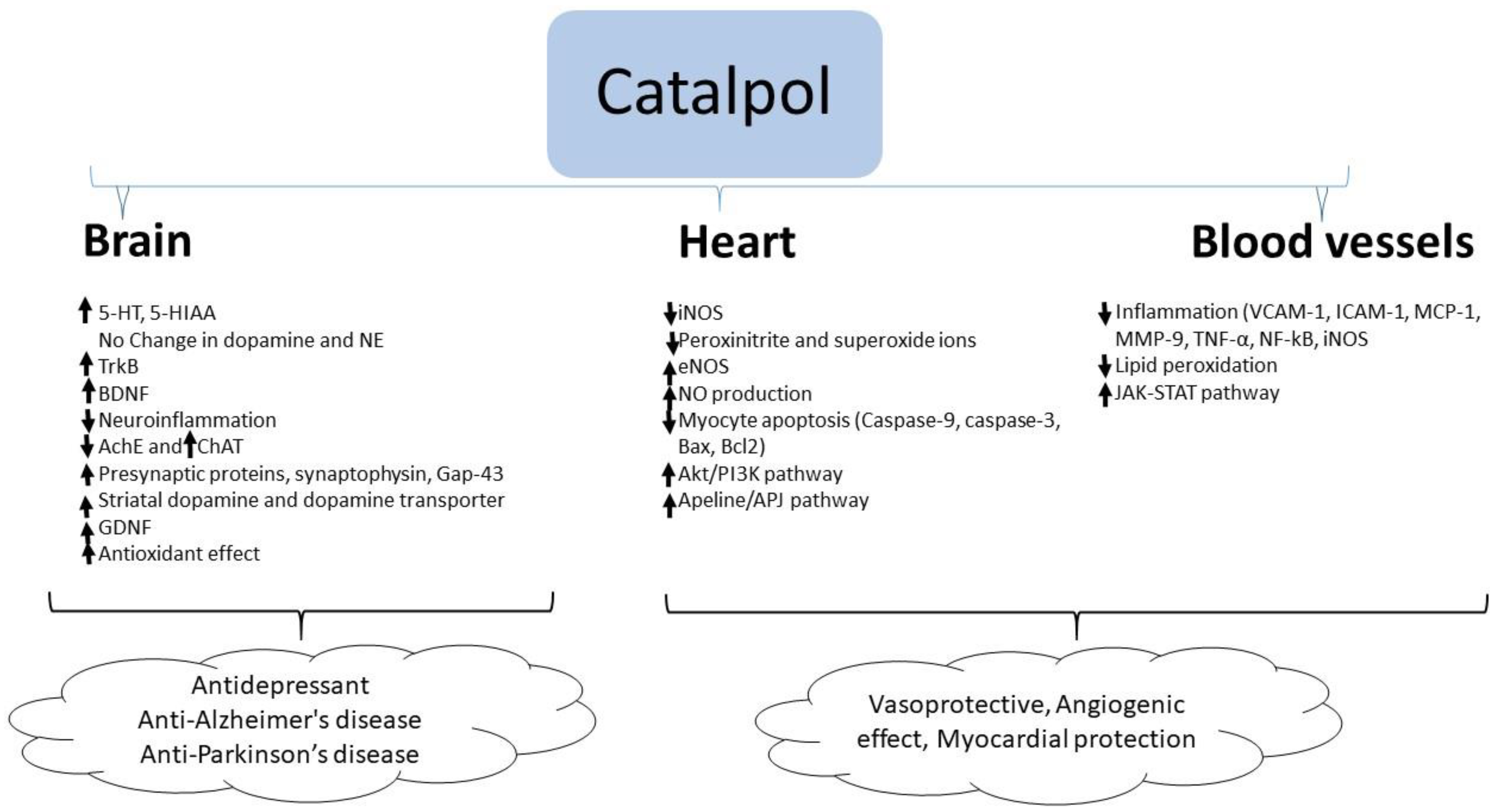
3. Effect of Catalpol in Neurological Disorders
3.1. Depression
3.2. Alzheimer’s Disease
3.3. Parkinson’s Disease
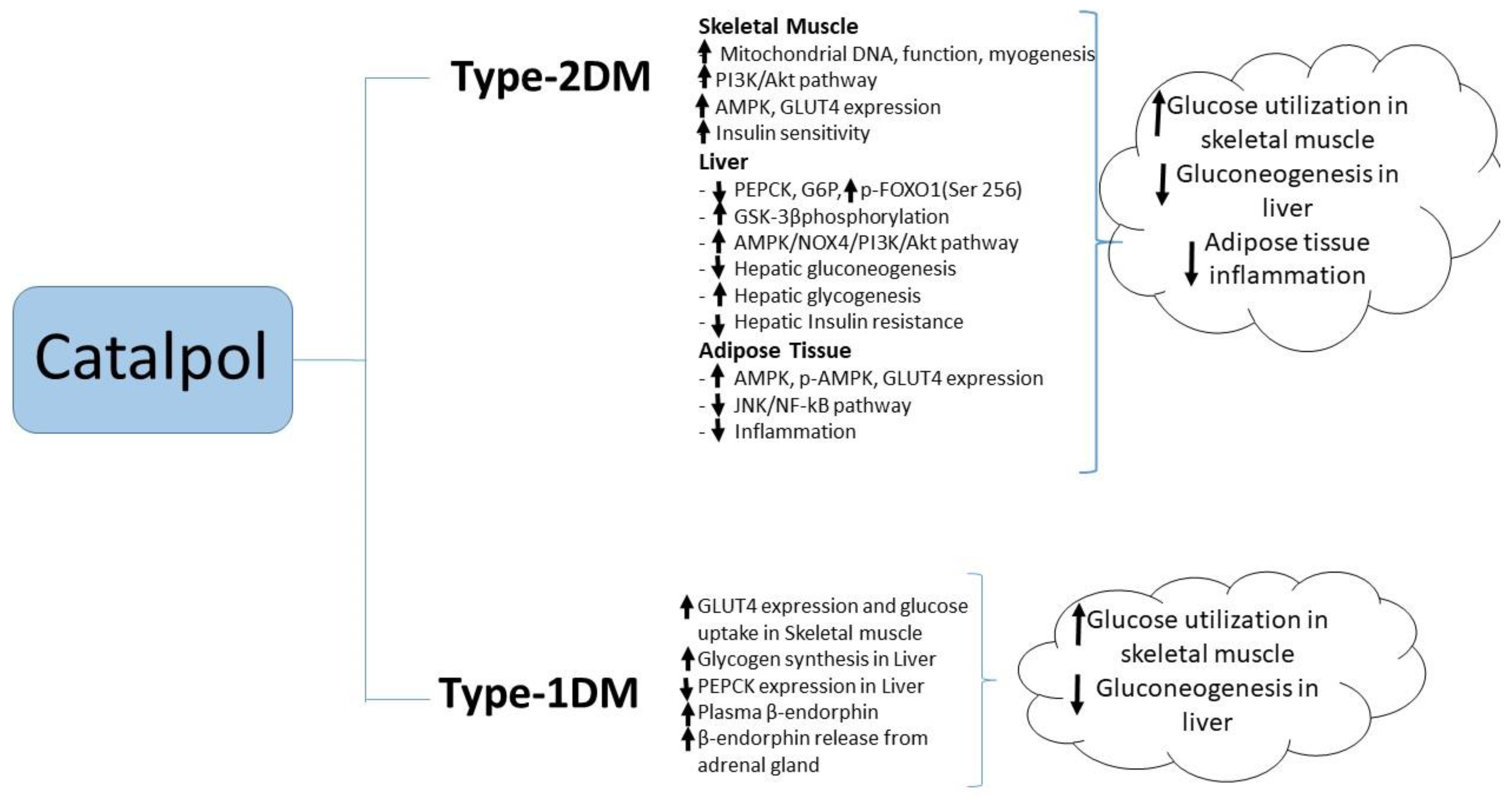
4. Effects of Catalpol in Diabetes Mellitus
4.1. Type-1 Diabetes Mellitus
4.2. Type-2 Diabetes Mellitus
4.3. Diabetic Complications
5. Effect of Catalpol in Cardiovascular Disorders
5.1. Myocardial Protection
5.2. Vascular Protection
6. Effect of Catalpol in Cancers
7. Other Biological Activities
7.1. Hepatoprotective Activity
7.2. Ovary Protective Effect
8. Pharmacokinetics of Catalpol
9. Conclusions
Author Contributors
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ±LVdp/dtmax | Left ventricular maximum rate of positive or negative pressure development |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | 5-hydroxy tryptophan |
| A2780 | Human ovarian cancer cell line |
| A549 | Adenocarcinomic human alveolar basal epithelial cell line |
| ACC | Acetyl-CoA carboxylase |
| Ach | Acetylcholine |
| AD | Alzheimer’s disease |
| ADAM10 | A Disintegrin and Metalloproteinase 10 |
| AGE | Advanced glycation end product |
| Akt | Protein kinase B |
| ALT | Alanine aminotransferase |
| AMPK | Adenosine Monophosphate Activated Protein Kinase |
| Ang II | Angiotensin II |
| APJ | Apelin receptor |
| APP | Amyloid precursor protein |
| AST | Aspartate transaminase |
| ATG5 | Autophagy-related gene 5 |
| ATP | Adenosine triphosphate |
| AUC | Area under the curve |
| Aβ | Amyloid β |
| Bax | BCL2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | Brain-derived neurotrophic factor |
| bFGF | Basic fibroblast growth factor |
| BUN | Blood urea nitrogen |
| CA 19-9 | Cancer antigen 19-9 |
| CA1 | Carbonic anhydrase 1 |
| CAT | Catalase |
| CCL4 | Carbon tetrachloride |
| CCR3 | CC chemokine receptor 3 |
| CD-1 | Cluster of differentiation 1 |
| CD133+ | Cluster of differentiation 1 |
| CD34+ | Cluster of differentiation 1 |
| CEA | Carcinoembryonic antigen |
| ChAT | Choline acetyltransferase |
| CK-MB | Creatine Kinase-MB |
| Clec7a | C-type lectin domain family 7-member A |
| Col IV | Collagen type IV |
| COX-2 | Cyclooxygenase-2 |
| CT26 | Colorectal cancer cell line |
| CTGF | Connective tissue growth factor |
| CXCR4 | Chemokine receptor 4 |
| DBP | Diastolic blood pressure |
| dNTP | Deoxynucleoside triphosphates |
| EMT | Epithelial mesenchymal transition |
| eNOS | Endothelial nitric oxide synthase |
| EPO | Erythropoietin |
| EPOR | Erythropoietin receptors |
| FBG | Fasting blood glucose |
| FN | Fibronectin |
| FOXO1 | Fork head box protein O1 |
| FPI | Fasting plasma insulin |
| FSH | Follicle-stimulating hormone |
| FST | Forced swim test |
| G6pase | Glucose-6 phosphatase |
| GAP-43 | Growth-associated protein 43 |
| GDNF | Glial cell-derived neurotrophic factor |
| GLUT4 | Glucose transporter 4 |
| Grb10 | Growth factor receptor-bound protein 10 |
| GS | Glycogen synthase |
| GSH-PX | glutathione peroxidase |
| GSK-3β | Glycogen synthase kinase beta |
| GSP | Glycated serum protein |
| H2O2 | Hydrogen peroxide |
| HBL-100 | Human breast mammary gland cell line |
| HCT116 | Human colorectal carcinoma cell line |
| HDL-C | High-density lipoprotein cholesterol |
| HFD | High fat diet |
| HIF-1α | Hypoxia-inducible factor 1α |
| HMGCR | Hydroxymethyl glutaric acid acyl CoA reductase |
| HO-1 | Haem oxygenase-1 |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| HPA | Hypothalamic-pituitary-adrenal |
| HSCs | Hepatic stellate cells |
| HUVECs | Human umbilical vein endothelial cells |
| HUVECs | Human umbilical vein endothelial cells |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IDO | Indoleamine 2,3- dioxygenase |
| IgE | Immunoglobulin E |
| IGF-1 | Insulin-like growth factor 1 |
| IGF-1R | Insulin-like growth factor 1 receptor |
| IKKβ | Inhibitor of nuclear factor kappa-B kinase subunit beta |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1β |
| IL-4 | Interleukin-4 |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| iNOS | Inducible nitric oxide synthase |
| Insig1 | Insulin-induced gene 1 |
| IPGTT | Intraperitoneal glucose tolerance test |
| IRS-1 | Insulin receptor substrate 1 |
| ISO | Isoproterenol |
| IκBα | Inhibitor of kappa B alpha |
| JAK2 | Janus kinase2 |
| JNK | c-Jun N-terminal kinases |
| KWI | Kidney weight index |
| LC3 | Microtubule-associated proteins 1A/1B light chain 3B |
| LDH | Lactate dehydrogenase |
| LDL-C | Low density lipoprotein cholesterol |
| LH | Luteinizing hormone |
| LPS | Lipopolysaccharide |
| LVEDP | Left ventricular end diastolic pressure |
| LVESP | Left ventricular end-systolic pressure |
| LY294002 | 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran- 4-one |
| MAPK | Mitogen activated protein kinase |
| MBP | Mean blood pressure |
| MCF-7 | Human breast adenocarcinoma cell line |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MDA | Malondialdehyde |
| MG63 | Human osteosarcoma cell line |
| MGL1 | Macrophage galactose-type lectin 1 |
| MI/R | Myocardial ischemia/reperfusion |
| MI | Myocardial infarction |
| MMP-16 | Matrix metalloproteinases-16 |
| MMP-2 | Matrix metalloproteinases-2 |
| MMP-9 | Matrix metalloproteinases-9 |
| MMR | Measles mumps and rubella vaccine |
| MPO | Myeloperoxidase |
| MPTP | 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine |
| mRNA | Messenger ribonucleic acid |
| mtDNA | Mitochondrial deoxyribonucleic acid |
| MyoD | Myoblast determination protein |
| MyoG | Myogenin |
| N2a | Neuroblastoma 2a cell line |
| NE | Norepinephrine |
| NF-κB | Nuclear factor-κB |
| NGF | Nerve growth factor |
| NO | Nitric oxide |
| Nox4 | NADPH oxidase 4 |
| NQO1 | NADPH quinone 1 |
| NRF-1 | Nuclear respiratory factor 1 |
| NSCLC | Non-small cell lung cancer |
| OGTT | Oral glucose tolerance test |
| p-AMPK | Phosphorylated Adenosine Monophosphate Activated Protein Kinase |
| PARP | Poly-ADP ribose polymerase |
| PC12 | Pheochromocytoma cell line |
| PDGF | Platelet derived growth factor |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1α |
| PGE2 | Prostaglandin E2 |
| PGF2α | Prostaglandin F2 alpha |
| PI3K | Phosphoinositide 3-kinase |
| PMA | Phorbol-12-myristate-13-acetate |
| RACK1 | Receptor of activated protein C kinase 1 |
| RBG | Random blood glucose |
| RhoA | Ras homolog gene family member A |
| ROCK1 | Rho associated coiled-coil containing protein kinase 1 |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| Scd2 | Steaoryl-coenzyme A desaturase 2 |
| SD | Sprague Dawley |
| SiRNA | Short interfering ribonucleic acid |
| Sirt1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducer and activator of transcription 3 |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
| T-47D | Human breast cancer cell line |
| TC | Total cholesterol |
| TG | Triglycerides |
| TGF-β1 | Transforming growth factor-β1 |
| TH | Tyrosine hydroxylase |
| THP-1 | Human leukemia monocytic cell line |
| TNF-α | Tumor necrosis factor-α |
| TP | Triptolide |
| TrkB | Tropomyosin receptor kinase B |
| TST | Tail suspension test |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP Nick-End labelling |
| U2OS | Human osteosarcoma cell line |
| ULK | Unc-51-like kinases |
| UPE | Urinary protein excretion |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| WiDr | Colon adenocarcinoma cell line |
| α-SMA | Alpha smooth muscle actin |
References
- Shieh, J.P.; Cheng, K.C.; Chung, H.H.; Kerh, Y.F.; Yeh, C.H.; Cheng, J.T. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2011, 59, 3747–3753. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Shen, R.F.; Bi, J.; Tian, X.S.; Hinchliffe, T.; Xia, Y. Catalpol: A potential therapeutic for neurodegenerative diseases. Curr. Med. Chem. 2015, 22, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.F.; Wan, D.; Luo, Y.; Zhou, J.L.; Chen, L.; Xu, X.Y. Catalpol increases brain angiogenesis and up-regulates VEGF and EPO in the rat after permanent middle cerebral artery occlusion. Int. J. Biol. Sci. 2010, 6, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duff, R.B.; Bacon, J.S.; Mundie, C.M.; Farmer, V.C.; Russell, J.D.; Forrester, A.R. Catalpol and methylcatalpol: Naturally occurring glycosides in Plantago and Buddleia species. Biochem. J. 1965, 96, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Pharmacopoeia, 8th ed.; EDQM Council of Europe: Strasbourg, France, 2014.
- Bone, K.; Mills, S. Principles and Practice of Phytotherapy: Modern Herbal Medicine, 2nd ed.; Churchill Livingstone Elsevier: New York, NY, USA, 2013; pp. 238–259. [Google Scholar]
- Raja, S.; Ramya, I. A review on ethnopharmacology, phytochemistry and pharmacology of Buddleja asiatica. Int. J. Pharm. Sci. Res. 2016, 7, 4697–4709. [Google Scholar]
- Bai, Y.; Zhu, R.; Tian, Y.; Li, R.; Chen, B.; Zhang, H.; Xia, B.; Zhao, D.; Mo, F.; Zhang, D.; et al. Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety. Molecules 2019, 24, 3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Liu, X.; Sun, H. Catalpol inhibits homocysteine-induced oxidation and inflammation via inhibiting Nox4/NF-κB and GRP78/PERK pathways in human aorta endothelial cells. Inflammation 2019, 42, 64–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, H.; Yang, X. Effects of catalpol on bronchial asthma and its relationship with cytokines. J. Cell. Biochem. 2018, 120, 8992–8998. [Google Scholar] [CrossRef]
- Fu, K.; Piao, T.; Wang, M.; Zhang, J.; Jiang, J.; Wang, X.; Liu, H. Protective effect of catalpol on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2014, 23, 400–406. [Google Scholar] [CrossRef]
- Xiao, W.; Yin, G.; Fan, Y.; Qiu, L.; Cang, X.; Yu, G.; Hu, Y.; Xing, M.; Wu, D.; Wang, X.; et al. Catalpol ameliorates sodium taurocholate-induced acute pancreatitis in rats via inhibiting activation of nuclear factor kappa B. Int. J. Mol. Sci. 2014, 15, 11957–11972. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, Y.; Xu, M.; Luan, J.; Piao, S.; Chi, S.; Wang, H. Catalpol alleviates ovalbumin-induced asthma in mice: Reduced eosinophil infiltration in the lung. Int. Immunopharmacol. 2017, 43, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Hong, J.; Liu, D.; Lai, G.; Ye, J.; Song, Y. Enhanced effect of catalpol on specific immune therapy in treatment of asthmatic mice. Am. J. Transl. Res. 2019, 11, 2463. [Google Scholar] [PubMed]
- Le, M.; Kim, M.; Song, Y.; Ryu, H.; Oh, S.; Yoon, D. 6-O-Veratroyl catalpol suppresses pro-inflammatory cytokines via regulation of extracellular signal-regulated kinase and nuclear factor-κB in human monocytic cells. Biochimie 2015, 119, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid. Med. Cell. Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; An, L.J.; Duan, Y.L.; Li, Y.C.; Jiang, B. Catalpol protects rat pheochromocytoma cells against oxygen and glucose deprivation-induced injury. Neurol. Res. 2008, 30, 106–112. [Google Scholar] [CrossRef]
- Humbles, A.A.; Lloyd, C.M.; McMillan, S.J.; Friend, D.S.; Xanthou, G.; McKenna, E.E.; Ghiran, S.; Gerard, N.P.; Yu, C.; Orkin, S.H.; et al. A critical role for eosinophils in allergic airways remodelling. Science 2004, 305, 1776–1779. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Liu, J.H.; Bao, Y.M.; An, L.J. Catalpol inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Toxicon 2004, 43, 53–59. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, H.; Morishima, N.; Li, E.; Xu, J.; Yankner, B.; Yuan, J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 2000, 403, 98–103. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Feng, W.; Zhang, Y.; Wang, G.; Wang, X.; Zhou, G. Involvement of the central monoaminergic system in the antidepressant-like effect of catalpol in mice. Biosci. Trends 2014, 8, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.M.; Yang, L.H.; Zhang, Y.Y.; Niu, C.L.; Cui, Y.; Feng, W.S.; Wang, G.F. BDNF and COX-2 participate in anti-depressive mechanisms of catalpol in rats undergoing chronic unpredictable mild stress. Physiol. Behav. 2015, 151, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Xue, L.; Zhu, H.; Luo, Y. Catalpol induces neuroprotection and prevents memory dysfunction through the cholinergic system and BDNF. Evid.-Based Complement. Altern. Med. 2013, 2013, 134852. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Zhang, R.; Liu, S.; Xia, Z.; Hu, Y. Catalpol ameliorates beta amyloid–induced degeneration of cholinergic neurons by elevating brain-derived neurotrophic factors. Neuroscience 2009, 163, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Q.J.; Zou, W.; Wang, H.X.; Bao, Y.M.; Liu, Y.X.; An, L.J. Catalpol increases hippocampal neuroplasticity and up-regulates PKC and BDNF in the aged rats. Brain Res. 2006, 1123, 68–79. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Zhao, P.; Zhao, L.; Wang, Z. Catalpol Inhibits amyloid-β generation through promoting α-cleavage of APP in Swedish mutant APP overexpressed N2a Cells. Front. Aging Neurosci. 2018, 10, 66. [Google Scholar] [CrossRef]
- Xu, G.; Xiong, Z.; Yong, Y.; Wang, Z.; Ke, Z.; Xia, Z.; Hu, Y. Catalpol attenuates MPTP induced neuronal degeneration of nigral-striatal dopaminergic pathway in mice through elevating glial cell derived neurotrophic factor in striatum. Neurosci 2010, 167, 174–184. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, B.; An, L.; Bao, Y. Neuroprotective effect of catalpol against MPP+-induced oxidative stress in mesencephalic neurons. Eur. J. Pharmacol. 2007, 568, 142–148. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, B.; Cui, Y.; Lim, M.; Liu, P.; Han, L.; Guo, H.; Lao, L. Effects of moxa smoke on monoamine neurotransmitters in SAMP8 mice. Evid.-Based Complement. Altern. Med. 2013, 2013, 178067. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, M.; Yan, J.; Cao, Y.; Liu, P. Antidepressant-like effect of the extracted of Kai Xin San, a traditional Chinese herbal prescription, is explained by modulation of the central monoaminergic neurotransmitter system in mouse. J. Ethnopharmacol. 2012, 139, 422–428. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, G.; Zhang, X.; Gong, Z.; Gu, C. Effects of 5-hydroxymethyl furfural extracted from Rehmannia glutinosa Libosch on the expression of signaling molecules relevant to learning and memory among hippocampal neurons exposed to high concentration of corticosterone. Chin. J. Integr. Med. 2014, 20, 844–849. [Google Scholar] [CrossRef]
- Kuwano, T.; Nakao, S.; Yamamoto, H.; Tsuneyoshi, M.; Yamamoto, T.; Kuwano, M.; Ono, M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004, 18, 300–310. [Google Scholar] [CrossRef]
- Rojas, A.; Jiang, J.; Ganesh, T.; Yang, M.; Lelutiu, N.; Gueorguieva, P.; Dingledine, R. Cyclooxygenase-2 in epilepsy. Epilepsia 2014, 55, 17–25. [Google Scholar] [CrossRef]
- Singh, D.; Chopra, K. Flavocoxid, dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, exhibits neuroprotection in rat model of ischaemic stroke. Pharmacol. Biochem. Behav. 2014, 120, 33–42. [Google Scholar] [CrossRef]
- Dhull, D.; Jindal, A.; Dhull, R.; Aggarwal, S.; Bhateja, D.; Padi, S. Neuroprotective effect of cyclooxygenase inhibitors in ICV-STZ induced sporadic Alzheimer’s disease in rats. J. Mol. Neurosci. 2012, 46, 223–235. [Google Scholar] [CrossRef]
- Muller, N. COX-2 inhibitors as antidepressants and antipsychotics: Clinical evidence. Curr. Opin. Investig. Drugs. 2010, 11, 31–42. [Google Scholar]
- Christmas, D.; Potokar, J.; Davies, S. A biological pathway linking inflammation and depression: Activation of indoleamine 2,3-dioxygenase. Neuropsychiatr. Dis. Treat. 2011, 7, 431. [Google Scholar]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsych. Dis. Treat. 2015, 11, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jin, C.; Li, Y.; Guan, S.; Han, F.; Zhang, S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by d-galactose. Food Chem. Toxicol. 2013, 58, 50–55. [Google Scholar] [CrossRef]
- Savitt, J. Diagnosis and treatment of Parkinson disease: Molecules to medicine. J. Clin. Investig. 2006, 116, 1744–1754. [Google Scholar] [CrossRef] [Green Version]
- Nagatsu, T.; Sawada, M. Cellular and molecular mechanisms of Parkinson’s disease: Neurotoxins, causative genes, and inflammatory cytokines. Cell. Mol. Neurobiol. 2006, 26, 779–800. [Google Scholar] [CrossRef]
- Yang, X.; Mertens, B.; Lehtonen, E.; Vercammen, L.; Bockstael, O.; Chtarto, A.; Levivier, M.; Brotchi, J.; Michotte, Y.; Baekelandt, V.; et al. Reversible neurochemical changes mediated by delayed intrastriatal glial cell line-derived neurotrophic factor gene delivery in a partial Parkinson’s disease rat model. J. Gene Med. 2009, 11, 899–912. [Google Scholar] [CrossRef]
- Li, D.; Duan, Y.; Bao, Y.; Liu, C.; Liu, Y.; An, L. Neuroprotection of catalpol in transient global ischemia in gerbils. Neurosci. Res. 2004, 50, 169–177. [Google Scholar] [CrossRef]
- Kitagawa, I.; Nishimura, T.; Furubayashi, A.; Yosioka, I. On the constituents of rhizome of Rehmannia glutinosa Libosch. forma hueichingensis Hsiao. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1971, 91, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.J.; Niu, H.S.; Lin, M.H.; Cheng, J.T.; Hsu, F.L. Antihyperglycemic effect of catalpol in streptozotocin-induced diabetic rats. J. Nat. Prod. 2010, 73, 1170–1172. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, D.Q.; Xue, H.Y.; Hu, B. Oral supplementation of catalpol ameliorates diabetic encephalopathy in rats. Brain Res. 2010, 1307, 158–165. [Google Scholar] [CrossRef]
- Lin, C.M.; Wang, B.W.; Fang, W.J.; Pan, C.M.; Shyu, K.G.; Hou, S.W. Catalpol ameliorates neointimal hyperplasia in diabetic rats. Planta Med. 2019, 85, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Deng, H.; Zhang, Q.; Xie, J.; Zeng, H.; Jin, X.; Ling, Z.; Shan, Q.; Liu, M.; Ma, Y.; et al. Amelioration of diabetic mouse nephropathy by catalpol correlates with down-regulation of Grb10 expression and activation of insulin-like growth factor 1/insulin-like growth factor 1 receptor signaling. PLoS ONE 2016, 11, e0151857. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, Z.; Jiang, Z.; Sun, L.; Ji, J.; Miao, J.; Zhang, X.; Li, X.; Huang, S.; Wang, T.; et al. Hypoglycemic effect of catalpol on high-fat diet/streptozotocin-induced diabetic mice by increasing skeletal muscle mitochondrial biogenesis. Acta. Biochim. Biophys. Sin. 2014, 46, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Wang, L.; Jiang, Z.; Zhao, G.; Hassan, H.M.; Sun, L.; Fan, S.; Zhou, Z.; Zhang, L.; Wang, T. A new hypoglycemic mechanism of catalpol revealed by enhancing MyoD/MyoG-mediated myogenesis. Life Sci. 2018, 209, 313–323. [Google Scholar] [CrossRef]
- Bao, Q.; Shen, X.; Qian, L.; Gong, C.; Nie, M.; Dong, Y. Anti-diabetic activities of catalpol in db/db mice. Korean J. Physiol. Pharmacol. 2016, 20, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, G.; Ma, S.; Li, F.; Yuan, M.; Xu, H.; Huang, K. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-κB pathways. Biochem. Biophys. Res. Commun. 2015, 467, 853–858. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Liu, Z.; Wang, J.; Wan, D.; Feng, S.; Yang, X.; Wang, T. Antidiabetic and antioxidant effects of catalpol extracted from Rehmannia glutinosa (Di Huang) on rat diabetes induced by streptozotocin and high-fat, high-sugar feed. Chin. Med. 2016, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Yap, K.; Gan, S.; Candasamy, M.; Md, S.; Majeed, A.A.; Bhattamisra, S. Effect of catalpol on liver glucose homeostasis in high fat diet/low dose streptozotocin-induced type 2 diabetes mellitus. Br. J. Pharmacol. 2019, 176, 3051–3052. [Google Scholar]
- Liu, J.Y. Catalpol protect diabetic vascular endothelial function by inhibiting NADPH oxidase. Zhongguo Zhong Yao Za Zhi 2014, 39, 2936–2941. [Google Scholar]
- Dong, Z.; Chen, C.X. Effect of catalpol on diabetic nephropathy in rats. Phytomedicine 2013, 20, 1023–1029. [Google Scholar] [CrossRef]
- Jiang, P.; Xiang, L.; Chen, Z.; Lu, H.; Zhou, L.; Yang, L.; Ji, Y.; Liu, Y.; Sun, X.; Deng, Y.; et al. Catalpol alleviates renal damage by improving lipid metabolism in diabetic db/db mice. Am. J. Transl. Res. 2018, 10, 1750. [Google Scholar]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef] [Green Version]
- St-Pierre, J.; Lin, J.; Krauss, S.; Tarr, P.T.; Yang, R.; Newgard, C.B.; Spiegelman, B.M. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J. Biol. Chem. 2003, 278, 26597–26603. [Google Scholar] [CrossRef] [Green Version]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Bi, R.; Meng, Q.; Wang, C.; Huo, X.; Liu, Z.; Wang, C.; Sun, P.; Sun, H.; Ma, X.; et al. Catalpol alleviates adriamycin-induced nephropathy by activating the SIRT1 signalling pathway in vivo and in vitro. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Vieitez, P.; Gomez, O.; Uceda, E.R.; Vera, M.E.; Molina-Holgado, E. Systemic and local effects of angiotensin II blockade in experimental diabetic nephropathy. J. Renin–Angio–Aldo S. 2008, 9, 96–102. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Huang, C.; Cui, Y.; Ji, L.; Zhang, W.; Li, R.; Ma, L.; Xing, W.; Zhou, H.; Chen, B.; Yu, J.; et al. Catalpol decreases peroxynitrite formation and consequently exerts cardioprotective effects against ischemia/reperfusion insult. Pharm. Biol. 2013, 51, 463–473. [Google Scholar] [CrossRef]
- Bi, F.J.; Zhang, H.; Xu, Y.J.; Hu, J. Protective effect of catalpol on isoproterenol-induced myocardial injury in Wistar rats. Afr. J. Biotechnol. 2012, 11, 9270–9275. [Google Scholar]
- Bi, F.; Xu, Y.; Sun, Q. Catalpol pretreatment attenuates cardiac dysfunction following myocardial infarction in rats. Anatol. J. Cardiol. 2018, 19, 296–302. [Google Scholar]
- Zeng, J.; Huang, F.; Tu, Y.; Wu, S.; Li, M.; Tong, X. Protective effect of catalpol on myocardium in rats with isoprenaline-induced myocardial infarcts via angiogenesis through endothelial progenitor cells and Notch1 signaling pathway. Pharm. Pharm. 2013, 4, 619. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Lu, Y.; Yan, X.; Wu, X.; Kuai, M.; Sun, X.; Chen, Q.; Kong, X.; Liu, Z.; Tang, Y.; et al. Catalpol protects glucose-deprived rat embryonic cardiac cells by inducing mitophagy and modulating estrogen receptor. Biomed. Pharm. 2017, 89, 973–982. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, D.J. Amelioration by catalpol of atherosclerotic lesions in hypercholesterolemic rabbits. Planta Med. 2015, 81, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Xian, Y.; Yuan, W.; Huifeng, Z.; Tao, W.; Zhiqiang, L.; Shan, F.; Ya, F.; Hongli, W.; Jinghuan, W.; et al. Catalpol stimulates VEGF production via the JAK2/STAT3 pathway to improve angiogenesis in rats’ stroke model. J. Ethnopharmacol. 2016, 191, 169–179. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Hu, J. Catalpol inhibits apoptosis in hydrogen peroxide-induced endothelium by activating the PI3K/Akt signaling pathway and modulating expression of Bcl-2 and Bax. Eur. J. Pharmacol. 2010, 628, 155–163. [Google Scholar] [CrossRef]
- Smith, C.C.; Yellon, D.M. Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharm. Ther. 2011, 129, 206–219. [Google Scholar] [CrossRef]
- Wang, Z.; Zhan-Sheng, H. Catalpol inhibits migration and induces apoptosis in gastric cancer cells and in athymic nude mice. Biomed. Pharmacother. 2018, 103, 1708–1719. [Google Scholar] [CrossRef]
- Garcia, C.; Leon, L.; Pungitore, C.; Rios-Luci, C.; Daranas, A.; Montero, J.C.; Pandiella, A.; Tonn, C.E.; Martín, V.S.; Padrón, J.M. Enhancement of antiproliferative activity by molecular simplification of catalpol. Bioorg. Med. Chem. 2010, 18, 2515–2523. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Sheng, B.; Ding, Y.; Cheng, X. Catalpol inhibits TGF-β1-induced epithelial-mesenchymal transition in human non–small-cell lung cancer cells through the inactivation of Smad2/3 and NF-κB signaling pathways. J. Cell. Biochem. 2019, 120, 2251–2258. [Google Scholar] [CrossRef]
- Liu, L.; Gao, H.; Wang, H.; Zhang, Y.; Xu, W.; Lin, S.; Wang, H.; Wu, Q.; Guo, J. Catalpol promotes cellular apoptosis in human HCT116 colorectal cancer cells via microRNA-200 and the downregulation of PI3K-Akt signaling pathway. Oncol. Lett. 2017, 14, 3741–3747. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Wu, Y.; Yang, A.; Fu, X.; Mao, M.; Liu, Z. Catalpol suppressed proliferation, growth and invasion of CT26 colon cancer by inhibiting inflammation and tumor angiogenesis. Biomed. Pharm. 2017, 95, 68–76. [Google Scholar] [CrossRef]
- Fei, B.; Dai, W.; Zhao, S. Efficacy, safety, and cost of therapy of the traditional Chinese medicine, catalpol, in patients following surgical resection for locally advanced colon cancer. Med. Sci. Monit. 2018, 24, 3184–3192. [Google Scholar] [CrossRef]
- Liu, C.; Wu, F.; Liu, Y.; Meng, C. Catalpol suppresses proliferation and facilitates apoptosis of MCF-7 breast cancer cells through upregulating microRNA-146a and downregulating matrix metalloproteinase-16 expression. Mol. Med. Rep. 2015, 12, 7609–7614. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xue, G. Catalpol suppresses osteosarcoma cell proliferation through blocking epithelial-mesenchymal transition (EMT) and inducing apoptosis. Biochem. Biophys. Res. Commun. 2018, 495, 27–34. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Liu, Q. Catalpol inhibits cell proliferation, invasion and migration through regulating miR-22–3p/MTA3 signalling in hepatocellular carcinoma. Exp. Mol. Pathol. 2019, 109, 51–60. [Google Scholar] [CrossRef]
- Pungitore, C.; León, L.; García, C.; Martín, V.; Tonn, C.; Padrón, J. Novel antiproliferative analogs of the Taq DNA polymerase inhibitor catalpol. Bioorg. Med. Chem. Lett. 2007, 17, 1332–1335. [Google Scholar] [CrossRef]
- Luo, J.B.; Feng, L.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q. The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish. Shellfish Immun. 2014, 40, 197–207. [Google Scholar] [CrossRef]
- Saxena, A.; Fayad, R.; Kaur, K.; Truman, S.; Greer, J.; Carson, J.A.; Chanda, A. Dietary selenium protects adiponectin knockout mice against chronic inflammation induced colon cancer. Cancer Biol. Ther. 2017, 18, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.; Hago, A.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef]
- Liu, F.; You, X.; Wang, Y.; Liu, Q.; Liu, Y.; Zhang, S.; Chen, L.; Zhang, X.; Ye, L. The oncoprotein HBXIP enhances angiogenesis and growth of breast cancer through modulating FGF8 and VEGF. Carcinogenesis 2014, 35, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, M.; Evered, C.; Pelling, J.; Damjanovski, S. Inhibition of MT1-MMP proteolytic function and ERK1/2 signalling influences cell migration and invasion through changes in MMP-2 and MMP-9 levels. J. Cell. Commun. Signal. 2017, 11, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Adams, B.; Kasinski, A.; Slack, F. Aberrant regulation and function of microRNAs in cancer. Curr. Biol. 2014, 24, R762–R776. [Google Scholar] [CrossRef] [Green Version]
- Seven, M.; Karatas, O.; Duz, M.; Ozen, M. The role of miRNAs in cancer: From pathogenesis to therapeutic implications. Future Oncol. 2014, 10, 1027–1048. [Google Scholar] [CrossRef]
- Sandhu, R.; Rein, J.; D’Arcy, M.; Herschkowitz, J.; Hoadley, K.; Troester, M. Overexpression of miR-146a in basal-like breast cancer cells confers enhanced tumorigenic potential in association with altered p53 status. Carcinogenesis 2014, 35, 2567–2575. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Liu, D.; Gao, J.; Liu, M.; Liu, S.; Jiang, M.; Liu, Y.; Zheng, D. TRAIL-induced miR-146a expression suppresses CXCR 4-mediated human breast cancer migration. FEBS J. 2013, 280, 3340–3353. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Niu, J.; Wang, J.; Zhao, P. Phytoestrogenic effects of catalpol in T47D and MDA-MB231 cells in culture. FASEB J. 2010, 24, 821–829. [Google Scholar]
- Gao, N.; Tian, J.; Shang, Y.; Zhao, D.; Wu, T. Catalpol suppresses proliferation and facilitates apoptosis of OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and downregulating MMP-2 expression. Int. J. Mol. Sci. 2014, 15, 19394–19405. [Google Scholar] [CrossRef] [Green Version]
- Trerotola, M.; Jernigan, D.; Liu, Q.; Siddiqui, J.; Fatatis, A.; Languino, L. Trop-2 promotes prostate cancer metastasis by modulating 1 integrin functions. Cancer Res. 2013, 73, 3155–3167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jia, R.; Wang, F.; Qiu, G.; Qiao, P.; Xu, X.; Wu, D. Catalpol protects mice against lipopolysaccharide/d-galactosamine-induced acute liver injury through inhibiting inflammatory and oxidative response. Oncotarget 2018, 9, 3887. [Google Scholar] [CrossRef]
- Feng, Z.; Zhou, C.; Dong, S.; Liu, Z.; Liu, T.; Zhou, L.; Zhou, X. Catalpol and panax notoginseng saponins synergistically alleviate triptolide-induced hepatotoxicity through Nrf2/ARE pathway. Toxicol. In Vitro 2019, 56, 141–149. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, P.; Zhang, L.; Xiong, B.; Tao, J.; Guan, W.; Li, C.; Chen, C.; Gu, J.; Duanmu, J.; et al. Autophagy inhibition attenuates the induction of anti-inflammatory effect of catalpol in liver fibrosis. Biomed. Pharmacother. 2018, 103, 1262–1271. [Google Scholar] [CrossRef]
- Wei, M.; Lu, Y.; Liu, D. and Ru, W. Ovarian failure-resistant effects of catalpol in aged female rats. Biol. Pharm. Bull. 2014, 37, 1444–1449. [Google Scholar] [CrossRef] [Green Version]
- Perrone, L.; Squillaro, T.; Napolitano, F.; Terracciano, C.; Sampaolo, S.; Melone, M.A. The Autophagy Signaling Pathway: A Potential Multifunctional Therapeutic Target of Curcumin in Neurological and Neuromuscular Diseases. Nutrients 2019, 11, 1881. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Kenny, S.J.; Ge, L.; Xu, K.; Schekman, R. Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife 2015, 4, e11205. [Google Scholar] [CrossRef]
- Joven, J.; Guirro, M.; Mariné-Casadó, R.; Rodríguez-Gallego, E.; Menéndez, J.A. Autophagy is an inflammation-related defensive mechanism against disease. In Oxidative Stress and Inflammation in Non-communicable Diseases-Molecular Mechanisms and Perspectives in Therapeutics; Springer: Cham, Switzerland, 2014; pp. 43–59. [Google Scholar]
- Tao, J.H.; Zhao, M.; Wang, D.G.; Yang, C.; Du, L.Y.; Qiu, W.Q.; Jiang, S. Biotransformation and metabolic profile of catalpol with human intestinal microflora by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2016, 1009, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, B.; Ma, B.; Zhang, Q.; Li, X.; Zhu, J.; Liu, M.; Wu, X.; Wang, C.; Wu, Z. Pharmacokinetics and tissue distribution of Aucubin, Ajugol and Catalpol in rats using a validated simultaneous LC–ESI-MS/MS assay. J. Chromatogr. B 2015, 1002, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.H.; Zhao, M.; Wang, D.G.; Yang, C.; Chen, G.T.; Zhao, X.; Pu, X.L.; Jiang, S. UPLC-Q-TOF/MS-based screening and identification of two major bioactive components and their metabolites in normal and CKD rat plasma, urine and feces after oral administration of Rehmannia glutinosa Libosch extract. J. Chromatogr. B 2015, 1001, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Gu, Y.; Si, D.; Liu, C. Quantitation of catalpol in rat plasma by liquid chromatography/electrospray ionization tandem mass spectrometry and its pharmacokinetic study. J. Chromatogr. B 2009, 877, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xing, M.; Chen, W.; Zhang, J.; Qi, H.; Xu, X. HPLC–APCI–MS/MS method for the determination of catalpol in rat plasma and cerebrospinal fluid: Application to an in vivo pharmacokinetic study. J. Pharm. Biomed. Anal. 2012, 70, 337–343. [Google Scholar] [CrossRef]
- Zhao, M.; Tao, J.; Qian, D.; Liu, P.; Shang, E.-X.; Jiang, S.; Guo, J.; Su, S.-L.; Duan, J.A.; Du, L. Simultaneous determination of loganin, morroniside, catalpol and acteoside in normal and chronic kidney disease rat plasma by UPLC–MS for investigating the pharmacokinetics of Rehmannia glutinosa and Cornus officinalis Sieb drug pair extract. J. Chromatogr. B 2016, 1009, 122–129. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, X.; Fang, C.; Zhang, Q.-Y. Regulation of Intestinal Cytochrome P450 Expression by Hepatic Cytochrome P450: Possible Involvement of Fibroblast Growth Factor 15 and Impact on Systemic Drug Exposure. Mol. Pharmacol. 2014, 85, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, V.; Surya Sandeep, M.; Ravindra Babu, P.; Naveen Babu, K. Evaluation of First-Pass Cytochrome P4503A (CYP3A) and P-glycoprotein Activities Using Felodipine and Hesperetin in Combination in Wistar Rats and Everted Rat Gut Sacs in Vitro. Phyther. Res. 2014, 28, 699–705. [Google Scholar] [CrossRef]
- Naud, J.; Laurin, L.P.; Michaud, J.; Beauchemin, S.; Leblond, F.A.; Pichette, V. Effects of Chronic Renal Failure on Brain Drug Transporters in Rats. Drug Metab. Dispos. 2012, 40, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Gai, Z.; Chu, L.; Hiller, C.; Arsenijevic, D.; Penno, C.A.; Montani, J.P.; Odermatt, A.; Kullak-Ublick, G.A. Effect of chronic renal failure on the hepatic, intestinal, and renal expression of bile acid transporters. Am. J. Physiol. Renal Physiol. 2014, 306, F130–F137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, F.; Zhang, J.F. Simultaneous determination of puerarin and catalpol in biological samples by LC-MS/MS*. Chin. J. Pharm. Anal. 2016, 36, 1596–1604. [Google Scholar]
| Experimental Model | Dose and Duration | Key Findings of Catalpol | References |
|---|---|---|---|
| Human aorta endothelial cells | Catalpol: 7.5, 15, or 30 µM for 24 h |
| [9] |
| BALB/c mice | Catalpol: 2.5, 5, or 10 mg/kg |
| [11] |
| BALB/c mice | Catalpol: 5, or 10 mg/kg for 2 weeks |
| [13] |
| Ovalbumin induced asthma in BALB/c mice | 5 mg/kg, i.p. 2 weeks |
| [14] |
| Human THP-1 monocyte and A549 cell | Catalpol: 5, 10, 20, or 50 µM for 24 h |
| [15] |
| Human aorta epithelial cells | Catalpol: 10, 20, or 40 µM for 24 h |
| [16] |
| Rat pheochromocytoma cells | Catalpol: 0.001, 0.01, 0.1, or 1 mM for 24 h |
| [17] |
| Experimental Model | Dose and Duration | Key Findings of Catalpol | References |
|---|---|---|---|
| Kunming mice | Catalpol: 5, 10, or 20 mg/kg, p.o., for 2 weeks Positive control: 10 mg/kg fluoxetine hydrochloride for 2 weeks |
| [22] |
| Sprague-Dawley rats | Catalpol: 5, 10, or 20 mg/kg, for 5 weeks Positive control: 10 mg/kg fluoxetine hydrochloride for 2 weeks |
| [23] |
| Kunming mice | Catalpol: 1, 3, or 9 mg/kg, for 3 days Positive control: 7.9 mg/kg edaravone or 105 mg/kg oxiracetam for 3 days |
| [24] |
| CD-1 mice | Catalpol: 50 mg/kg, for 60 days |
| [25] |
| Sprague-Dawley rats | Catalpol: 5 mg/kg, for 10 days |
| [26] |
| Swedish mutant APP overexpressed N2a cells | Catalpol: 200 or 400 µ m for 18 h |
| [27] |
| Catalpol: 50 mg/kg, for 8 weeks Catalpol: 5, 15, or 50 mg/kg, for 8 weeks |
| [28] |
| Mesencephalic neurons | Catalpol: 0.05, 0.1, or 0.5 mM for 30 min |
| [29] |
| Experimental Model | Dose and Duration | Key Findings of Catalpol | References |
|---|---|---|---|
| Rats (STZ, 65 mg/kg) | 0.1 mg/kg, i.v. injection, three days |
| [1] |
| Rats (STZ, 60 mg/kg) | 0.01–0.1 mg/kg, i.v. injection |
| [46] |
| Male Sprague–Dawley rats (STZ, 65 mg/kg, i.p.) |
10, 50, and 100 mg/kg, intra-gastric infusion, Six (6) weeks |
| [47] |
| STZ-induced diabetes rats with balloon-injured carotid arteries |
| [48] | |
| Male C57BL/6 mice (STZ, 180 mg/kg) | 10 mg/kg/d, i.p. 14 days |
| [49] |
| C57BL/6 J male mice (HFD/STZ, 85 mg/kg, i.p.) | 50, 100, or 200 mg/kg, p.o.; 4 weeks |
| [50] |
| db/db mice | 200 mg/kg, p.o. 8 weeks |
| [51] |
| C2C12 cells | 10, 30, or 100 μM | ||
| db/db mice | 40, 80, 160 mg/kg, p.o. 4 weeks |
| [52] |
| C57BL/6 J mice (HFD, 60% calorie with STZ, 40 mg/kg, for five consecutive days) | 100 and 200 mg/kg/d, p.o.; 4 weeks |
| [53] |
| glucosamine-induced HepG2 cells | 20, 40, and 80 µM catalpol | ||
| C57BL/6 mice (HFD, 45% calorie) | 100 mg/kg, p.o. 4 weeks |
| [54] |
| Rats with initial high-fat and high-sugar diet (3 weeks), followed by STZ (30 mg/kg, i.p.) for 3 days | 5, 10, 20, or 50 mg/kg, i.v. 2 weeks |
| [55] |
| Male C57/BL6N (HFD and low dose STZ (50 m/kg, i.p.) | 200 mg/kg, p.o. 4 weeks |
| [56] |
| Rats (HFD/STZ) | 10, 50, 100 mg/kg, Six (6) weeks |
| [57] |
| Male Sprague-Dawley (SD) rats (HFD/STZ, 35 mg/kg, i.p.) | 30, 60, and 120 mg/kg, p.o. 10 weeks |
| [58] |
| C57BLKS/J db/db mice | Chow diet supplemented with catalpol (1 g/kg), Sixteen (16) weeks |
| [59] |
| Experimental Model | Dose and Duration | Key Findings of Catalpol | References |
|---|---|---|---|
| Adult male rats (30 min of myocardial ischemia and 3 h of reperfusion) | 5 mg/kg, i.p., 5 min before reperfusion |
| [66] |
| Isoproterenol (ISO)-induced myocardial infarction (MI) ISO (85 mg/kg, s.c. 2 days) | 10 mg/kg, i.p. 10 days |
| [67] |
| Isoproterenol (ISO)-induced myocardial infarction (MI) ISO (85 mg/kg, s.c. 2 days) | 5 and 10 mg/kg/day, i.p. 10 days |
| [68] |
| Isoprenaline (10 mg/kg, s.c) induced MI in SD rats | 10, 20, 40 mg/kg, p.o. Three (3) weeks |
| [69] |
| H9c2 embryonic rat cardiac cells | 0.1, 1, and 10 mg/mL 24 h |
| [70] |
| High-cholesterol fed diet to male New Zealand White rabbits | 5 mg/kg/day, 12 weeks |
| [71] |
| Middle cerebral artery occlusion in Rats | 5 mg/kg, i.p. 7 days |
| [72] |
| Hydrogen peroxide (H2O2) induced apoptosis in Human umbilical vein endothelial cells (HUVECs) | 0.1, 1, and 10 μg/mL, 48 h |
| [73] |
| Experimental Model | Dose and Duration | Key Findings of Catalpol | References |
|---|---|---|---|
| Catalpol (in vitro): 2.5, 5, 10, 20, 40, 80, or 160 µM for 24 h Catalpol (in vivo): 10, 20, or 40 mg/kg, for 3 weeks |
| [75] |
| Human solid tumor cell lines (A2780, HBL-100, HeLa, SW1573, T-47D and WiDr) | Catalpol: 1, 2, 3, or 5 µm for 24 h |
| [76] |
| Human non–small-cell lung cancer (NSCLC) cells- A549 cells |
| [77] | |
| Human colorectal cancer cells (HCT116) | Catalpol: 0, 25, 50, or 100 µg/mL for 48 h |
| [78] |
| Catalpol: 2.5, 5, 10, 20, 40, or 80 µM for 24 and 48 h |
| [79] |
| Randomized, placebo-controlled parallel clinical study in patients that had undergone surgical resection for locally advanced colon adenocarcinoma (n = 345) | Catalpol: 10 mg/kg twice daily for 12 weeks Positive control: 5 mg/kg bevacizumab twice weekly for 12 weeks Placebo group: no treatment |
| [80] |
| Human breast cancer cells (MCF-7) | Catalpol: 0, 25, 50, or 100 µg/mL for 24, 48 and 72 h |
| [81] |
| Human osteosarcoma cancer cells (MG63 and U2OS) | Catalpol: 20, 40, or 80 µm for 48 h |
| [82] |
| Experimental Model | Dose and Duration | Key Findings of Catalpol | References |
|---|---|---|---|
| Lipopolysaccharide (50 μg/kg, i.p.)/d-galactosamine (800 mg/kg, i.p.) -induced acute liver injury in mice | 2.5, 5, 10 mg/kg, i.p. Three (3) days |
| [97] |
| Triptolide (TP) induced hepatotoxicity in Human normal hepatocytes (L-02 cells) | 2, 10, 50, and 250 μg/mL |
| [98] |
| Catalpol (in vitro): 0.625, 1.25, 2.5, 5, 10, 20, or 40 µM for 24 h Catalpol (in vivo): 10, 20, or 40 mg/kg for 4 weeks |
| [99] |
| 14 months old SD female rats (ageing model) | 1, 3, and 5 mg/kg, p.o. Four (4) weeks |
| [100] |
| Sample | Rat Plasma | Rat Plasma | Rat Plasma | Rat Plasma | Rat CSF |
|---|---|---|---|---|---|
| Dose | 50 mg/kg, p.o. | 6 mg/kg, i.v. | 10 mg/kg, i.v. | 8 mg/kg, p.o. | 6 mg/kg, i.v. |
| Tmax (h) | 1.333 ± 0.408 | - | - | 2.8 ± 0.837 | 0.08 ± 0.02 |
| Cmax (ng/mL) | 23,318 ± 10,468 | 23 617.4 ± 914.7 | - | 1680 ± 120 | 675.9 ± 198.4 |
| T1/2 (h) | 1.212 ± 0.388 | 0.71 ± 0.23 | 0.984 ± 0.229 | 3.275 ± 1.192 | 1.52 ± 0.74 |
| AUC (0–t) | - | 11 432.3 ± 1582.5 ng·h/mL | 5951.125 ± 1247.247 µg·h/L | 584.80 ± 107.29 µg·min/mL | 594.5 ± 81.3 ng·h/mL |
| AUC (0–∞) | 69,520 ± 22,927 ng·h/mL | 11 532.9 ± 1643.0 ng·h/mL | 5954.076 ± 1248.205 µg·h/L | 666.30 ± 194.60 µg·min/mL | 671.5 ± 109.1 ng·h/mL |
| MRT (0–∞) (h) | 3.273 ± 0.365 | 0.70 ± 0.20 | 0.0454 ± 0.140 | - | 2.12 ± 1.0 |
| V (L/kg) | 1.428 ± 0.681 | - | 0.348 ± 0.075 | - | - |
| CL (L/h/kg) | 0.824 ± 0.317 | - | 0.348 ± 0.075 | 0.9 ± 0.24 | - |
| Method | LC/MS/MS | HPLC–APCI–MS/MS | LC–ESI-MS/MS | UPLC–MS | HPLC–APCI–MS/MS |
| Reference | [107] | [108] | [105] | [109] | [108] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattamisra, S.K.; Yap, K.H.; Rao, V.; Choudhury, H. Multiple Biological Effects of an Iridoid Glucoside, Catalpol, and Its Underlying Molecular Mechanisms. Biomolecules 2020, 10, 32. https://doi.org/10.3390/biom10010032
Bhattamisra SK, Yap KH, Rao V, Choudhury H. Multiple Biological Effects of an Iridoid Glucoside, Catalpol, and Its Underlying Molecular Mechanisms. Biomolecules. 2020; 10(1):32. https://doi.org/10.3390/biom10010032
Chicago/Turabian StyleBhattamisra, Subrat Kumar, Kah Heng Yap, Vikram Rao, and Hira Choudhury. 2020. "Multiple Biological Effects of an Iridoid Glucoside, Catalpol, and Its Underlying Molecular Mechanisms" Biomolecules 10, no. 1: 32. https://doi.org/10.3390/biom10010032