Protective Role of Peroxiredoxins against Reactive Oxygen Species in Neonatal Rat Testicular Gonocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Gonocyte Isolation and in Vitro Culture and Treatments
2.4. Cell Viability
2.5. RNA Extraction and Real-Time Quantitative PCR (Q-PCR) Analysis
2.6. Gene Array Analysis
2.7. Lipid Peroxidation Measurement by Bodipy Labelling
2.8. Immunohistochemistry (IHC)/Immunocytochemistry (ICC)
2.9. Statistical Analysis
3. Results
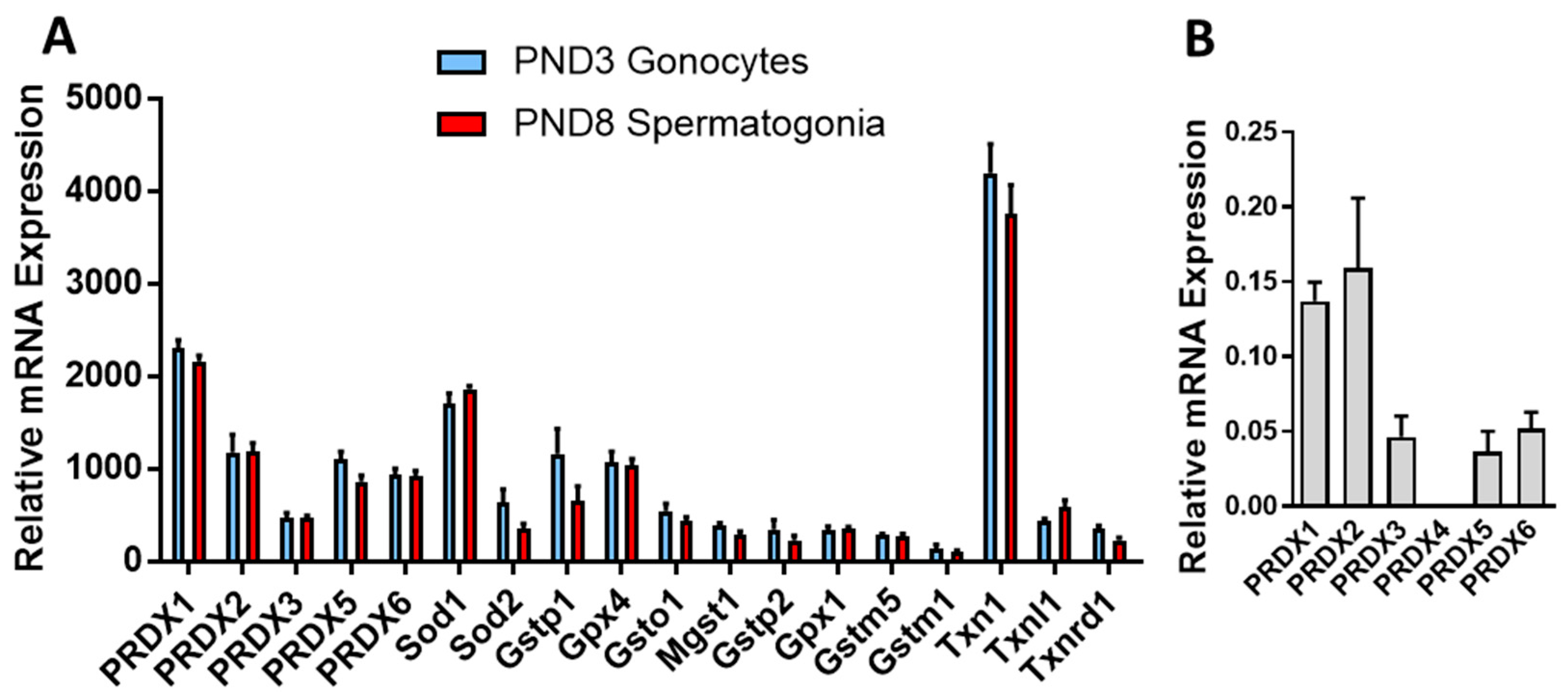
3.1. PRDXs Are among the Highest Expressed Antioxidant Genes in Neonatal Gonocytes and in Spermatogonia
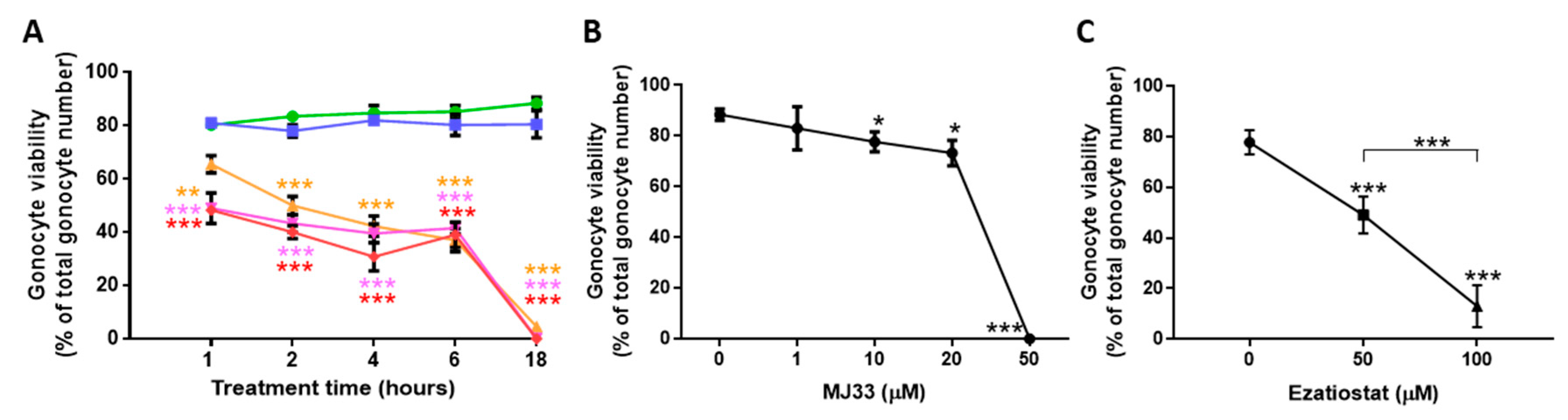
3.2. PRDXs Are Required for PND3 Gonocyte Survival in Basal Conditions
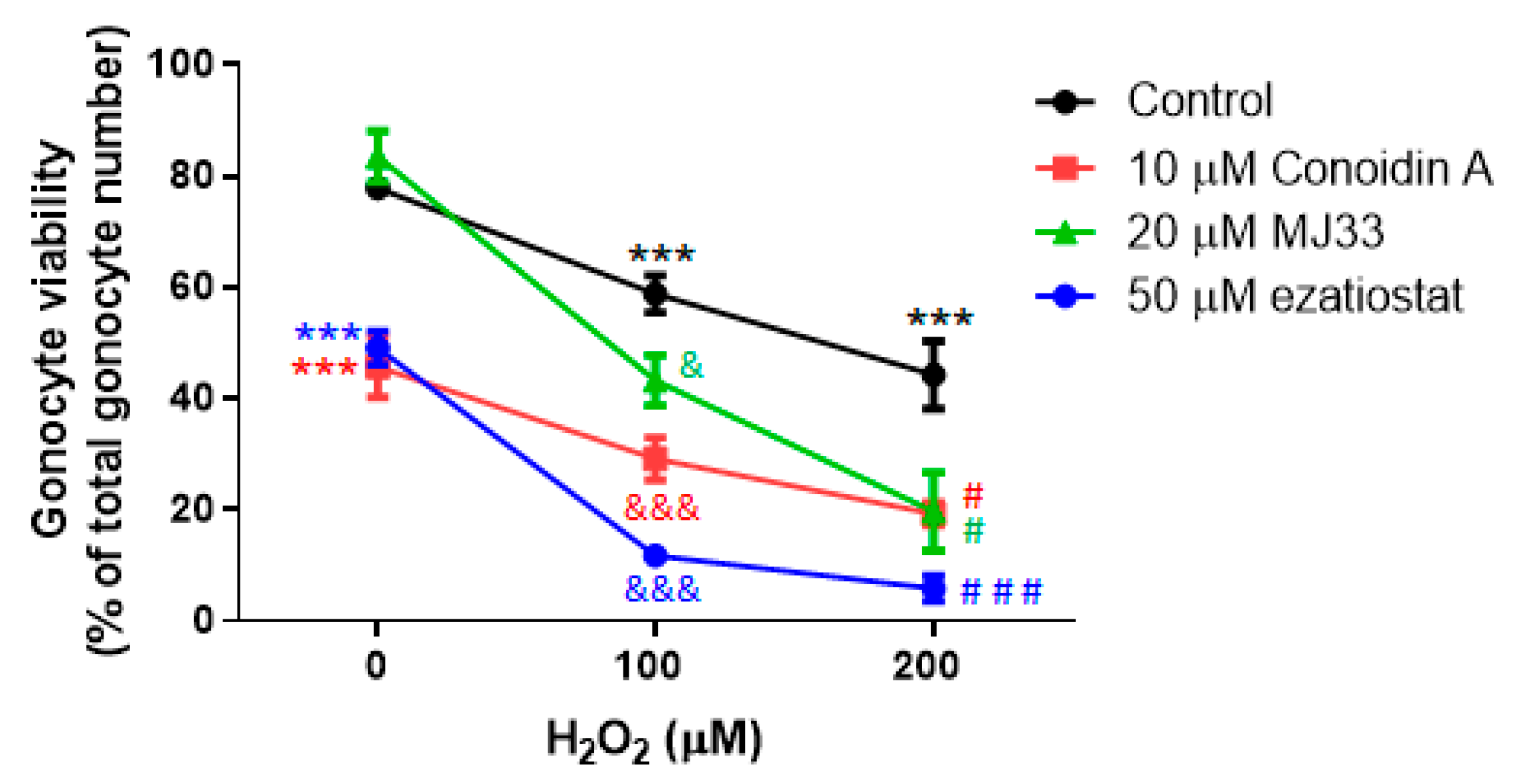
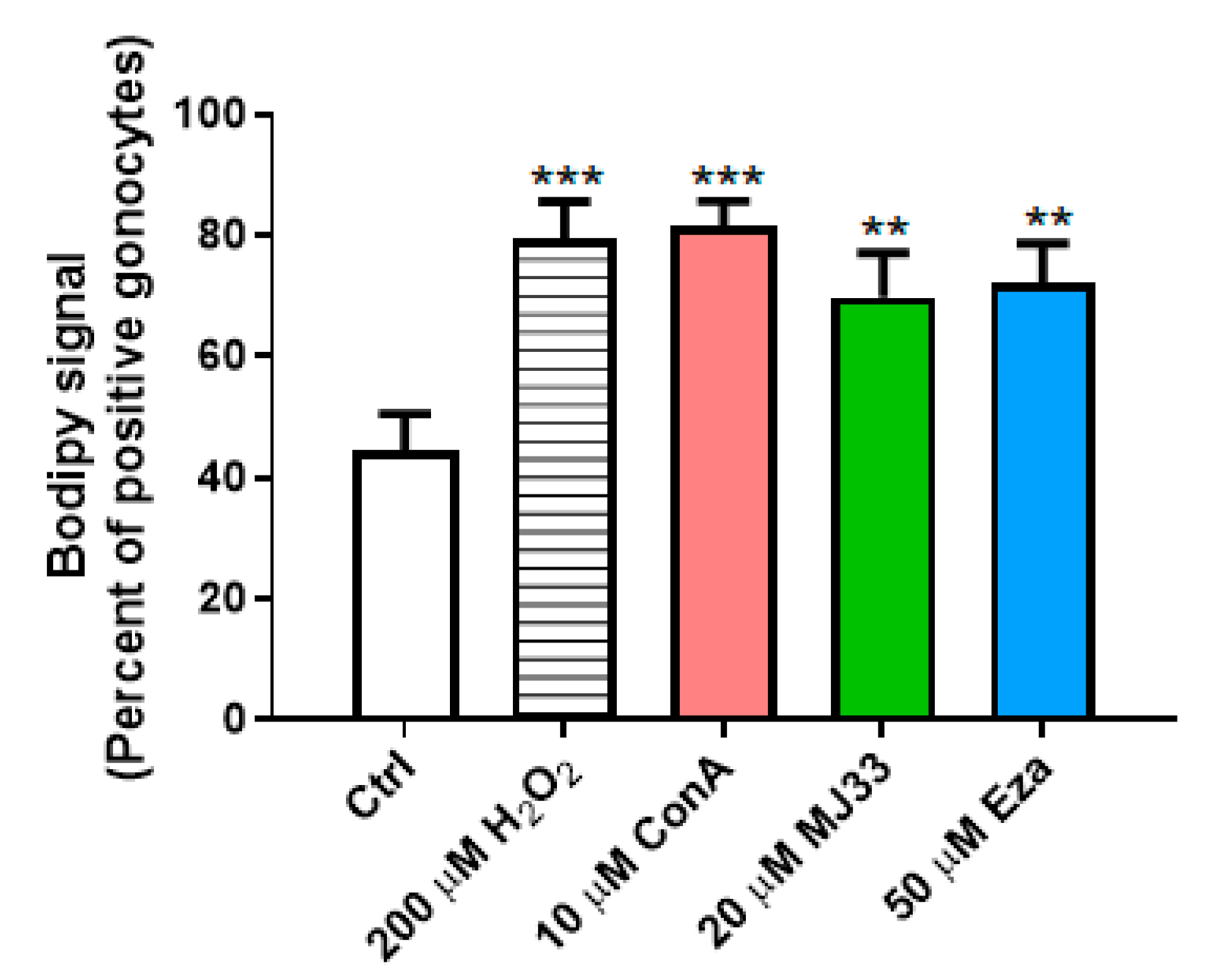
3.3. H2O2 Induces Lipid Peroxidation, and PRDXs Prevent Endogenous ROS-Induced Lipid Peroxidation in PND3 Gonocytes
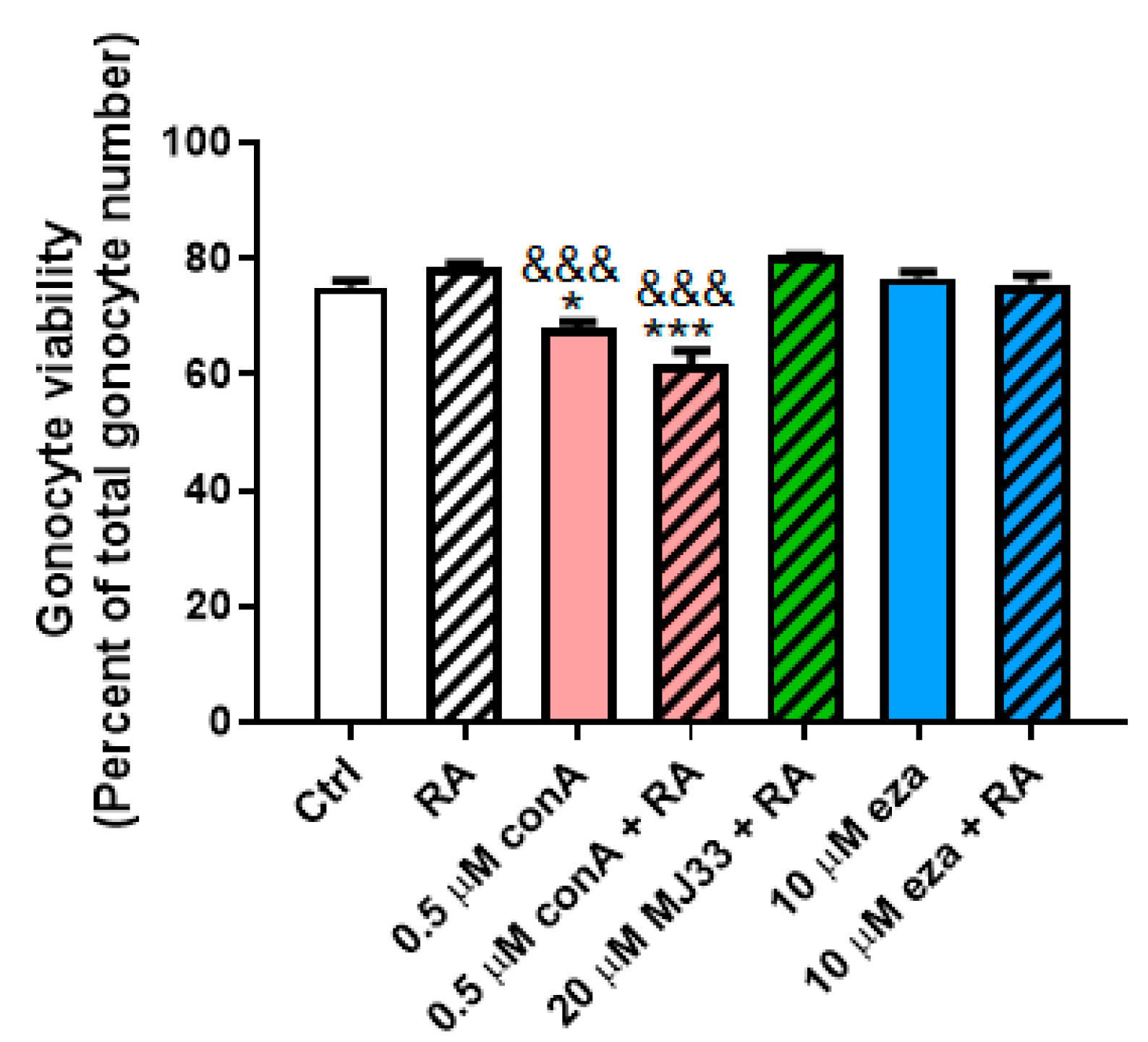
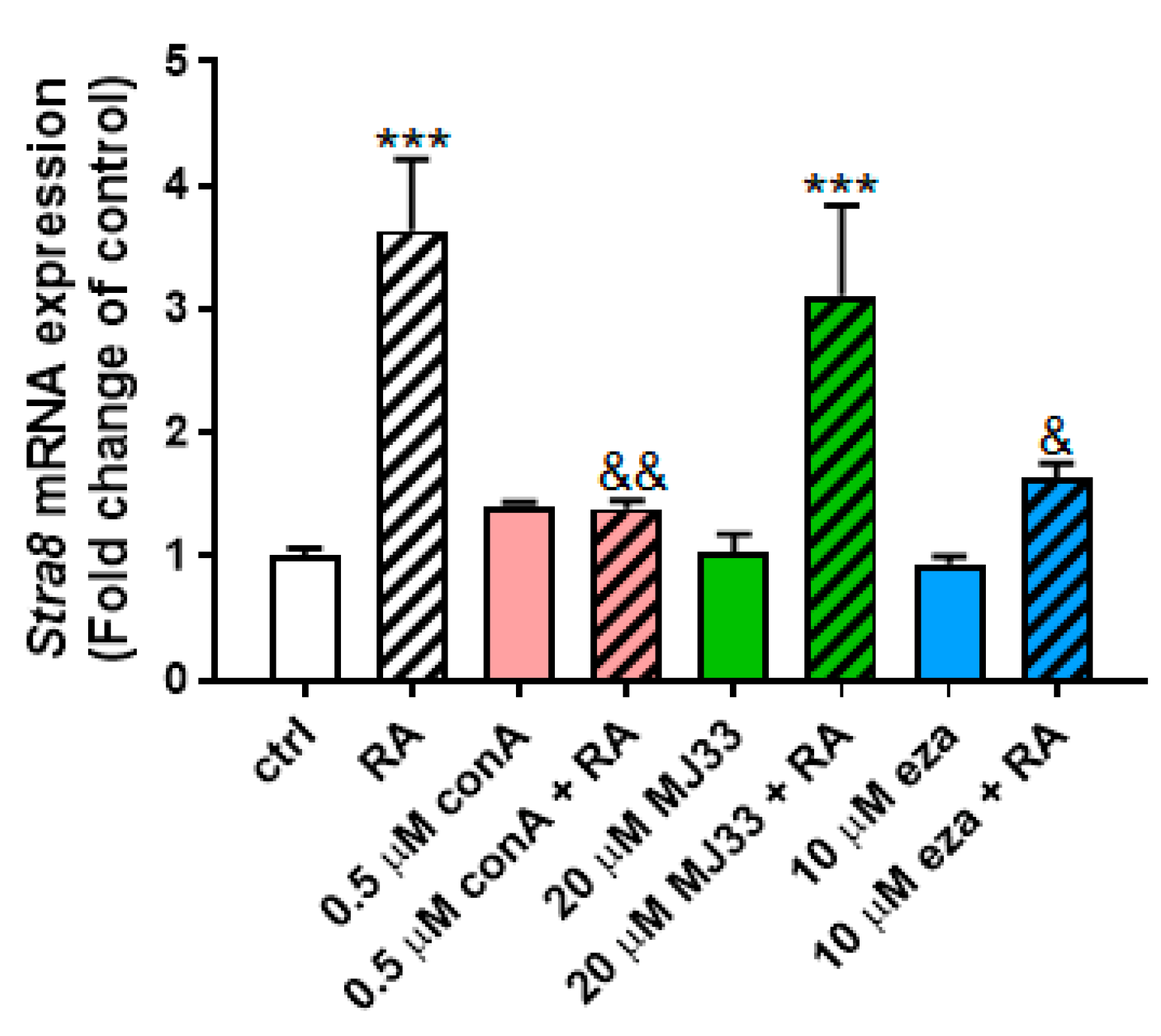
3.4. PRDX Inhibition Blocks RA-Induced Differentiation in PND3 Gonocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rhee, S.G.; Kil, I.S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2017, 86, 749–775. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar] [PubMed]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Peroxiredoxins: Hidden players in the antioxidant defence of human spermatozoa. Basic Clin. Androl. 2014, 24, 4. [Google Scholar] [CrossRef]
- Karlenius, T.C.; Tonissen, K.F. Thioredoxin and Cancer: A Role for Thioredoxin in all States of Tumor Oxygenation. Cancers (Basel) 2010, 2, 209–232. [Google Scholar] [CrossRef]
- O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Absence of Peroxiredoxin 6 Amplifies the Effect of Oxidant Stress on Mobility and SCSA/CMA3 Defined Chromatin Quality and Impairs Fertilizing Ability of Mouse Spermatozoa. Biol. Reprod. 2016, 94, 68. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- Liu, Y.; O’Flaherty, C. In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality. Asian J. Androl. 2017, 19, 73–79. [Google Scholar]
- Lee, D.; Moawad, A.R.; Morielli, T.; Fernandez, M.C.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef]
- Moawad, A.R.; Fernandez, M.C.; Scarlata, E.; Dodia, C.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Deficiency of peroxiredoxin 6 or inhibition of its phospholipase A2 activity impair the in vitro sperm fertilizing competence in mice. Sci. Rep. 2017, 7, 12994. [Google Scholar] [CrossRef]
- Gong, S.; San Gabriel, M.C.; Zini, A.; Chan, P.; O’Flaherty, C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J. Androl. 2012, 33, 1342–1351. [Google Scholar] [CrossRef]
- Culty, M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res. Part C 2009, 87, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Culty, M. Gonocytes, from the Fifties to the Present: Is There a Reason to Change the Name? Biol. Reprod. 2013, 89, 46. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Culty, M. Mammalian gonocyte and spermatogonia differentiation: Recent advances and remaining challenges. Reproduction 2015, 149, R139–R157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Culty, M. Identification and distribution of a novel platelet-derived growth factor receptor beta variant. Effect of retinoic acid and involvement in cell differentiation. Endocrinology 2007, 148, 2233–2250. [Google Scholar] [CrossRef]
- Manku, G.; Wang, Y.; Merkbaoui, V.; Boisvert, A.; Ye, X.; Blonder, J.; Culty, M. Role of Retinoic Acid and Platelet-Derived Growth Factor Receptor crosstalk in the regulation of neonatal gonocyte and embryonal carcinoma cell differentiation. Endocrinology 2015, 156, 346–359. [Google Scholar] [CrossRef]
- Manku, G.; Wing, S.S.; Culty, M. Expression of the Ubiquitin Proteasome System in Neonatal Rat Gonocytes and Spermatogonia: Role in Gonocyte Differentiation. Biol. Reprod. 2012, 87, 44. [Google Scholar] [CrossRef]
- Manku, G.; Papadopoulos, P.; Boisvert, A.; Culty, M. Cyclooxygenase 2 (COX2) Expression and prostaglandin synthesis in Neonatal Rat Testicular Germ Cells: Effects of Acetaminophen and Ibuprofen. Andrology 2019, in press. [Google Scholar] [CrossRef]
- Rossi, S.P.; Windschüttl, S.; Matzkin, M.E.; Rey-Ares, V.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.S.; Mayerhofer, A.; et al. Reactive oxygen species (ROS) production triggered by prostaglandin D2 (PGD2) regulates lactate dehydrogenase (LDH) expression/activity in TM4 Sertoli cells. Mol. Cellular Endoc. 2016, 434, 154–165. [Google Scholar] [CrossRef]
- Kiritoshi, S.; Nishikawa, T.; Sonoda, K.; Kukidome, D.; Senokuchi, T.; Matsuo, T.; Matsumura, T.; Tokunaga, H.; Brownlee, M.; Araki, E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: Potential role in diabetic nephropathy. Diabetes 2003, 52, 2570–2577. [Google Scholar] [CrossRef]
- Thuillier, R.; Mazer, M.; Manku, G.; Boisvert, A.; Wang, Y.; Culty, M. Interdependence of PDGF and estrogen signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol. Reprod. 2010, 82, 825–836. [Google Scholar] [CrossRef]
- Manku, G.; Mazer, M.; Culty, M. Neonatal testicular gonocytes isolation and processing for immunocytochemical analysis. Methods Mol. Biol. 2012, 825, 17–29. [Google Scholar] [PubMed]
- Culty, M.; Liu, Y.; Manku, G.; Chan, W.-Y. Papadopoulos. Expression of steroidogenesis-related genes in murine male germ cells. Steroids 2015, 103, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, A.; Jones, S.; Issop, L.; Erythropel, H.C.; Papadopoulos, V.; Culty, M. In vitro Functional screening as a means to identify new plasticizers devoid of reproductive toxicity. Environ. Res. 2016, 150, 496–512. [Google Scholar] [CrossRef]
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995, 7, 659–668. [Google Scholar] [CrossRef]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 activates maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Crawford, L.A.; Weerapana, E. A tyrosine-reactive irreversible inhibitor for glutathione S-transferase Pi (GSTP1). Mol. Biosyst. 2016, 12, 1768–1771. [Google Scholar] [CrossRef]
- Liu, X.; An, B.H.; Kim, M.J.; Park, J.H.; Kang, Y.S.; Chang, M. Human glutathione S-transferase P1-1 functions as an estrogen receptor alpha signaling modulator. Biochem. Biophys. Res. Commun. 2014, 452, 840–844. [Google Scholar] [CrossRef]
- Mahadevan, D.; Sutton, G.R. Ezatiostat hydrochloride for the treatment of myelodysplastic syndromes. Expert Opin. Investig. Drugs 2015, 24, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Peker, E.; Amponsah, P.S.; Hoehne, M.N.; Riemer, T.; Mai, M.; Bienert, G.P.; Deponte, M.; Morgan, B.; Riemer, J. Hyperoxidation of mitochondrial peroxiredoxin limits H2O2-induced cell death in yeast. EMBO J. 2019, 38, e101552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lien, Y.C.; Shuvaeva, T.; DeBolt, K.; Feinstein, S.I.; Fisher, A.B. Functional interaction of glutathione S-transferase pi and peroxiredoxin 6 in intact cells. Int. J. Biochem. Cell Biol. 2013, 45, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Munoz-Palma, E.; González-Billault, C. From birth to death: A role for reactive oxygen species in neuronal development. Semin. Cell Dev. Biol. 2018, 80, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Niedenberger, B.A.; Busada, J.T.; Geyer, C.B. Marker expression reveals heterogeneity of spermatogonia in the neonatal mouse testis. Reproduction 2015, 149, 329–338. [Google Scholar] [CrossRef]
- Hermann, B.P.; Mutoji, K.N.; Velte, E.K.; Ko, D.; Oatley, J.M.; Geyer, C.B.; McCarrey, J.R. Transcriptional and Translational Heterogeneity among Neonatal Mouse Spermatogonia. Biol. Reprod. 2015, 92, 54. [Google Scholar] [CrossRef]
- Fernandez, M.C.; Yu, A.; Moawad, A.R.; O’Flaherty, C. Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Mol. Hum. Reprod. 2019, 25, 787–796. [Google Scholar] [CrossRef]

| Gene | Accession Number | Forward and Reverse Primers |
|---|---|---|
| 18S rRNA | X01117.1 | Cgggtgctcttagctgagtgtcccg ctcgggcctgctttgaacac |
| Stra8 | XM_575429.2 | tgcttttgatgtggcgagct gcgctgatgttagacagacgct |
| Peroxiredoxin 1 (PRDX1) | NM_057114.1 | acctgtagctcgactctgctg aacagccgtggctttgaa |
| PRDX2 | NM_017169.1 | gactctcagttcacccacctg tattcagtgggcccaagc |
| PRDX3 | NM_022540.1 | agaagaacctgcttgacagaca caggggtgtggaatgaaga |
| PRDX4 | NM_053512.2 | tgagacactgcgtttggttc tgtttcactaccaggtttccag |
| PRDX5 | NM_053610.1 | agtgccgcggtgactatg caaaacacctttcttgtccttga |
| PRDX6 | NM_053576.2 | ttgattgctctttcaatagactctg ctgcaccattgtaagcattga |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Flaherty, C.; Boisvert, A.; Manku, G.; Culty, M. Protective Role of Peroxiredoxins against Reactive Oxygen Species in Neonatal Rat Testicular Gonocytes. Antioxidants 2020, 9, 32. https://doi.org/10.3390/antiox9010032
O’Flaherty C, Boisvert A, Manku G, Culty M. Protective Role of Peroxiredoxins against Reactive Oxygen Species in Neonatal Rat Testicular Gonocytes. Antioxidants. 2020; 9(1):32. https://doi.org/10.3390/antiox9010032
Chicago/Turabian StyleO’Flaherty, Cristian, Annie Boisvert, Gurpreet Manku, and Martine Culty. 2020. "Protective Role of Peroxiredoxins against Reactive Oxygen Species in Neonatal Rat Testicular Gonocytes" Antioxidants 9, no. 1: 32. https://doi.org/10.3390/antiox9010032
APA StyleO’Flaherty, C., Boisvert, A., Manku, G., & Culty, M. (2020). Protective Role of Peroxiredoxins against Reactive Oxygen Species in Neonatal Rat Testicular Gonocytes. Antioxidants, 9(1), 32. https://doi.org/10.3390/antiox9010032







