From Past to Present: The Link Between Reactive Oxygen Species in Sperm and Male Infertility
Abstract
:1. Introduction
2. The Foundation of the Link between ROS and Human Sperm
2.1. Spermatozoa and Their Susceptibility toward ROS
2.2. Polyunsaturated Fatty Acids Quantity and Sperm Susceptibility
2.3. Leukocytes and Their Contribution to ROS Generation
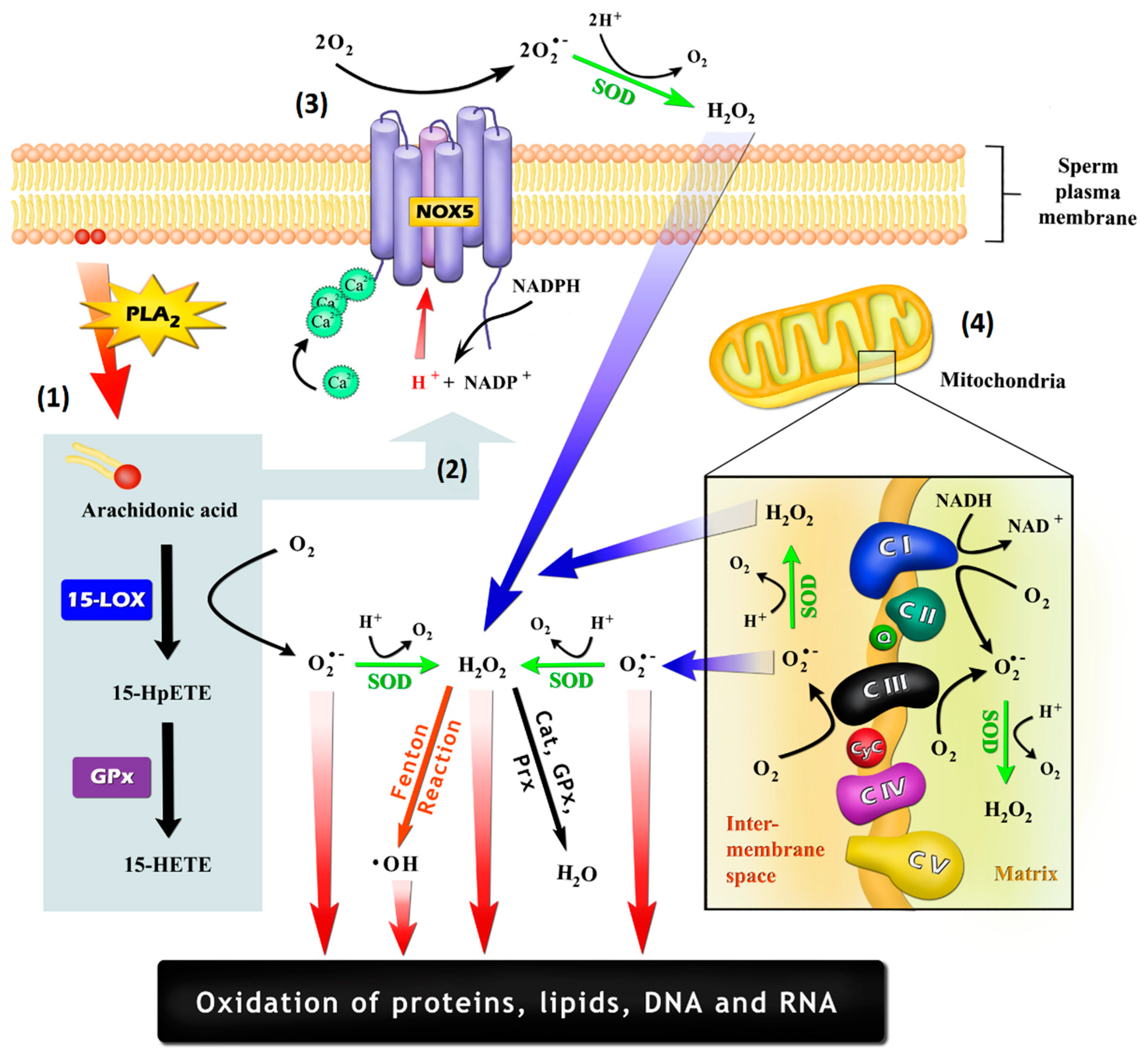
3. The Free Radical-Generating Systems in Sperm
3.1. The Potential for an NADPH–Oxidase System in Sperm
3.2. Other Enzymatic Sources of ROS in Sperm
3.3. Sperm Mitochondria and ROS Generation
4. ROS Measurement Techniques and Their Reliability
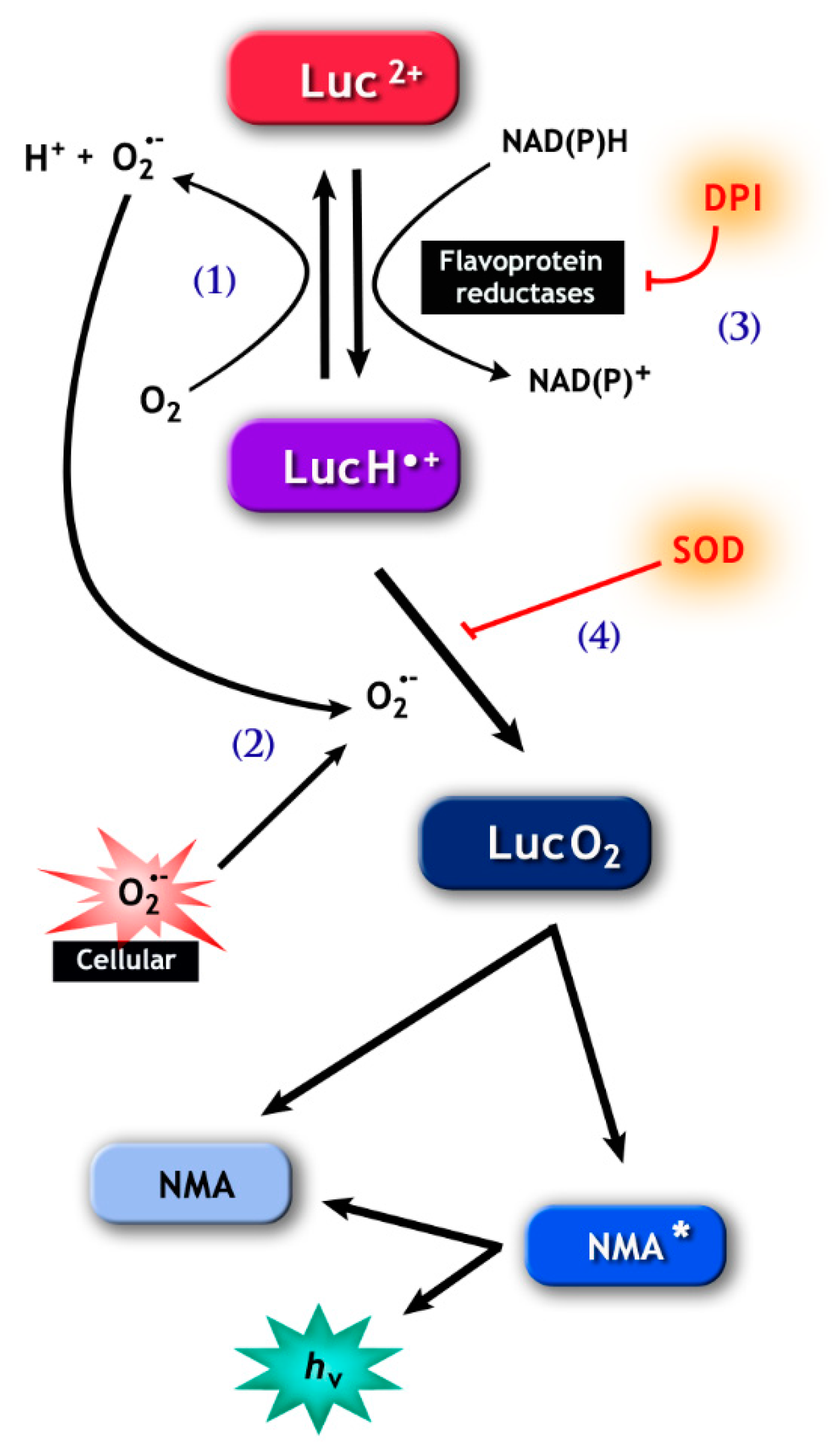
4.1. Lucigenin and Tetrazolium Salts
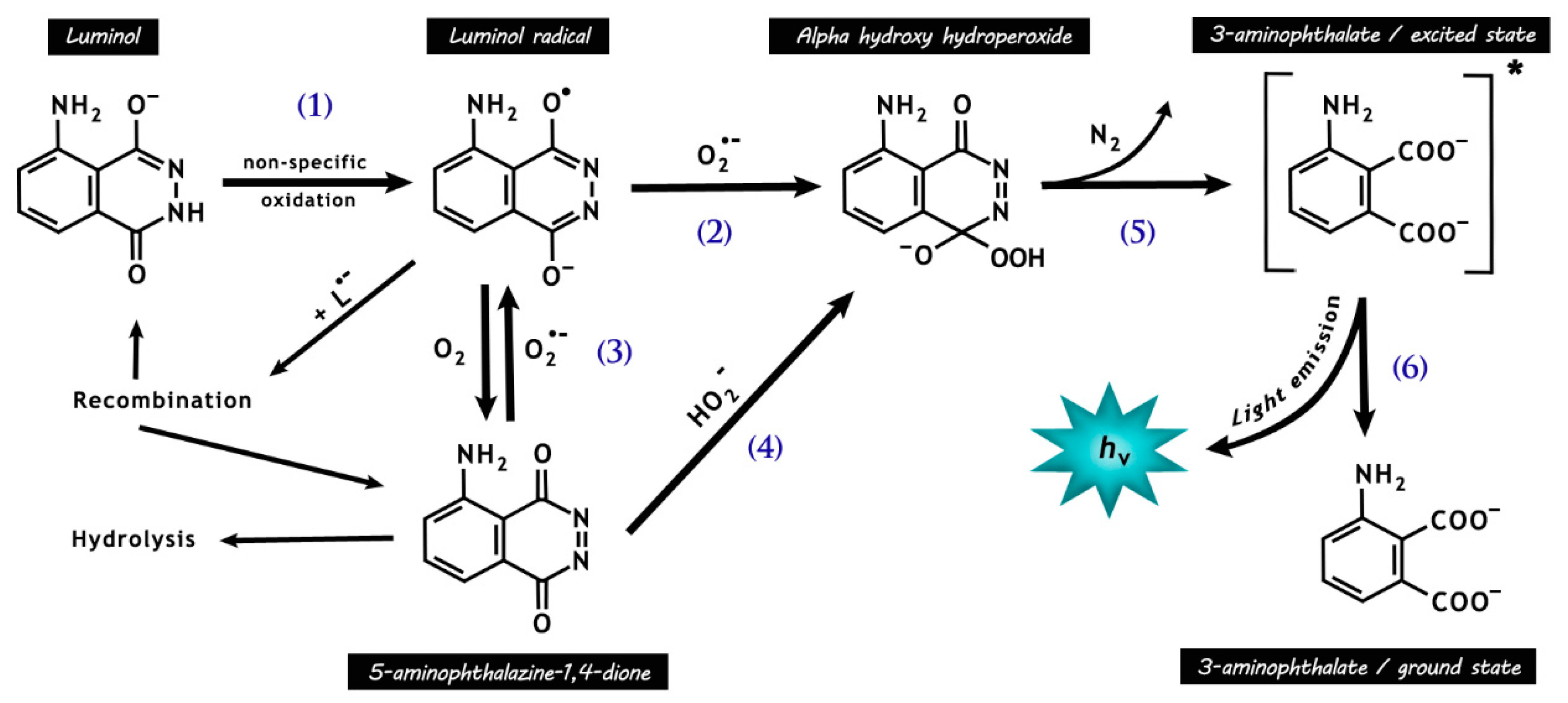
4.2. Luminol/HRP
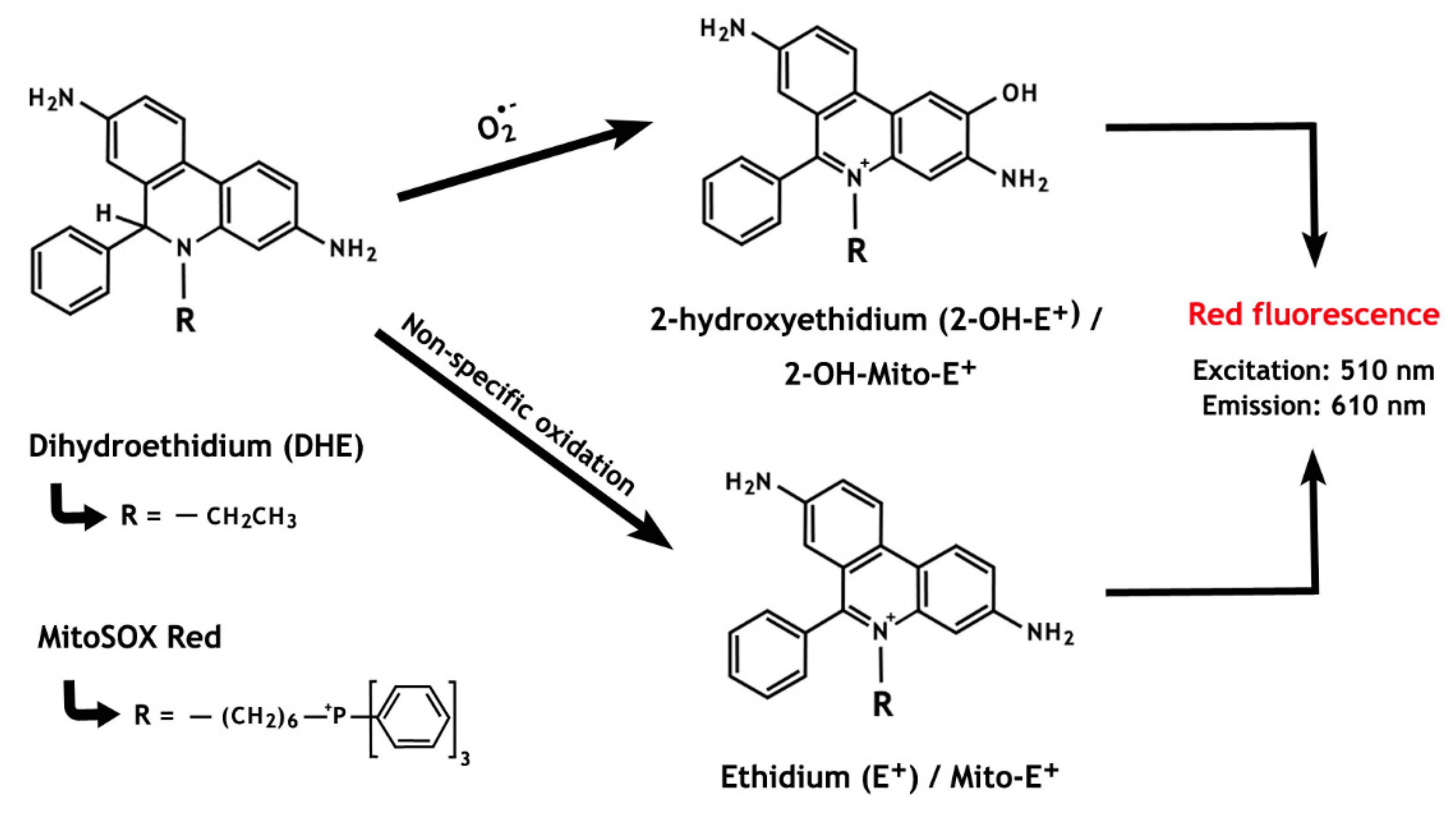
4.3. Dihydroethidium
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- MacLeod, J. The role of oxygen in the matabolism and motility of human spermatozoa. Am. J. Physiol. 1943, 138, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, E.; Boveris, A.; Ragan, C.I.; Stoppani, A.O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977, 80, 248–257. [Google Scholar] [CrossRef]
- Aitken, J.; Fisher, H. Reactive oxygen species generation and human spermatozoa: The balance of benefit and risk. Bioessays 1994, 16, 259–267. [Google Scholar] [CrossRef]
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995, 7, 659–668. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Ko, E.Y.; Sabanegh, E.S.; Agarwal, A. Male infertility testing: Reactive oxygen species and antioxidant capacity. Fertil. Steril. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Kessopoulou, E.; Tomlinson, M.J.; Barratt, C.L.; Bolton, A.E.; Cooke, I.D. Origin of reactive oxygen species in human semen: Spermatozoa or leucocytes? J. Reprod. Fertil. 1992, 94, 463–470. [Google Scholar] [CrossRef]
- Kovalski, N.N.; de Lamirande, E.; Gagnon, C. Reactive oxygen species generated by human neutrophils inhibit sperm motility: Protective effect of seminal plasma and scavengers. Fertil. Steril. 1992, 58, 809–816. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D.W.; Brindle, J.; Gomez, E.; Baker, H.W.; Irvine, D.S. Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum. Reprod. 1995, 10, 2061–2071. [Google Scholar] [CrossRef]
- Baehner, R.L.; Nathan, D.G. Leukocyte oxidase: Defective activity in chronic granulomatous disease. Science 1967, 155, 835–836. [Google Scholar] [CrossRef]
- Whittington, K.; Ford, W. The effect of incubation periods under 95% oxygen on the stimulated acrosome reaction and motility of human spermatozoa. Mol. Hum. Reprod. 1998, 4, 1053–1057. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T.; Sherins, R. Adverse effects of peroxidized lipid on human spermatozoa. Proc. R. Soc. Lond. B Biol. Sci. 1978, 201, 413–417. [Google Scholar]
- Kenaston, C.B.; Wilbur, K.M.; Ottolenghi, A.; Bernheim, F. Comparison of methods for determining fatty acid oxidation produced by ultraviolet irradiation. J. Am. Oil Chem. Soc. 1955, 32, 33–35. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehyde derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K.; Stadtman, E.R. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra- and intermolecular cross-linking reaction. J. Biol. Chem. 1993, 268, 6388–6393. [Google Scholar]
- Musatov, A.; Carroll, C.A.; Liu, Y.C.; Henderson, G.I.; Weintraub, S.T.; Robinson, N.C. Identification of bovine heart cytochrome c oxidase subunits modified by the lipid peroxidation product 4-hydroxy-2-nonenal. Biochemistry 2002, 41, 8212–8220. [Google Scholar] [CrossRef]
- Baker, M.A.; Weinberg, A.; Hetherington, L.; Villaverde, A.I.; Velkov, T.; Baell, J.; Gordon, C.P. Defining the mechanism by which the reactive oxygen species by-product, 4-hydroxynonenal, affects human sperm cell function. Biol. Reprod. 2015, 92, 108–112. [Google Scholar] [CrossRef]
- Neesen, J.; Kirschner, R.; Ochs, M.; Schmiedl, A.; Habermann, B.; Mueller, C.; Holstein, A.F.; Nuesslein, T.; Adham, I.; Engel, W. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum. Mol. Genet. 2001, 10, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Miki, K.; Willis, W.D.; Brown, P.R.; Goulding, E.H.; Fulcher, K.D.; Eddy, E.M. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev. Biol. 2002, 248, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.; Mann, T. Lipid peroxidation in spermatozoa. Proc. R. Soc. Lond. B Biol. Sci. 1973, 184, 103–107. [Google Scholar]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Rao, B.; Soufir, J.; Martin, M.; David, G. Lipid peroxidation in human spermatozoa as relate to midpiece abnormalities and motility. Mol. Reprod. Dev. 1989, 24, 127–134. [Google Scholar]
- Guthrie, H.D.; Welch, G.R. Using fluorescence-activated flow cytometry to determine reactive oxygen species formation and membrane lipid peroxidation in viable boar spermatozoa. Methods Mol. Biol. 2010, 594, 163–171. [Google Scholar]
- Nissen, H.; Kreysel, H. Superoxide dismutase in human semen. Klin. Wochenschr. 1983, 61, 63–65. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa: I. Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992, 13, 368. [Google Scholar]
- Aitken, R.; Buckingham, D.; Harkiss, D. Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. J. Reprod. Fertil. 1993, 97, 441–450. [Google Scholar] [CrossRef] [Green Version]
- De Lamirande, E.; Cagnon, C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic. Biol. Med. 1993, 14, 157–166. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar]
- Bize, I.; Santander, G.; Cabello, P.; Driscoll, D.; Sharpe, C. Hydrogen peroxide is involved in hamster sperm capacitation in vitro. Biol. Reprod. 1991, 44, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Duru, N.K.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Zini, A.; de Lamirande, E.; Gagnon, C. Low levels of nitric oxide promote human sperm capacitation in vitro. J. Androl. 1995, 16, 424–431. [Google Scholar]
- De Iuliis, G.N.; Wingate, J.K.; Koppers, A.J.; McLaughlin, E.A.; Aitken, R.J. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J. Clin. Endocrinol. Metab. 2006, 91, 1968–1975. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Whiting, S.; de Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [Green Version]
- Moazamian, R.; Polhemus, A.; Connaughton, H.; Fraser, B.; Whiting, S.; Gharagozloo, P.; Aitken, R.J. Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol. Hum. Reprod. 2015, 21, 502–515. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.E.; Aitken, R.J.; Nixon, B.; Smith, N.D.; Gibb, Z. Electrophilic aldehyde products of lipid peroxidation selectively adduct to heat shock protein 90 and arylsulfatase A in stallion spermatozoa. Biol. Reprod. 2017, 96, 107–121. [Google Scholar]
- Alvarez, J.G.; Storey, B.T. Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: Its effect on sperm motility. Biol. Reprod. 1982, 27, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Storey, B.T. Assessment of cell damage caused by spontaneous lipid peroxidation in rabbit spermatozoa. Biol. Reprod. 1984, 30, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Storey, B.T. Spontaneous lipid peroxidation in rabbit and mouse epididymal spermatozoa: Dependence of rate on temperature and oxygen concentration. Biol. Reprod. 1985, 32, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Curry, B.J.; Hall, S.E.; Aitken, R.J. Aldehyde dehydrogenase plays a pivotal role in the maintenance of stallion sperm motility. Biol. Reprod. 2016, 94, 133. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D.W.; Carreras, A.; Irvine, D.S. Superoxide dismutase in human sperm suspensions: Relationship with cellular composition, oxidative stress, and sperm function. Free Radic. Biol. Med. 1996, 21, 495–504. [Google Scholar] [CrossRef]
- Maneesh, M.; Jayalekshmi, H. Role of reactive oxygen species and antioxidants on pathophysiology of male reproduction. Indian J. Clin. Biochem. 2006, 21, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, A.; Picardo, M.; Gandini, L.; Dondero, F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.; Ballabriga, A.; Gil-Gibernau, J.J. Lipids of the developing human retina: I. Total fatty acids, plasmalogens, and fatty acid composition of ethanolamine and choline phosphoglycerides. J. Neurosci. Res. 1988, 20, 484–490. [Google Scholar] [CrossRef]
- Al, M.D.; Hornstra, G.; van der Schouw, Y.T.; Bulstra-Ramakers, M.T.; Huisjes, H.J. Biochemical EFA status of mothers and their neonates after normal pregnancy. Early Hum. Dev. 1990, 24, 239–248. [Google Scholar] [CrossRef]
- Yehuda, S.; Rabinovitz, S.; Mostofsky, D.I. Essential fatty acids are mediators of brain biochemistry and cognitive functions. J. Neurosci. Res. 1999, 56, 565–570. [Google Scholar] [CrossRef]
- Gardner, H.W. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic. Biol. Med. 1989, 7, 65–86. [Google Scholar] [CrossRef]
- Koppenol, W.H. Oxyradical reactions: From bond-dissociation energies to reduction potentials. FEBS Lett. 1990, 264, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Neill, A.R.; Masters, C.J. Metabolism of fatty acids by bovine spermatozoa. Biochem. J. 1972, 127, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Neill, A.R.; Masters, C.J. Metabolism of fatty acids by ovine spermatozoa. J. Reprod. Fertil. 1973, 34, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.E. Lipids of ocular tissues. IV. A comparison of the phospholipids from the retina of six mammalian species. Exp. Eye Res. 1970, 10, 339–344. [Google Scholar] [CrossRef]
- Breckenridge, W.C.; Gombos, G.; Morgan, I.G. The lipid composition of adult rat brain synaptosomal plasma membranes. Biochim. Biophys. Acta 1972, 266, 695–707. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Roqueta-Rivera, M.; Stroud, C.K.; Haschek, W.M.; Akare, S.J.; Segre, M.; Brush, R.S.; Agbaga, M.P.; Anderson, R.E.; Hess, R.A.; Nakamura, M.T. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J. Lipid Res. 2010, 51, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, J.P.; Church, D.F.; Pryor, W.A. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids 1987, 22, 299–304. [Google Scholar] [CrossRef]
- Catalá, A. Five decades with polyunsaturated fatty acids: Chemical synthesis, enzymatic formation, lipid peroxidation and its biological effects. J. Lipids 2013, 2013, 710290. [Google Scholar] [CrossRef] [Green Version]
- Kristal, B.S.; Park, B.K.; Yu, B.P. 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J. Biol. Chem. 1996, 271, 6033–6038. [Google Scholar] [CrossRef] [Green Version]
- Ortega Ferrusola, C.; González Fernández, L.; Salazar Sandoval, C.; Macías García, B.; Rodríguez Martínez, H.; Tapia, J.A.; Peña, F.J. Inhibition of the mitochondrial permeability transition pore reduces “apoptosis like” changes during cryopreservation of stallion spermatozoa. Theriogenology 2010, 74, 458–465. [Google Scholar] [CrossRef]
- Uribe, P.; Cabrillana, M.E.; Fornés, M.W.; Treulen, F.; Boguen, R.; Isachenko, V.; Isachenko, E.; Sánchez, R.; Villegas, J.V. Nitrosative stress in human spermatozoa causes cell death characterized by induction of mitochondrial permeability transition-driven necrosis. Asian J. Androl. 2018, 20, 600–607. [Google Scholar]
- Aitken, R.J.; Gibb, Z.; Mitchell, L.A.; Lambourne, S.R.; Connaughton, H.S.; de Iuliis, G.N. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol. Reprod. 2012, 87, 110. [Google Scholar] [CrossRef] [Green Version]
- Martin Muñoz, P.; Ortega Ferrusola, C.; Vizuete, G.; Plaza Dávila, M.; Rodriguez Martinez, H.; Peña, F.J. Depletion of intracellular thiols and increased production of 4-hydroxynonenal that occur during cryopreservation of stallion spermatozoa lead to caspase activation, loss of motility, and cell death. Biol. Reprod. 2015, 93, 143. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2015, 5, 123–127. [Google Scholar]
- Suleiman, S.A.; Ali, M.E.; Zaki, Z.; El-Malik, E.; Nasr, M. Lipid peroxidation and human sperm motility: Protective role of vitamin E. J. Androl. 1996, 17, 530–537. [Google Scholar]
- Keskes-Ammar, L.; Feki-Chakroun, N.; Rebai, T.; Sahnoun, Z.; Ghozzi, H.; Hammami, S.; Zghal, K.; Fki, H.; Damak, J.; Bahloul, A. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch. Androl. 2003, 49, 83–94. [Google Scholar] [CrossRef]
- Tavilani, H.; Doosti, M.; Saeidi, H. Malondialdehyde levels in sperm and seminal plasma of asthenozoospermic and its relationship with semen parameters. Clin. Chim. Acta 2005, 356, 199–203. [Google Scholar] [CrossRef]
- Wolff, H.; Anderson, D.J. Immunohistologic characterization and quantitation of leukocyte subpopulations in human semen. Fertil. Steril. 1988, 49, 497–504. [Google Scholar] [CrossRef]
- Wolff, H.; Politch, J.A.; Martinez, A.; Haimovici, F.; Hill, J.A.; Anderson, D.J. Leukocytospermia is associated with poor semen quality. Fertil. Steril. 1990, 53, 528–536. [Google Scholar] [CrossRef]
- Kaleli, S.; Öçer, F.; Irez, T.; Budak, E.; Aksu, M.F. Does leukocytospermia associate with poor semen parameters and sperm functions in male infertility? The role of different seminal leukocyte concentrations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 89, 185–191. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar]
- El-Demiry, M.I.; Young, H.; Elton, R.A.; Hargreave, T.B.; James, K.; Chisholm, G.D. Leucocytes in the ejaculate from fertile and infertile men. Br. J. Urol. 1986, 58, 715–720. [Google Scholar] [CrossRef]
- Kung, A.; Ho, P.; Wang, C. Seminal leucocyte subpopulations and sperm function in fertile and infertile Chinese men. Int. J. Androl. 1993, 16, 189–194. [Google Scholar] [CrossRef]
- Harrison, P.; Barratt, C.; Robinson, A.; Kessopoulou, E.; Cooke, I. Detection of white blood cell populations in the ejaculates of fertile men. Am. J. Reprod. Immunol. 1991, 19, 95–98. [Google Scholar] [CrossRef]
- WHO, Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interation; Cambridge University Press: Cambridge, UK, 2010.
- Wolff, H. The biologic significance of white blood cells in semen. Fertil. Steril. 1995, 63, 1143–1157. [Google Scholar]
- Flickinger, C.J.; Bush, L.A.; Howards, S.S.; Herr, J.C. Distribution of leukocytes in the epithelium and interstitium of four regions of the Lewis rat epididymis. Anat. Rec. 1997, 248, 380–390. [Google Scholar] [CrossRef]
- Mohammad Eid Hammadeh, M.E.; Filippos, A.A.; Hamad, M.F. Reactive oxygen species and antioxidant in seminal plasma and their impact on male fertility. Int. J. Fertil. Steril. 2009, 3, 87–110. [Google Scholar]
- Aitken, R.J.; Baker, M.A. Oxidative stress, spermatozoa and leukocytic infiltration: Relationships forged by the opposing forces of microbial invasion and the search for perfection. Am. J. Reprod. Immunol. 2013, 100, 11–19. [Google Scholar] [CrossRef]
- Krausz, C.; Mills, C.; Rogers, S.; Tan, S.; Aitken, R.J. Stimulation of oxidant generation by human sperm suspensions using phorbol esters and formyl peptides: Relationships with motility and fertilization in vitro. Fertil. Steril. 1994, 62, 599–605. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Buckingham, D.W.; West, K.M. Reactive oxygen species and human spermatozoa: Analysis of the cellular mechanisms involved in luminol- and lucigenin-dependent chemiluminescence. J. Cell. Physiol. 1992, 151, 466–477. [Google Scholar] [CrossRef]
- Gavella, M.; Lipovac, V. NADH-dependent oxido-reductase (diaphorase) activity and isozyme pattern of sperm in infertile men. Arch. Androl. 1992, 28, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Clarkson, J.S.; Hargreave, T.B.; Irvine, D.S.; Wu, F.C. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J. Androl. 1989, 10, 214–220. [Google Scholar] [CrossRef]
- De Lamirande, E.; Tsai, C.; Harakat, A.; Gagnon, C. Involvement of reactive oxygen species in human sperm acrosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J. Androl. 1998, 19, 585–594. [Google Scholar]
- Aitken, R.J.; Fisher, H.M.; Fulton, N.; Gomez, E.; Knox, W.; Lewis, B.; Irvine, S. Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol. Reprod. Dev. 1997, 47, 468–482. [Google Scholar] [CrossRef]
- Vernet, P.; Fulton, N.; Wallace, C.; Aitken, R.J. Analysis of reactive oxygen species generating systems in rat epididymal spermatozoa. Biol. Reprod. 2001, 65, 1102–1113. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Vernet, P. Maturation of redox regulatory mechanisms in the epididymis. J. Reprod. Fertil. Suppl. 1998, 53, 109–118. [Google Scholar]
- Aitken, R.J.; Ryan, A.L.; Baker, M.A.; McLaughlin, E.A. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radic. Biol. Med. 2004, 36, 994–1010. [Google Scholar] [CrossRef]
- Bánfi, B.; Molnár, G.; Maturana, A.; Steger, K.; Hegedûs, B.; Demaurex, N.; Krause, K.H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001, 276, 37594–37601. [Google Scholar] [CrossRef] [Green Version]
- Sabeur, K.; Ball, B.A. Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction 2007, 134, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Musset, B.; Clark, R.A.; DeCoursey, T.E.; Petheo, G.L.; Geiszt, M.; Chen, Y.; Cornell, J.E.; Eddy, C.A.; Brzyski, R.G.; El Jamali, A. NOX5 in human spermatozoa expression, function, and regulation. J. Biol. Chem. 2012, 287, 9376–9388. [Google Scholar] [CrossRef] [Green Version]
- Bánfi, B.; Tirone, F.; Durussel, I.; Knisz, J.; Moskwa, P.; Molnár, G.Z.; Krause, K.H.; Cox, J.A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J. Biol. Chem. 2004, 279, 18583–18591. [Google Scholar] [CrossRef] [Green Version]
- Vatannejad, A.; Tavilani, H.; Sadeghi, M.R.; Karimi, M.; Lakpour, N.; Amanpour, S.; Shabani Nashtaei, M.; Doosti, M. Evaluation of the NOX5 protein expression and oxidative stress in sperm from asthenozoospermic men compared to normozoospermic men. J. Endocrinol. Invest. 2019, 42, 1181–1189. [Google Scholar] [CrossRef]
- Armstrong, J.S.; Bivalacqua, T.J.; Chamulitrat, W.; Sikka, S.; Hellstrom, W.J. A comparison of the NADPH oxidase in human sperm and white blood cells. Int. J. Androl. 2002, 25, 223–229. [Google Scholar] [CrossRef]
- Baker, M.A.; Reeves, G.; Hetherington, L.; Müller, J.; Baur, I.; Aitken, R.J. Identification of gene products present in Triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis. Proteom. Clin. Appl. 2007, 1, 524–532. [Google Scholar] [CrossRef]
- Baker, M.A.; Naumovski, N.; Hetherington, L.; Weinberg, A.; Velkov, T.; Aitken, R.J. Head and flagella subcompartmental proteomic analysis of human spermatozoa. Proteomics 2013, 13, 61–74. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Zhou, T.; Shi, X.; Yu, J.; Yang, Y.; Wu, Y.; Wang, J.; Liu, M.; Chen, X.; et al. In-depth proteomic analysis of the human sperm reveals complex protein compositions. J. Proteom. 2013, 79, 114–122. [Google Scholar] [CrossRef]
- De Lamirande, E.; Harakat, A.; Gagnon, C. Human sperm capacitation induced by biological fluids and progesterone, but not by NADH or NADPH, is associated with the production of superoxide anion. J. Androl. 1998, 19, 215–225. [Google Scholar]
- Richer, S.C.; Ford, W.C. A critical investigation of NADPH oxidase activity in human spermatozoa. Mol. Hum. Reprod. 2001, 7, 237–244. [Google Scholar] [CrossRef]
- Baker, M.A.; Krutskikh, A.; Curry, B.J.; McLaughlin, E.A.; Aitken, R.J. Identification of cytochrome P450-reductase as the enzyme responsible for NADPH-dependent lucigenin and tetrazolium salt reduction in rat epididymal sperm preparations. Biol. Reprod. 2004, 71, 307–318. [Google Scholar] [CrossRef]
- Baker, M.A.; Krutskikh, A.; Curry, B.J.; Hetherington, L.; Aitken, R.J. Identification of cytochrome-b5 reductase as the enzyme responsible for NADH-dependent lucigenin chemiluminescence in human spermatozoa. Biol. Reprod. 2005, 73, 334–342. [Google Scholar] [CrossRef]
- Katsuki, H.; Okuda, S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995, 46, 607–636. [Google Scholar] [CrossRef]
- Shiose, A.; Sumimoto, H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J. Biol. Chem. 2000, 275, 13793–13801. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Dinauer, M.C. Impaired NADPH oxidase activity in Rac2-deficient murine neutrophils does not result from defective translocation of p47phox and p67phox and can be rescued by exogenous arachidonic acid. J. Biol. Chem. 2006, 79, 223–234. [Google Scholar]
- De Carvalho, D.D.; Sadok, A.; Bourgarel-Rey, V.; Gattacceca, F.; Penel, C.; Lehmann, M.; Kovacic, H. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. Int. J. Cancer 2008, 122, 1757–1764. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.J.; Seo, J.M.; Kim, J.H. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol. Cells 2011, 32, 1–5. [Google Scholar] [CrossRef]
- Bromfield, E.G.; Mihalas, B.P.; Dun, M.D.; Aitken, R.J.; McLaughlin, E.A.; Walters, J.L.; Nixon, B. Inhibition of arachidonate 15-lipoxygenase prevents 4-hydroxynonenal-induced protein damage in male germ cells. Biol. Reprod. 2017, 96, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.A.; Arunasree, K.M.; Roy, K.R.; Reddy, N.P.; Aparna, A.; Reddy, G.V.; Reddanna, P. Effects of (15S)-hydroperoxyeicosatetraenoic acid and (15S)-hydroxyeicosatetraenoic acid on the acute- lymphoblastic-leukaemia cell line Jurkat: Activation of the Fas-mediated death pathway. Biotechnol. Appl. Biochem. 2009, 52, 121–133. [Google Scholar] [CrossRef]
- Tahara, E.B.; Navarete, F.D.; Kowaltowski, A.J. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic. Biol. Med. 2009, 46, 1283–1297. [Google Scholar] [CrossRef]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Dröse, S.; Brandt, U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012, 748, 145–169. [Google Scholar]
- Koppers, A.J.; de Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [Green Version]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The paradoxical relationship between stallion fertility and oxidative stress. Biol. Reprod. 2014, 91, 77. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell. Physiol. 2008, 217, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Aitken, R.J. Nitroblue tetrazolium (NBT) assay. Reprod. Biomed. Online 2018, 36, 90–91. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Daia, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 38, 1–17. [Google Scholar] [CrossRef]
- Said, T.M.; Agarwal, A.; Sharma, R.K.; Mascha, E.; Sikka, S.C.; Thomas, A.J., Jr. Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility. Fertil. Steril. 2004, 82, 871–877. [Google Scholar] [CrossRef]
- Tunc, O.; Thompson, J.; Tremellen, K. Development of the NBT assay as a marker of sperm oxidative stress. Int. J. Androl. 2010, 33, 13–21. [Google Scholar] [CrossRef]
- Pujol, A.; Obradors, A.; Esteo, E.; Costilla, B.; García, D.; Vernaeve, V.; Vassena, R. Oxidative stress level in fresh ejaculate is not related to semen parameters or to pregnancy rates in cycles with donor oocytes. J. Assist. Reprod. Genet. 2016, 33, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Donà, G.; Fiore, C.; Andrisani, A.; Ambrosini, G.; Brunati, A.; Ragazzi, E.; Armanini, D.; Bordin, L.; Clari, G. Evaluation of correct endogenous reactive oxygen species content for human sperm capacitation and involvement of the NADPH oxidase system. Hum. Reprod. 2011, 26, 3264–3273. [Google Scholar] [CrossRef] [Green Version]
- McKinney, K.A.; Lewis, S.E.; Thompson, W. Reactive oxygen species generation in human sperm: Luminol and lucigenin chemiluminescence probes. Arch. Androl. 1996, 36, 119–125. [Google Scholar] [CrossRef]
- Said, T.M.; Agarwal, A.; Sharma, R.K.; Thomas, A.J.; Sikka, S.C. Impact of sperm morphology on DNA damage caused by oxidative stress induced by β-nicotinamide adenine dinucleotide phosphate. Fertil. Steril. 2005, 83, 95–103. [Google Scholar] [CrossRef]
- Gosálvez, J.; Coppola, L.; Fernández, J.L.; López-Fernández, C.; Góngora, A.; Faundez, R.; Kim, J.; Sayme, N.; de la Casa, M.; Santiso, R.; et al. Multi-centre assessment of nitroblue tetrazolium reactivity in human semen as a potential marker of oxidative stress. Reprod. Biomed. Online 2017, 34, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Merényi, G.; Lind, J.; Eriksen, T.E. Luminol chemiluminescence: Chemistry, excitation, emitter. J. Biolumin. Chemilumin. 1990, 5, 53–56. [Google Scholar] [CrossRef]
- Faulkner, K.; Fridovich, I. Luminol and lucigenin as detectors for O2.-. Free Radic. Biol. Med. 1993, 15, 447–451. [Google Scholar] [CrossRef]
- Prichard, P.M.; Cormier, M.J. Studies on the mechanism of the horseradish peroxidase catalyzed luminescent peroxidation of luminol. Biochem. Biophys. Res. Commun. 1968, 3, 131–136. [Google Scholar] [CrossRef]
- Vilim, V.; Wilhelm, J. What do we measure by a luminol-dependent chemiluminescence of phagocytes? Free Radic. Biol. Med. 1989, 6, 623–629. [Google Scholar] [CrossRef]
- Gil-Guzman, E.; Ollero, M.; Lopez, M.C.; Sharma, R.K.; Alvarez, J.G.; Thomas, A.J., Jr.; Agarwal, A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod. 2001, 16, 1922–1930. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.; Saleh, R.A.; Sharma, R.K.; Lewis-Jones, I.; Esfandiari, N.; Thomas, A.J., Jr.; Agarwal, A. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004, 81, 349–354. [Google Scholar] [CrossRef]
- Aitken, J.; Krausz, C.; Buckingham, D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol. Reprod. Dev. 1994, 39, 268–279. [Google Scholar] [CrossRef]
- Zhao, H.; Kalivendi, S.; Zhang, H.; Joseph, J.; Nithipatikom, K.; Vásquez-Vivar, J.; Kalyanaraman, B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: Potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003, 34, 1359–1368. [Google Scholar] [CrossRef]
- Zielonka, J.; Kalyanaraman, B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic. Biol. Med. 2010, 48, 983–1001. [Google Scholar] [CrossRef] [Green Version]
- Burnaugh, L.; Sabeur, K.; Ball, B. Generation of superoxide anion by equine spermatozoa as detected by dihydroethidium. Theriogenology 2007, 67, 580–589. [Google Scholar] [CrossRef]
- Espinoza, J.; Schulz, M.; Sánchez, R.; Villegas, J. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia 2009, 41, 51–54. [Google Scholar] [CrossRef]
- Aitken, R.J.; Hanson, A.R.; Kuczera, L. Electrophoretic sperm isolation: Optimization of electrophoresis conditions and impact on oxidative stress. Hum. Reprod. 2011, 26, 1955–1964. [Google Scholar] [CrossRef] [Green Version]
- Mahfouz, R.Z.; du Plessis, S.S.; Aziz, N.; Sharma, R.; Sabanegh, E.; Agarwal, A. Sperm viability, apoptosis, and intracellular reactive oxygen species levels in human spermatozoa before and after induction of oxidative stress. Fertil. Steril. 2010, 93, 814–821. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Fales, H.M.; Sokoloski, E.A.; Levine, R.L.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 2005, 102, 5727–5732. [Google Scholar] [CrossRef] [Green Version]
- Kalyanaraman, B.; Dranka, B.P.; Hardy, M.; Michalski, R.; Zielonka, J. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes—The ultimate approach for intra- and extracellular superoxide detection. Biochim. Biophys. Acta 2014, 1840, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Netherton, J.K.; Hetherington, L.; Ogle, R.A.; Mazloumi, M.; Velkov, T.; Villaverde, A.I.S.B.; Tanphaichitr, N.; Baker, M.A. Mass Spectrometry reveals new insights into the production of superoxide anions and 4-hydroxynonenal adducted proteins in human sperm. Proteomics 2019, accepted. [Google Scholar]
- Kodama, H.; Yamaguchi, R.; Fukuda, J.; Kasai, H.; Tanaka, T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil. Steril. 1997, 68, 519–524. [Google Scholar] [CrossRef]
- Aitken, R.J.; de Iuliis, G.N.; Finnie, J.M.; Hedges, A.; McLachlan, R.I. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Hum. Reprod. 2010, 25, 2415–2426. [Google Scholar] [CrossRef] [Green Version]
- Guz, J.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Zarakowska, E.; Siomek, A.; Szpila, A.; Kotzbach, M.; Kotzbach, R.; Olinski, R. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS ONE 2013, 8, e68490. [Google Scholar] [CrossRef] [Green Version]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]

| Probe | Method | Characteristics and Limiting Factors |
|---|---|---|
| Tetrazolium salts | Colorimetric | Nitro blue tetrazolium (NBT) is the most commonly used one Low sensitivity to detect ROS Low specificity for O2•− detection, with various intracellular reductases being able to generate the same response Autoxidation can generate O2•− |
| Lucigenin | Chemiluminescence | More specific for extracellular O2•− Inability to detect O2•− at low level Low specificity for O2•− detection. Signal can be triggered by various nucleophiles and reducing agents, being sensitive to changes in the reductase activity within the tested systems. Reduced radical can generate O2•− |
| Luminol/HRP | Chemiluminescence | Allows the detection of both intra- and extracellular ROS Reacts with several electron-donor compounds, showing indiscriminate recognition of numerous free radicals The luminol radical formed by various univalent oxidants can form O2•− through autoxidation Susceptible to various interferences in biological systems, such as poor ROS detection at neutral pH and absorption of the emitted light (400 nm) by some biomolecules |
| DHE | Fluorescence HPLC and LC–MS | Used to detect intracellular O2•− Highly specific for O2•− detection, producing 2-hydroxyethidium (2-OH-E+); however, the majority of DHE reacts with other oxidants, resulting in the production of ethidium (E+) Both by-products of non-specific (E+) and specific (2-OH-E+) oxidation have overlapping fluorescence properties, thus not allowing distinction by fluorescence methods. For specific O2•− quantification, 2-OH-E+ must be measured by techniques such as HPLC and LC-MS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From Past to Present: The Link Between Reactive Oxygen Species in Sperm and Male Infertility. Antioxidants 2019, 8, 616. https://doi.org/10.3390/antiox8120616
Villaverde AISB, Netherton J, Baker MA. From Past to Present: The Link Between Reactive Oxygen Species in Sperm and Male Infertility. Antioxidants. 2019; 8(12):616. https://doi.org/10.3390/antiox8120616
Chicago/Turabian StyleVillaverde, Ana Izabel Silva Balbin, Jacob Netherton, and Mark A. Baker. 2019. "From Past to Present: The Link Between Reactive Oxygen Species in Sperm and Male Infertility" Antioxidants 8, no. 12: 616. https://doi.org/10.3390/antiox8120616




