Influence of a Concurrent Exercise Training Intervention during Pregnancy on Maternal and Arterial and Venous Cord Serum Cytokines: The GESTAFIT Project
Abstract
1. Introduction
2. Methods
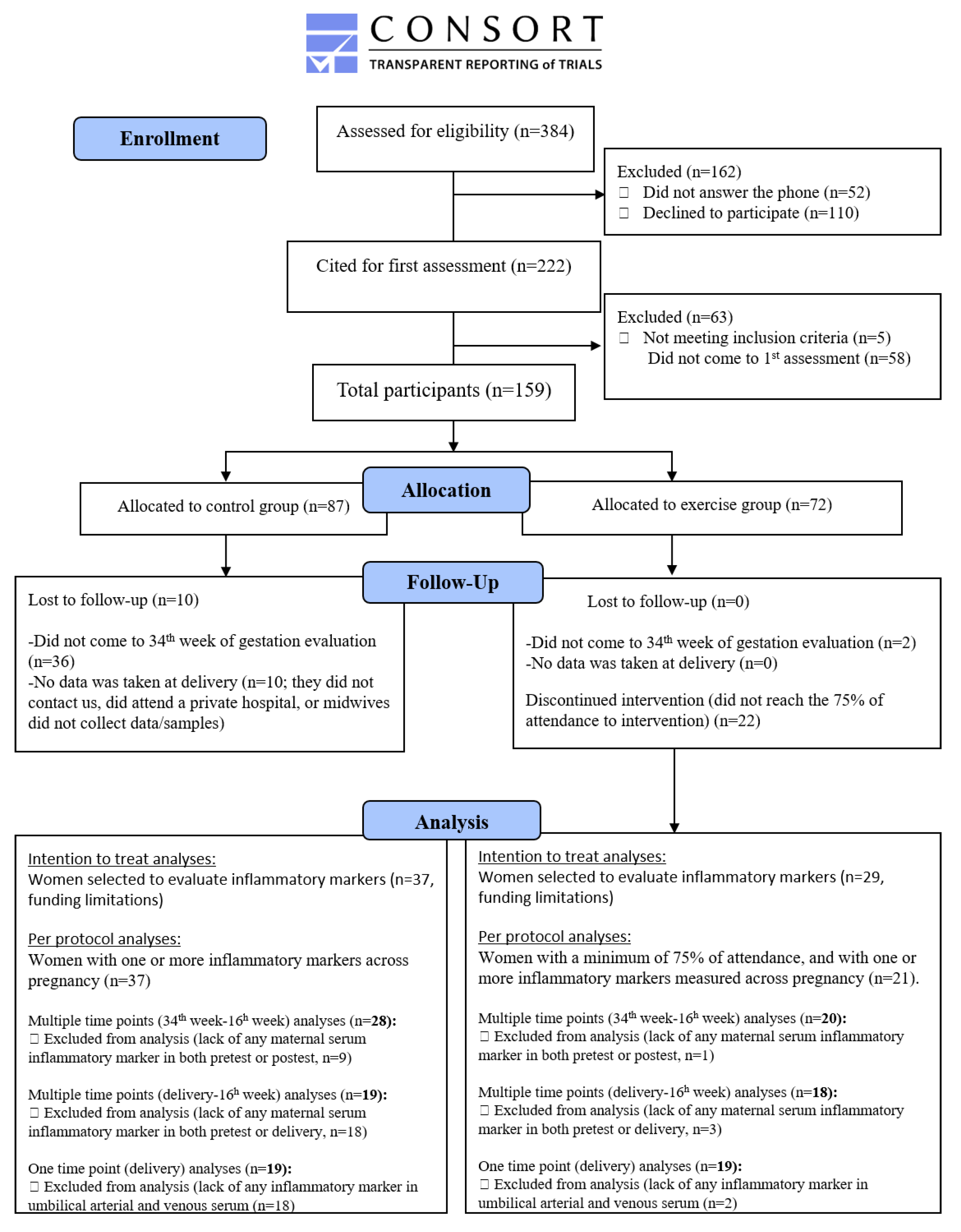
2.1. Settings and Eligibility Criteria
2.2. Sample Size
2.3. Randomization
2.4. General Procedure
2.5. Intervention
2.5.1. Exercise Group
2.5.2. Control Group
2.6. Outcome Measures
2.6.1. Sociodemographic and Clinical Data
2.6.2. Perinatal Outcomes
2.6.3. Weight Status
2.6.4. Blood Pressure and Resting Heart Rate
2.6.5. Mediterranean Diet Score
2.6.6. Sedentary Time and Physical Activity
2.6.7. Blood Collection
2.6.8. Cytokines
2.7. Statistical Analyses
2.8. Patient and Public Involvement
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Exercise Sessions
Appendix B. Talks Provided to Pregnant Women
Appendix C. Detailed Information about the Blood Samples Analyses
Appendix D. Outlier Detection and Management
Appendix E. Reasons for Losses and Exclusions during the Enrollment and Follow-Up
Appendix F. Effect of the Concurrent Exercise-Training Program on Maternal and Arterial and Venous Cord Serum Cytokines (Non-Statistically SIGNIFICANT Associations, but Evidence of STATISTICAL Significance)
References
- Kalagiri, R.R.; Carder, T.; Choudhury, S.; Vora, N.; Ballard, A.R.; Govande, V.; Dreyer, N.; Beeram, M.R.; Uddin, M.N. Inflammation in Complicated Pregnancy and Its Outcome. Am. J. Perinatol. 2016, 33, 1337–1356. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. In Reproductive Science; Guller, S., Bulletti, C., DeZiegler, D., Eds.; Blackwell Science Publ: Oxford, UK, 2011; Volume 1221, pp. 80–87. [Google Scholar]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Sharma, S. Interleukin-10: A Pleiotropic Regulator in Pregnancy. Am. J. Reprod. Immunol. 2015, 73, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Tayade, C.; Ashkar, A.A.; Hatta, K.; Zhang, J.H.; Croy, B.A. Interferon Gamma in Successful Pregnancies. Biol. Reprod. 2009, 80, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Knöfler, M. Human Tumour Necrosis Factor: Physiological and Pathological Roles in Placenta and Endometrium. Placenta 2009, 30, 111–123. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Hauguel-De Mouzon, S.; Lepercq, J.; Challier, J.C.; Huston-Presley, L.; Friedman, J.E.; Kalhan, S.C.; Catalano, P.M. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 2002, 51, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.N.; Chalak, L.F.; Liao, J.; Johnson-Welch, S.; Brown, L.S.; Longoria, C.; Savani, R.C.; Rosenfeld, C.R. Fetal-placental crosstalk occurs through fetal cytokine synthesis and placental clearance. Placenta 2018, 69, 1–8. [Google Scholar] [CrossRef]
- Dube, C.; Aguer, C.; Adamo, K.; Bainbridge, S. A role for maternally derived myokines to optimize placental function and fetal growth across gestation. Appl. Physiol. Nutr. Metab. 2017, 42, 459–469. [Google Scholar] [CrossRef]
- Zaretsky, M.V.; Alexander, J.M.; Byrd, W.; Bawdon, R.E. Transfer of inflammatory cytokines across the placenta. Obstet. Gynecol. 2004, 103, 546–550. [Google Scholar] [CrossRef]
- Tribe, R.M.; Moriarty, P.; Dalrymple, A.; Hassoni, A.A.; Poston, L. Interleukin-1β Induces Calcium Transients and Enhances Basal and Store Operated Calcium Entry in Human Myometrial Smooth Muscle1. Biol. Reprod. 2003, 68, 1842–1849. [Google Scholar] [CrossRef]
- Aaltonen, R.; Heikkinen, T.A.; Hakala, K.; Laine, K.; Alanen, A. Transfer of proinflammatory cytokines across term placenta. Obstet. Gynecol. 2005, 106, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.-M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Catoire, M.; Kersten, S. The search for exercise factors in humans. FASEB J. 2015, 29, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Catoire, M.; Mensink, M.; Kalkhoven, E.; Schrauwen, P.; Kersten, S. Identification of human exercise-induced myokines using secretome analysis. Physiol. Genom. 2014, 46, 256–267. [Google Scholar] [CrossRef]
- Cronin, O.; Keohane, D.M.; Molloy, M.G.; Shanahan, F. The effect of exercise interventions on inflammatory biomarkers in healthy, physically inactive subjects: A systematic review. QJM 2017, 110, 629–637. [Google Scholar] [CrossRef]
- Aparicio, V.A.; Ocon, O.; Padilla-Vinuesa, C.; Soriano-Maldonado, A.; Romero-Gallardo, L.; Borges-Cosic, M.; Coll-Risco, I.; Ruiz-Cabello, P.; Acosta-Manzano, P.; Estevez-Lopez, F.; et al. Effects of supervised aerobic and strength training in overweight and grade I obese pregnant women on maternal and foetal health markers: The GESTAFIT randomized controlled trial. BMC Pregnancy Childbirth 2016, 16, 13. [Google Scholar] [CrossRef]
- Kehler, A.K.; Heinrich, K.M. A selective review of prenatal exercise guidelines since the 1950s until present: Written for women, health care professionals, and female athletes. Women Birth 2015, 28, e93–e98. [Google Scholar] [CrossRef]
- ACOG Committee Opinion. Exercise during pregnancy and the postpartum period. Number 267, January 2002. American College of Obstetricians and Gynecologists. Int. J. Gynaecol. Obstet. 2002, 77, 79–81. [Google Scholar] [CrossRef]
- Aparicio, V.A.; Ocon, O.; Diaz-Castro, J.; Acosta-Manzano, P.; Coll-Risco, I.; Borges-Cosic, M.; Romero-Gallardo, L.; Moreno-Fernandez, J.; Ochoa-Herrera, J.J. Influence of a Concurrent Exercise Training Program During Pregnancy on Colostrum and Mature Human Milk Inflammatory Markers: Findings From the GESTAFIT Project. J. Hum. Lact. 2018, 34, 789–798. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Carbiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Mataix, J.L.; Martinez de Victoria, E. Valoración Del Estado Nutricional de la Comunidad Autónoma de Andalucía; Consejería de Salud, Junta de Andalucía: Seville, Spain, 2000. [Google Scholar]
- Acosta-Manzano, P.; Acosta, F.M.; Femia, P.; Coll-Risco, I.; Segura-Jiménez, V.; Díaz-Castro, J.; Ochoa-Herrera, J.J.; Van Poppel, M.N.M.; Aparicio, V.A. Association of sedentary time and physical activity levels with immunometabolic markers in early pregnancy: The GESTAFIT project. Scand. J. Med. Sci. Sports 2019. [Google Scholar] [CrossRef] [PubMed]
- Little, R.J.A.; Rubin, D.B. Statistical Analysis with Missing Data; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Clapp, J.F.; Kiess, W. Effects of pregnancy and exercise on concentrations of the metabolic markers tumor necrosis factor alpha and leptin. Am. J. Obstet. Gynecol. 2000, 182, 300–306. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Fraser, L.M.; Sundernathan, T.; Deussen, A.R.; Louise, J.; Yelland, L.N.; Grivell, R.M.; Macpherson, A.; Gillman, M.W.; Robinson, J.S.; et al. The effect of an antenatal lifestyle intervention in overweight and obese women on circulating cardiometabolic and inflammatory biomarkers: Secondary analyses from the LIMIT randomised trial. BMC Med. 2017, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Hjorth, M.; Febbraio, M.A. IL-1β delivers a sweet deal. Nat. Immunol. 2017, 18, 247. [Google Scholar] [CrossRef]
- Menon, R.; Bonney, E.A.; Condon, J.; Mesiano, S.; Taylor, R.N. Novel concepts on pregnancy clocks and alarms: Redundancy and synergy in human parturition. Hum. Reprod. Update 2016, 22, 535–560. [Google Scholar] [CrossRef]
- Castillo-Castrejon, M.; Meraz-Cruz, N.; Gomez-Lopez, N.; Flores-Pliego, A.; Beltran-Montoya, J.; Viveros-Alcaraz, M.; Vadillo-Ortega, F. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am. J. Reprod. Immunol. 2014, 71, 86–93. [Google Scholar] [CrossRef]
- Ferriter, M.; Huband, N. Does the non-randomized controlled study have a place in the systematic review? A pilot study. Crim. Behav. Mental Health 2005, 15, 111–120. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Castelo, P.M. Improving the quality of data presentation in health sciences. Arch. Oral Biol. 2019, 98, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Kovach, C.R.; Ke, W. Handling Those Pesky Statistical Outliers. Res. Gerontol. Nurs. 2016, 9, 206–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aguinis, H.; Gottfredson, R.K.; Joo, H. Best-Practice Recommendations for Defining, Identifying, and Handling Outliers. Organ. Res. Methods 2013, 16, 270–301. [Google Scholar] [CrossRef]
- Ray, S.; Nishimura, R.; Clarke, S.; Simpson, I. Maintaining good clinical practice: Publication of outcomes and handling of outliers. Heart 2016, 102, 1518–1519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weichle, T.; Hynes, D.M.; Durazo-Arvizu, R.; Tarlov, E.; Zhang, Q. Impact of alternative approaches to assess outlying and influential observations on health care costs. Springerplus 2013, 2, 614. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Tonascia, J.; Brown, K.M.; Canner, P.L. The routine use of winsorization in data monitoring reports to reduce the effects of influential observations. Controll. Clin. Trials 1986, 7, 229. [Google Scholar] [CrossRef]
- Altman, N.; Krzywinski, M. Analyzing outliers: Influential or nuisance? Nat. Methods 2016, 13, 281. [Google Scholar] [CrossRef]
- Hackshaw, A.; Kirkwood, A. Interpreting and reporting clinical trials with results of borderline significance. BMJ 2011, 343, d3340. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Kochanowski, J.; Pyzlak, M.; Szewczyk, G.; Stangret, A.; Mittal, T.K. Fractalkine (CX3CL1) and Its Receptor CX3CR1 May Contribute to Increased Angiogenesis in Diabetic Placenta. Mediat. Inflamm. 2013, 2013, 437576. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski-Lenoir, D.; Chen, S.Z.; Feng, L.L.; Maki, R.; Bacon, K.B. Characterization of fractalkine in rat brain cells: Migratory and activation signals for CX3CR-1-expressing microglia. J. Immunol. 1999, 163, 1628–1635. [Google Scholar] [PubMed]
- Verheggen, R.; Poelkens, F.; Roerink, S.; Ramakers, R.; Catoire, M.; Hermus, A.; Thijssen, D.H.J.; Hopman, M.T.E. Exercise Improves Insulin Sensitivity in the Absence of Changes in Cytokines. Med. Sci. Sports Exerc. 2016, 48, 2378–2386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palmefors, H.; DuttaRoy, S.; Rundqvist, B.; Borjesson, M. The effect of physical activity or exercise on key biomarkers in atherosclerosis—A systematic review. Atherosclerosis 2014, 235, 150–161. [Google Scholar] [CrossRef]
| Total (n = 48) | Control (n = 28) | Intervention (n = 20) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Age (years) | 33.5 | (4.7) | 33.5 | (4.7) | 33.5 | (4.8) | 0.97 |
| Gestational age in the 1st assessment (weeks), 16th week | 16.8 | (1.4) | 16.9 | (1.1) | 16.6 | (1.7) | 0.68 |
| Gestational age in the 2nd assessment (weeks), 34th week | 33.0 | (1.7) | 32.1 | (1.7) | 31.6 | (1.7) | 0.28 |
| Gestational age at delivery (weeks) | 39.4 | (1.4) | 39.1 | (1.6) | 39.8 | (1) | 0.16 |
| Percentage of attendance | 83.9 | (8.2) | |||||
| Cohabitation, n (%) | |||||||
| Living alone | 0 | (0) | 0 | (0) | 0 | (0) | |
| Living with partner | 48 | (100) | 28 | (100) | 20 | (100) | |
| Educational level, n (%) | |||||||
| Non-university degree | 20 | (41.7) | 11 | (39.3) | 9 | (45) | 0.92 |
| University degree | 28 | (58.3) | 17 | (60.7) | 11 | (55) | |
| Professional status, n (%) | |||||||
| Work full/part time | 31 | (64.6) | 21 | (75) | 10 | (50) | 0.14 |
| Unemployed/Retired/Housekeeper | 17 | (35.4) | 7 | (25) | 10 | (50) | |
| Parity status, n (%) | |||||||
| Primarious | 28 | (58.3) | 14 | (50) | 14 | (70) | 0.28 |
| Multiparious | 20 | (41.7) | 14 | (50) | 6 | (30) | |
| Number of abortions | 0.5 | (0.8) | 0.5 | (0.8) | 0.5 | (0.8) | 0.64 |
| Type of deliver a, n (%) | |||||||
| Spontaneous | 27 | (57.4) | 16 | (59.3) | 11 | (55) | 0.20 |
| Vacuum extraction | 9 | (19.1) | 3 | (11.1) | 6 | (30) | |
| Caesarean section | 11 | (23.4) | 8 | (29.6) | 3 | (15) | |
| Offspring sex b, n (%) | |||||||
| Male | 24 | (52.2) | 13 | (50) | 11 | (55) | 0.97 |
| Female | 22 | (47.8) | 13 | (50) | 9 | (45) | |
| Body mass index pre-pregnancy e (kg/m2) | 23.2 | (3.8) | 22.8 | (3.5) | 24.0 | (4.4) | 0.32 |
| Body mass index (kg/m2), 16th week | 23.6 | (4.1) | 23.3 | (3.5) | 24.0 | (4.9) | 0.98 |
| Gestational weight gain from pre-pregnancy to 16th week e (kg) | 1.1 | (2.8) | 1.1 | (3.2) | 0.9 | (2) | 0.81 |
| Gestational weight gain from 16th to 34th week c (kg) | 9.5 | (3.2) | 10.1 | (2.8) | 8.7 | (3.5) | 0.234 |
| Cardiovascular function b, 16th week | |||||||
| Systolic blood pressure (mmHg) | 105.2 | (8.8) | 106.1 | (9.1) | 103.8 | (8.4) | 0.38 |
| Diastolic blood pressure (mmHg) | 61.9 | (7.5) | 61.5 | (7.9) | 62.4 | (7) | 0.70 |
| Resting heart rate (bpm) | 81.7 | (10.8) | 81.9 | (10.7) | 81.5 | (11.3) | 0.57 |
| Smoking during pregnancy (cigarettes per day), 16th week | 0.4 | (1.6) | 0.5 | (2) | 0.2 | (0.9) | 0.50 |
| Adherence to the Mediterranean Diet Score (0–55), 16th week | 29.1 | (3.8) | 29.3 | (3.9) | 28.8 | (3.9) | 0.73 |
| Sedentary lifestyle and physical activity d, 16th week | |||||||
| Sedentary time (min/day) | 503.9 | (98.5) | 486.0 | (116.5) | 526.4 | (65.7) | 0.19 |
| Light PA (min/day) | 392.5 | (89.9) | 420.8 | (99.2) | 356.7 | (61.9) | 0.01 |
| Moderate-vigorous PA (min/day) | 37.8 | (23) | 37.2 | (26.1) | 38.6 | (19.0) | 0.48 |
| Bouted moderate-vigorous PA (min/week) | 99.4 | (120.1) | 106.1 | (141.6) | 90.8 | (89.1) | 0.95 |
| Total PA (min/day) | 430.3 | (93.0) | 458.0 | (99.1) | 395.3 | (73.0) | 0.03 |
| Average accelerometer wear time (min/day) | 934.2 | (53.5) | 944.0 | (61.5) | 921.7 | (39.3) | 0.18 |
| Relative percentage of daily sedentary time (%) | 53.9 | (9.7) | 51.3 | (10.7) | 57.2 | (7.4) | 0.05 |
| Relative percentage of total daily PA (%) | 46.1 | (9.7) | 48.7 | (10.7) | 42.8 | (7.4) | 0.05 |
| Cytokines | 17th Week of Gestation (n = 48) | 35th Week of Gestation (n = 48) | Delivery (n = 38) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 28) | Intervention (n = 20) | p-Value | Control (n = 28) | Intervention (n = 20) | p-Value | Control (n = 19) | Intervention (n = 19) | p-Value | |||||||
| Maternal Serum | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Fractalkine (pg/mL) | 376.1 | 149.0 | 371.4 | 152.7 | 0.85 | 375.8 | 107.3 | 391.4 | 107.2 | 0.74 | 345.5 | 64.6 | 387.2 | 109.6 | 0.17 |
| Interleukin 1 beta (pg/mL) | 6.8 | 2.6 | 6.1 | 3.2 | 0.17 | 7.5 | 3.0 | 6.3 | 3.6 | 0.20 | 9.6 | 3.9 | 7.2 | 3.5 | 0.06 |
| Interleukin 6 (pg/mL) | 5.6 | 2.8 | 5.9 | 3.0 | 0.70 | 6.3 | 2.5 | 5.2 | 2.6 | 0.15 | 32.9 | 9.5 | 33.2 | 19.1 | 0.95 |
| Interleukin 8 (pg/mL) | 21.5 | 10.7 | 18.6 | 7.6 | 0.50 | 19.8 | 8.5 | 21.6 | 10.7 | 0.62 | 34.4 | 6.8 | 37.8 | 15.2 | 0.39 |
| Interleukin 10 (pg/mL) | 24.0 | 9.6 | 22.3 | 12.2 | 0.59 | 24.5 | 8.8 | 29.1 | 9.8 | 0.10 | 40.7 | 11.0 | 50.4 | 16.7 | 0.05 |
| Interferon gamma (pg/mL) | 23.5 | 11.0 | 22.6 | 12.8 | 0.57 | 22.9 | 11.5 | 18.4 | 9.3 | 0.15 | 18.8 | 6.6 | 15.5 | 7.0 | 0.14 |
| Tumor necrosis factor alpha (pg/mL) | 5.6 | 1.7 | 5.2 | 2.7 | 0.11 | 7.1 | 1.7 | 6.1 | 1.4 | 0.03 | 10.1 | 2.7 | 9.0 | 2.6 | 0.25 |
| Umbilical arterial serum a | |||||||||||||||
| Fractalkine (pg/mL) | 314.6 | 91.1 | 372.3 | 103.9 | 0.07 | ||||||||||
| Interleukin 1 beta (pg/mL) | 1.2 | 0.9 | 1.5 | 0.5 | 0.03 | ||||||||||
| Interleukin 6 (pg/mL) | 18.9 | 5.0 | 15.0 | 4.3 | 0.02 | ||||||||||
| Interleukin 8 (pg/mL) | 51.8 | 31.6 | 56.6 | 24.2 | 0.62 | ||||||||||
| Interleukin 10 (pg/mL) | 10.2 | 2.6 | 12.5 | 4.0 | 0.06 | ||||||||||
| Interferon gamma (pg/mL) | 3.2 | 1.5 | 2.6 | 1.1 | 0.15 | ||||||||||
| Tumor necrosis factor-alpha (pg/mL) | 15.8 | 3.5 | 14.3 | 2.9 | 0.17 | ||||||||||
| Umbilical venous serum | |||||||||||||||
| Fractalkine (pg/mL) | 265.8 | 113.1 | 300.4 | 117.3 | 0.36 | ||||||||||
| Interleukin 1 beta (pg/mL) | 1.4 | 0.8 | 1.7 | 1.0 | 0.58 | ||||||||||
| Interleukin 6 (pg/mL) | 13.3 | 5.4 | 12.6 | 4.8 | 0.65 | ||||||||||
| Interleukin 8 (pg/mL) | 59.1 | 22.8 | 60.8 | 17.4 | 0.80 | ||||||||||
| Interleukin 10 (pg/mL) | 12.9 | 4.1 | 13.3 | 3.8 | 0.78 | ||||||||||
| Interferon gamma (pg/mL) | 2.4 | 1.0 | 2.8 | 1.4 | 0.41 | ||||||||||
| Tumor necrosis factor-alpha (pg/mL) | 19.0 | 5.4 | 13.9 | 3.9 | 0.002 | ||||||||||
| Changes in Control Group | Changes in Exercise Group | Model Unadjusted | Model 1 | Model 2 | Adjusted R2 * | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | B | SE | β | p-Value | B | SE | β | p-value | B | SE | β | p-Value | ||
| 35th week-17th week (maternal serum, n = 48) | (n = 28) | (n = 20) | |||||||||||||||
| Fractalkine | −0.35 | 101.10 | 19.98 | 91.67 | 20.33 | 28.49 | 0.11 | 0.48 | 17.92 | 20.72 | 0.09 | 0.39 | 15.11 | 23.16 | 0.08 | 0.52 | −0.011 |
| Interleukin 1 beta | 0.67 | 3.13 | 0.17 | 2.12 | −0.50 | 0.81 | −0.09 | 0.54 | −0.79 | 0.76 | −0.14 | 0.31 | −1.17 | 0.82 | −0.22 | 0.16 | −0.013 |
| Interleukin 6 | 0.74 | 3.27 | −0.67 | 3.11 | −1.41 | 0.94 | −0.22 | 0.14 | −1.19 | 0.73 | −0.18 | 0.11 | −1.11 | 0.78 | −0.17 | 0.16 | 0.047 |
| Interleukin 8 | −1.68 | 9.48 | 3.05 | 7.40 | 4.73 | 2.54 | 0.26 | 0.07 | 3.38 | 2.23 | 0.19 | 0.14 | 4.51 | 2.60 | 0.25 | 0.09 | 0.070 |
| Interleukin 10 | 0.55 | 13.74 | 6.80 | 8.88 | 6.25 | 3.50 | 0.25 | 0.08 | 4.66 | 2.47 | 0.19 | 0.07 | 4.39 | 2.66 | 0.18 | 0.11 | 0.044 |
| Interferon gamma | −0.55 | 9.97 | −4.21 | 9.65 | −3.65 | 2.88 | −0.18 | 0.21 | −4.11 | 2.50 | −0.21 | 0.11 | −5.56 | 2.81 | −0.27 | 0.06 | 0.013 |
| Tumor necrosis factor alpha | 1.51 | 2.29 | 0.86 | 2.52 | −0.66 | 0.70 | −0.14 | 0.35 | −1.03 | 0.43 | −0.22 | 0.02 | −0.86 | 0.44 | −0.19 | 0.06 | 0.019 |
| Delivery-17th week (maternal serum, n = 37) | (n = 19) | (n = 18) | |||||||||||||||
| Fractalkine | −3.22 | 69.74 | 4.36 | 101.34 | 7.57 | 28.47 | 0.05 | 0.79 | 24.09 | 20.9 | 0.14 | 0.26 | 26.66 | 22.00 | 0.16 | 0.23 | −0.026 |
| Interleukin 1 beta | 3.24 | 2.86 | 0.86 | 3.78 | −2.38 | 2.10 | −0.34 | 0.04 | −2.38 | 1.06 | −0.34 | 0.03 | −2.09 | 1.10 | −0.30 | 0.07 | 0.093 |
| Interleukin 6 | 26.91 | 9.86 | 27.06 | 18.93 | 0.15 | 4.92 | 0.01 | 0.98 | 0.23 | 4.97 | 0.01 | 0.96 | −0.90 | 5.2 | −0.03 | 0.86 | −0.029 |
| Interleukin 8 | 14.53 | 14.57 | 19.31 | 16.10 | 4.78 | 5.04 | 0.16 | 0.35 | 3.33 | 3.91 | 0.11 | 0.40 | 3.20 | 4.18 | 0.11 | 0.45 | 0.025 |
| Interleukin 10 | 18.65 | 13.98 | 27.30 | 16.93 | 8.65 | 0.10 | 0.28 | 0.10 | 9.40 | 4.55 | 0.30 | 0.05 | 7.92 | 4.37 | 0.25 | 0.08 | 0.076 |
| Interferon gamma | −2.50 | 9.08 | −8.26 | 12.21 | −5.76 | 3.53 | −0.27 | 0.11 | −3.97 | 2.03 | −0.18 | 0.06 | −3.31 | 2.09 | −0.15 | 0.12 | 0.044 |
| Tumor necrosis factor alpha | 4.66 | 2.72 | 3.62 | 2.97 | −1.04 | 0.94 | −0.19 | 0.27 | −1.03 | 085 | −0.18 | 0.23 | −0.83 | 0.87 | −0.15 | 0.35 | 0.007 |
| Model Unadjusted | Model 1 | Model 2 | Adjusted R2 b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | p-value | B | SE | β | p-value | B | SE | β | p-Value | ||
| Umbilical arterial serum (delivery) a | |||||||||||||
| Fractalkine * | 0.63 | 0.32 | 0.33 | 0.06 | 0.53 | 0.32 | 0.28 | 0.11 | 0.52 | 0.33 | 0.27 | 0.13 | 0.081 |
| Interleukin 1 beta * | 0.66 | 0.29 | 0.38 | 0.03 | 0.69 | 0.30 | 0.39 | 0.03 | 0.72 | 0.27 | 0.41 | 0.01 | 0.113 |
| Interleukin 6 * | −0.83 | 0.32 | −0.42 | 0.02 | −0.79 | 0.33 | −0.40 | 0.02 | −0.80 | 0.34 | −0.40 | 0.03 | 0.147 |
| Interleukin 8 | 4.83 | 9.56 | 0.09 | 0.61 | 6.67 | 9.85 | 0.12 | 0.50 | 7.25 | 9.93 | 0.13 | 0.47 | −0.023 |
| Interleukin 10 | 2.32 | 1.20 | 0.32 | 0.06 | 2.14 | 1.24 | 0.30 | 0.10 | 2.14 | 1.23 | 0.30 | 0.09 | 0.076 |
| Interferon gamma | −0.65 | 0.44 | −0.25 | 0.15 | −0.70 | 0.46 | −0.27 | 0.14 | −0.68 | 0.46 | −0.27 | 0.15 | 0.034 |
| Tumor necrosis factor alpha | −1.55 | 1.10 | −0.24 | 0.17 | −1.63 | 1.14 | −0.25 | 0.17 | −1.63 | 1.14 | −0.26 | 0.16 | 0.029 |
| Umbilical venous serum (delivery) | |||||||||||||
| Fractalkine | 34.58 | 37.38 | 0.15 | 0.36 | 27.29 | 36.93 | 0.12 | 0.47 | 23.68 | 38.24 | 0.10 | 0.54 | −0.004 |
| Interleukin 1 beta * | 0.21 | 0.32 | 0.11 | 0.53 | 0.18 | 0.33 | 0.10 | 0.58 | 0.17 | 0.33 | 0.09 | 0.62 | 0.011 |
| Interleukin 6 | −0.77 | 1.67 | −0.08 | 0.65 | −0.90 | 1.70 | −0.09 | 0.60 | −1.03 | 1.77 | −0.10 | 0.56 | 0.006 |
| Interleukin 8 * | 0.20 | 0.32 | 0.11 | 0.53 | 0.22 | 0.33 | 0.11 | 0.50 | 0.16 | 0.33 | 0.08 | 0.64 | −0.016 |
| Interleukin 10 | 0.37 | 1.28 | 0.05 | 0.78 | 0.31 | 1.31 | 0.04 | 0.82 | 0.23 | 1.36 | 0.03 | 0.87 | −0.025 |
| Interferon gamma | 0.34 | 0.41 | 0.14 | 0.41 | 0.24 | 0.39 | 0.10 | 0.54 | 0.29 | 0.41 | 0.12 | 0.49 | −0.008 |
| Tumor necrosis factor alpha | −5.07 | 1.54 | −0.48 | 0.002 | −5.53 | 1.45 | −0.53 | 0.001 | −5.21 | 1.45 | −0.50 | 0.001 | 0.211 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Manzano, P.; Coll-Risco, I.; Van Poppel, M.N.M.; Segura-Jiménez, V.; Femia, P.; Romero-Gallardo, L.; Borges-Cosic, M.; Díaz-Castro, J.; Moreno-Fernández, J.; Ochoa-Herrera, J.J.; et al. Influence of a Concurrent Exercise Training Intervention during Pregnancy on Maternal and Arterial and Venous Cord Serum Cytokines: The GESTAFIT Project. J. Clin. Med. 2019, 8, 1862. https://doi.org/10.3390/jcm8111862
Acosta-Manzano P, Coll-Risco I, Van Poppel MNM, Segura-Jiménez V, Femia P, Romero-Gallardo L, Borges-Cosic M, Díaz-Castro J, Moreno-Fernández J, Ochoa-Herrera JJ, et al. Influence of a Concurrent Exercise Training Intervention during Pregnancy on Maternal and Arterial and Venous Cord Serum Cytokines: The GESTAFIT Project. Journal of Clinical Medicine. 2019; 8(11):1862. https://doi.org/10.3390/jcm8111862
Chicago/Turabian StyleAcosta-Manzano, Pedro, Irene Coll-Risco, Mireille N. M. Van Poppel, Víctor Segura-Jiménez, Pedro Femia, Lidia Romero-Gallardo, Milkana Borges-Cosic, Javier Díaz-Castro, Jorge Moreno-Fernández, Julio J. Ochoa-Herrera, and et al. 2019. "Influence of a Concurrent Exercise Training Intervention during Pregnancy on Maternal and Arterial and Venous Cord Serum Cytokines: The GESTAFIT Project" Journal of Clinical Medicine 8, no. 11: 1862. https://doi.org/10.3390/jcm8111862
APA StyleAcosta-Manzano, P., Coll-Risco, I., Van Poppel, M. N. M., Segura-Jiménez, V., Femia, P., Romero-Gallardo, L., Borges-Cosic, M., Díaz-Castro, J., Moreno-Fernández, J., Ochoa-Herrera, J. J., & Aparicio, V. A. (2019). Influence of a Concurrent Exercise Training Intervention during Pregnancy on Maternal and Arterial and Venous Cord Serum Cytokines: The GESTAFIT Project. Journal of Clinical Medicine, 8(11), 1862. https://doi.org/10.3390/jcm8111862










