Hypovitaminosis D Influences the Clinical Presentation of Immune Thrombocytopenia in Children with Newly Diagnosed Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Variables
2.4. Statistics
2.4.1. Calculation of the Minimal Sample Size
2.4.2. Statistical Tests Used in the Study
3. Results
3.1. Correlation between 25(OH)D and SMOG Score
3.2. Correlation between 25(OH)D and IBLS Score
3.3. Correlation between 25(OH)D and Platelet Count
3.4. Correlation between Patient Gender and Age with 25(OH)D and SMOG Score
4. Discussion
4.1. Demographics and Clinical Presentation of ITP
4.2. Correlation between Hypovitaminosis D and ITP
4.3. Correlation between VD and ITP Clinical Presentation
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bussel, B.J. Immune Thrombocytopenia (ITP) in Children. Available online: http://www.uptodate.com/ (accessed on 20 October 2016).
- Kühne, T.; Buchanan, G.R.; Zimmerman, S.; Michaels, L.A.; Kohan, R.; Berchtold, W.; Imbach, P.; Intercontinental Childhood ITP Study Group. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J. Pediatr. 2003, 143, 605–608. [Google Scholar]
- Kühne, T.; Berchtold, W.; Michaels, L.A.; Wu, R.; Donato, H.; Espina, B.; Tamary, H.; Rodeghiero, F.; Chitlur, M.; Rischewski, J.; et al. Newly diagnosed immune thrombocytopenia in children and adults: A comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica 2011, 96, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Labarque, V.; Van Geet, C. Clinical practice: Immune thrombocytopenia in paediatrics. Eur. J. Pediatr. 2014, 173, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Kilpatrick, K.; Eisen, M.; Tarantino, M. The incidence and clinical burden of immune thrombocytopenia in pediatric patients in the United States. Platelets 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef]
- Donato, H.; Picón, A.; Martinez, M.; Rapetti, M.C.; Rosso, A.; Gomez, S.; Rossi, N.; Bacciedoni, V.; Schvartzman, G.; Riccheri, C.; et al. Demographic data, natural history, and prognostic factors of idiopathic thrombocytopenic purpura in children: A multicentered study from Argentina. Pediatr. Blood Cancer 2009, 52, 491–496. [Google Scholar] [CrossRef]
- Imbach, P.; Kühne, T.; Müller, D.; Berchtold, W.; Zimmerman, S.; Elalfy, M.; Buchanan, G.R. Childhood ITP: 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS). Pediatr. Blood Cancer 2006, 46, 351–356. [Google Scholar] [CrossRef]
- Blanchette, V.; Bolton-Maggs, P. Childhood immune thrombocytopenic purpura: Diagnosis and management. Hematol. Oncol. Clin. N. Am. 2010, 24, 249–273. [Google Scholar] [CrossRef]
- Roganović, J. Idiopathic thrombocytopenic purpura in children. Acta Med. Acad. 2009, 38, 21–34. [Google Scholar]
- Neunert, C.E.; Buchanan, G.R.; Imbach, P.; Bolton-Maggs, P.H.B.; Bennett, C.M.; Neufeld, E.J.; Vesely, S.K.; Adix, L.; Blanchette, V.S.; Kühne, T. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood 2008, 112, 4003–4008. [Google Scholar] [CrossRef]
- Psaila, B.; Petrovic, A.; Page, L.K.; Menell, J.; Schonholz, M.; Bussel, J.B. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): Study of 40 cases. Blood 2009, 114, 4777–4783. [Google Scholar] [CrossRef] [PubMed]
- Neunert, C.; Noroozi, N.; Norman, G.; Buchanan, G.R.; Goy, J.; Nazi, I.; Kelton, J.G.; Arnold, D.M. Severe bleeding events in adults and children with primary immune thrombocytopenia: A systematic review. J. Thromb Haemost 2015, 13, 457–464. [Google Scholar] [CrossRef] [PubMed]
- British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br. J. Haematol. 2003, 120, 574–596. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Maggs, P.H.; Moon, I. Assessment of UK practice for management of acute childhood idiopathic thrombocytopenic purpura against published guidelines. Lancet 1997, 350, 620–623. [Google Scholar] [CrossRef]
- Buchanan, G.R.; Adix, L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. J. Pediatr. 2002, 141, 683–688. [Google Scholar] [CrossRef]
- Page, L.K.; Psaila, B.; Provan, D.; Michael Hamilton, J.; Jenkins, J.M.; Elish, A.S.; Lesser, M.L.; Bussel, J.B. The immune thrombocytopenic purpura (ITP) bleeding score: Assessment of bleeding in patients with ITP. Br. J. Haematol 2007, 138, 245–248. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Michel, M.; Gernsheimer, T.; Ruggeri, M.; Blanchette, V.; Bussel, J.B.; Cines, D.B.; Cooper, N.; Godeau, B.; Greinacher, A.; et al. Standardization of bleeding assessment in immune thrombocytopenia: Report from the International Working Group. Blood 2013, 121, 2596–2606. [Google Scholar] [CrossRef]
- Vesely, S.; Buchanan, G.R.; Cohen, A.; Raskob, G.; George, J. Self-reported diagnostic and management strategies in childhood idiopathic thrombocytopenic purpura: Results of a survey of practicing pediatric hematology/oncology specialists. J. Pediatr. Hematol. Oncol. 2000, 22, 55–61. [Google Scholar] [CrossRef]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L.; Crowther, M.A.; American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef]
- Provan, D.; Stasi, R.; Newland, A.C.; Blanchette, V.S.; Bolton-Maggs, P.; Bussel, J.B.; Chong, B.H.; Cines, D.B.; Gernsheimer, T.B.; Godeau, B.; et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010, 115, 168–186. [Google Scholar] [CrossRef]
- Rosthøj, S.; Hedlund-Treutiger, I.; Rajantie, J.; Zeller, B.; Jonsson, O.G.; Elinder, G.; Wesenberg, F.; Henter, J.I.; NOPHO ITP Working Group. Duration and morbidity of newly diagnosed idiopathic thrombocytopenic purpura in children: A prospective Nordic study of an unselected cohort. J. Pediatr. 2003, 143, 302–307. [Google Scholar]
- Tarantino, M.D.; Young, G.; Bertolone, S.J.; Kalinyak, K.A.; Shafer, F.E.; Kulkarni, R.; Weber, L.C.; Davis, M.L.; Lynn, H.; Nugent, D.J.; et al. Single dose of anti-D immune globulin at 75 microg/kg is as effective as intravenous immune globulin at rapidly raising the platelet count in newly diagnosed immune thrombocytopenic purpura in children. J. Pediatr. 2006, 148, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, V.S.; Luke, B.; Andrew, M.; Sommerville-Nielsen, S.; Barnard, D.; de Veber, B.; Gent, M. A prospective, randomized trial of high-dose intravenous immune globulin G therapy, oral prednisone therapy, and no therapy in childhood acute immune thrombocytopenic purpura. J. Pediatr. 1993, 123, 989–995. [Google Scholar] [CrossRef]
- Treutiger, I.; Rajantie, J.; Zeller, B.; Henter, J.I.; Elinder, G.; Rosthøj, S.; Group, N.I.S. Does treatment of newly diagnosed idiopathic thrombocytopenic purpura reduce morbidity? Arch. Dis. Child. 2007, 92, 704–707. [Google Scholar] [CrossRef]
- Blanchette, V.; Imbach, P.; Andrew, M.; Adams, M.; McMillan, J.; Wang, E.; Milner, R.; Ali, K.; Barnard, D.; Bernstein, M. Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet 1994, 344, 703–707. [Google Scholar] [CrossRef]
- Grainger, J.D.; Locatelli, F.; Chotsampancharoen, T.; Donyush, E.; Pongtanakul, B.; Komvilaisak, P.; Sosothikul, D.; Drelichman, G.; Sirachainan, N.; Holzhauer, S.; et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): A randomised, multicentre, placebo-controlled trial. Lancet 2015, 386, 1649–1658. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Yee, Y.K.; Chintalacharuvu, S.R.; Lu, J.; Nagpal, S. Vitamin D receptor modulators for inflammation and cancer. Mini Rev. Med. Chem. 2005, 5, 761–778. [Google Scholar] [CrossRef]
- Fernandes de Abreu, D.A.; Eyles, D.; Féron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009, 34, S265–S277. [Google Scholar] [CrossRef]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef]
- Jørgensen, S.P.; Bartels, L.E.; Agnholt, J.; Glerup, H.; Nielsen, S.L.; Hvas, C.L.; Dahlerup, J.F. Vitamin D insufficiency—Possible etiologic factor of autoimmune diseases. Ugeskr. Laeger 2007, 169, 3655–3660. [Google Scholar] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Zhu, Y.; Froicu, M.; Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 2004, 80, 1717S–1720S. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.; Martini, L.A.; Rogero, M.M. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition 2011, 27, 399–404. [Google Scholar] [CrossRef]
- Cippitelli, M.; Santoni, A. Vitamin D3: A transcriptional modulator of the interferon-gamma gene. Eur. J. Immunol. 1998, 28, 3017–3030. [Google Scholar] [CrossRef]
- Prentice, A.; Goldberg, G.R.; Schoenmakers, I. Vitamin D across the lifecycle: Physiology and biomarkers. Am. J. Clin. Nutr. 2008, 88, 500S–506S. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Antico, A.; Tampoia, M.; Tozzoli, R.; Bizzaro, N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 2012, 12, 127–136. [Google Scholar] [CrossRef]
- Deluca, H.F.; Cantorna, M.T. Vitamin D: Its role and uses in immunology. FASEB J. 2001, 15, 2579–2585. [Google Scholar] [CrossRef]
- Adorini, L. Immunomodulatory effects of vitamin D receptor ligands in autoimmune diseases. Int. Immunopharmacol. 2002, 2, 1017–1028. [Google Scholar] [CrossRef]
- Arnson, Y.; Amital, H.; Shoenfeld, Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007, 66, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Zold, E.; Barta, Z.; Bodolay, E. Vitamin D deficiency and connective tissue disease. Vitam. Horm. 2011, 86, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Fattizzo, B.; Zaninoni, A.; Giannotta, J.A.; Binda, F.; Cortelezzi, A.; Barcellini, W. Reduced 25-OH vitamin D in patients with autoimmune cytopenias, clinical correlations and literature review. Autoimmun. Rev. 2016, 15, 770–775. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Čulić, S.; Markić, J.; Petrović, D.; Konjevoda, P.; Pavelić, J. Serum vitamin D levels in children with newly diagnosed and chronic immune thrombocytopenia. Semin. Hematol. 2016, 53 (Suppl. 1), S67–S69. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologist. Available online: http://www.lacbiosafety.org/wp-content/uploads/2011/09/experimental-design-and-data-analysis-for-biologists1.pdf (accessed on 20 July 2017).
- Alam, M.M. Idiopathic thrombocytopenic purpura in children: A 10 years experience at tertiary care hospital. J. Pak. Med. Assoc. 2014, 64, 1358–1362. [Google Scholar]
- Paling, A.; Stefan, D.C. Idiopathic thrombocytopenic purpura in childhood: A 10-year audit. Hematology 2008, 13, 175–180. [Google Scholar] [CrossRef]
- Kumar, M.; Dutta, S.; Bhattyacharyya, M. Application of ITP-BAT bleeding score in clinical practice. Int. J. Hematol. 2015, 101, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M. Platelet count or bleeding as the outcome in ITP trials? Am. J. Hematol. 2012, 87, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, G.; Antico, A.; Fortunato, A.; Bizzaro, N. Vitamin D and Autoimmune Diseases: Is Vitamin D Receptor (VDR) Polymorphism the Culprit? Isr. Med. Assoc. J. 2017, 19, 438–443. [Google Scholar] [PubMed]
- Yesil, S.; Tanyildiz, H.G.; Tekgunduz, S.A.; Toprak, S.; Fettah, A.; Dikmen, A.U.; Sahin, G. Vitamin D receptor polymorphisms in immune thrombocytopenic purpura. Pediatr. Int. 2017, 59, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, H.; Hao, Y.; Li, Y.; Lv, M.; Xue, F.; Liu, X.; Zhang, L.; Yang, R. Decreased immunosuppressive actions of 1α, 25-dihydroxyvitamin D3 in patients with immune thrombocytopenia. Mol. Immunol. 2016, 78, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Smek, L. Climate Atlas of Croatia; Zaninovic, K., Ed.; Meterological and Hydrological Service of Croatia: Zagreb, Croatia, 2008; pp. 83–85. [Google Scholar]
- Lassandro, G.; Carriero, F.; Palmieri, V.; Palladino, V.; Amoruso, A.; Gallone, M.F.; Del Vecchio, G.C.; Tafuri, S.; Russo, G.; Giordano, P. Serum D vitamin levels in children with immune thrombocytopenia. Endocr. Metab. Immune Disord. Drug Targets 2019, 31203812. [Google Scholar] [CrossRef]
- Hafez, M.; Hassan, M.; Musa, N.; Abdel Atty, S.; Azim, S.A. Vitamin D status in Egyptian children with type 1 diabetes and the role of vitamin D replacement in glycemic control. J. Pediatr. Endocrinol. Metab. 2017, 30, 389–394. [Google Scholar] [CrossRef]
- Simsek, Y.; Cakır, I.; Yetmis, M.; Dizdar, O.S.; Baspinar, O.; Gokay, F. Effects of Vitamin D treatment on thyroid autoimmunity. J. Res. Med. Sci. 2016, 21, 85. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef]
- Lu, C.Z.; Jensen, M.A.; Arnason, B.G. Interferon gamma- and interleukin-4-secreting cells in multiple sclerosis. J. Neuroimmunol. 1993, 46, 123–128. [Google Scholar] [CrossRef]
- Amital, H.; Szekanecz, Z.; Szucs, G.; Danko, K.; Nagy, E.; Csepany, T.; Kiss, E.; Rovensky, J.; Tuchynova, A.; Kozakova, D.; et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: Is it time to routinely supplement patients with SLE with vitamin D? Ann. Rheum. Dis. 2010, 69, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.B.; Shults, J.; Leonard, M.B.; Zemel, B.S.; Burnham, J.M. Hypovitaminosis D is associated with greater body mass index and disease activity in pediatric systemic lupus erythematosus. J. Pediatr. 2009, 155, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Song, G.G.; Bae, S.C.; Lee, Y.H. Association between vitamin D intake and the risk of rheumatoid arthritis: A meta-analysis. Clin. Rheumatol. 2012, 31, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, J.; Davies, M.L.; Chen, W. Serum Vitamin D Level and Rheumatoid Arthritis Disease Activity: Review and Meta-Analysis. PLoS ONE 2016, 11, e0146351. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.S.; Sabahi, I.; Richards, J.S.; Caplan, L.; Cannon, G.W.; Reimold, A.; Thiele, G.M.; Johnson, D.; Mikuls, T.R. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J. Rheumatol. 2011, 38, 53–59. [Google Scholar] [CrossRef]
- Sadeghian, M.; Saneei, P.; Siassi, F.; Esmaillzadeh, A. Vitamin D status in relation to Crohn’s disease: Meta-analysis of observational studies. Nutrition 2016, 32, 505–514. [Google Scholar] [CrossRef]
- Skaaby, T.; Husemoen, L.L.; Thuesen, B.H.; Linneberg, A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 2015, 50, 231–238. [Google Scholar] [CrossRef]
- Andjelkovic, Z.; Vojinovic, J.; Pejnovic, N.; Popovic, M.; Dujic, A.; Mitrovic, D.; Pavlica, L.; Stefanovic, D. Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 1999, 17, 453–456. [Google Scholar]
- Huckins, D.; Felson, D.T.; Holick, M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: A pilot study. Arthritis Rheum. J. Am. Coll. Rheumatol. 1990, 33, 1723–1727. [Google Scholar] [CrossRef]
- Bockow, B.; Kaplan, T.B. Refractory immune thrombocytopenia successfully treated with high-dose vitamin D supplementation and hydroxychloroquine: Two case reports. J. Med. Case Rep. 2013, 7, 91. [Google Scholar] [CrossRef]
- Hill, Q.A.; Grainger, J.D.; Thachil, J.; Provan, D.; Evans, G.; Garg, M.; Bradbury, C.; Bagot, C.; Kanis, J.A.; Compston, J.E. The prevention of glucocorticoid-induced osteoporosis in patients with immune thrombocytopenia receiving steroids: A British Society for Haematology Good Practice Paper. Br. J. Haematol. 2019, 185, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, F.; Wang, S.; Shang, X.; Luo, S.; Zhou, H.; Shi, H.; Cai, L. Serum Vitamin D Level is Inversely Associated With Anti-Cyclic Citrullinated Peptide Antibody Level and Disease Activity in Rheumatoid Arthritis Patients. Arch. Rheumatol. 2016, 31, 64–70. [Google Scholar] [CrossRef] [PubMed]
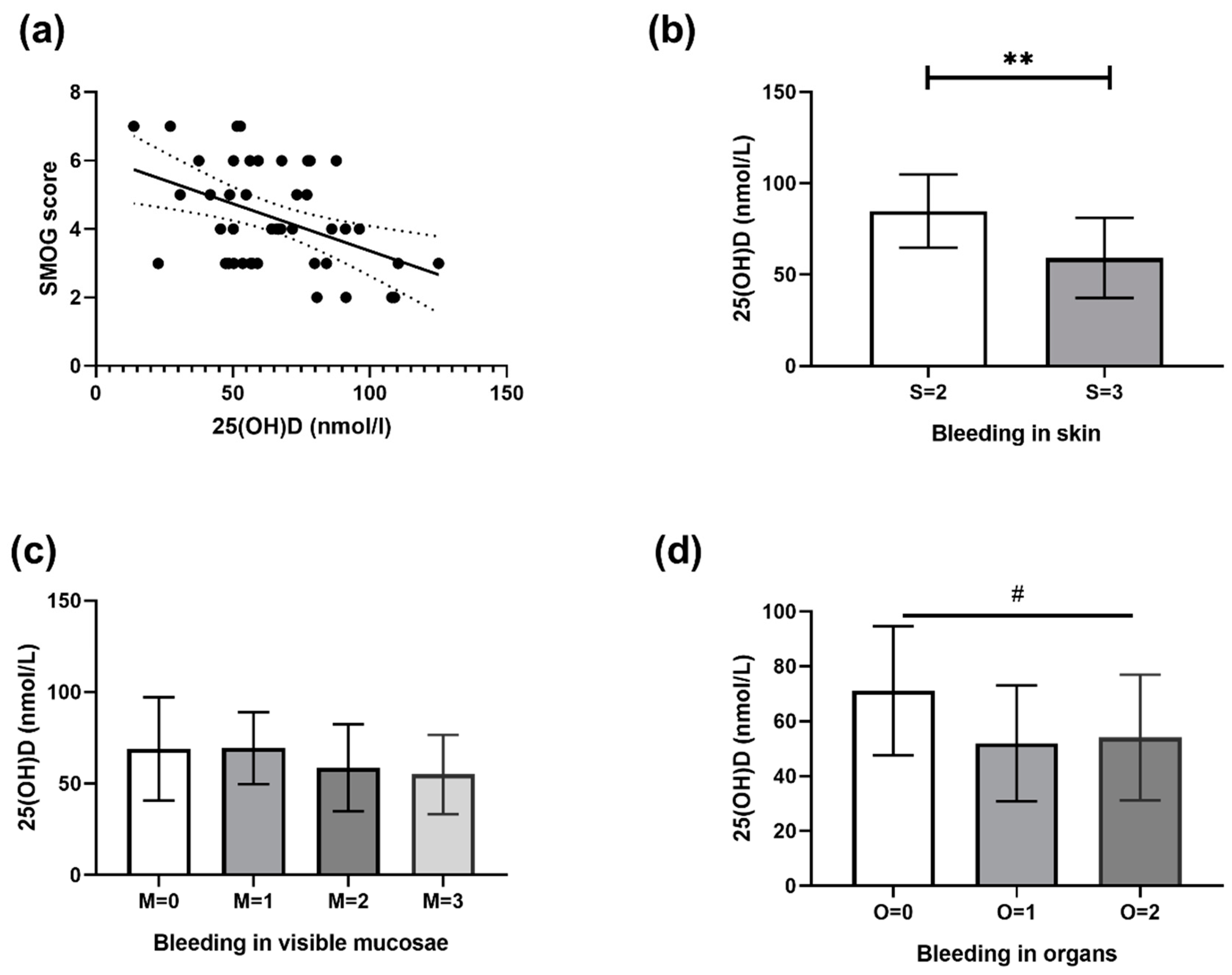
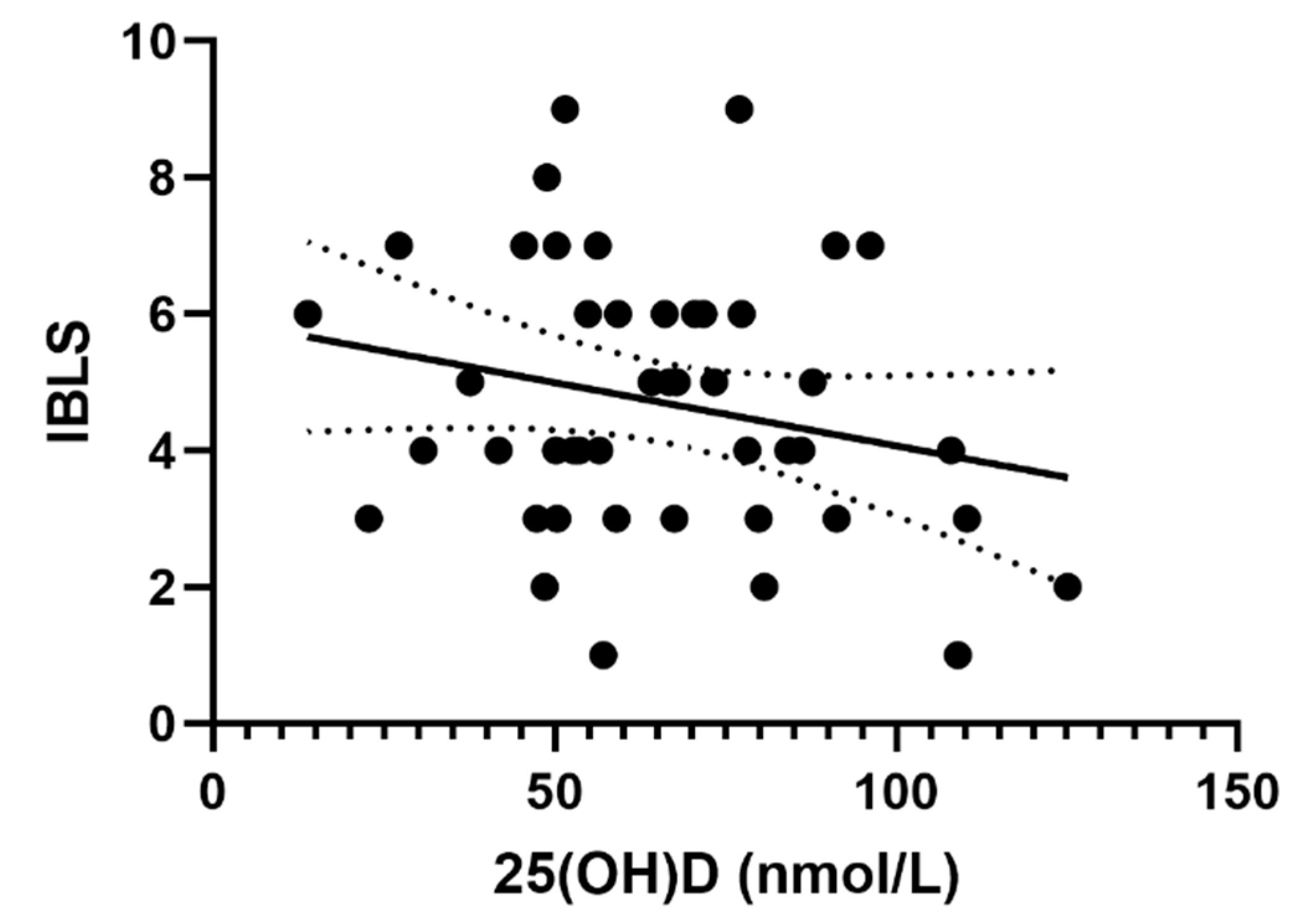
| Variable | All Patients (n = 45) | Sufficient 25(OH)D Value (n = 15) | Hypovitaminosis D (n = 30) |
|---|---|---|---|
| Mean ± SD | |||
| Age at presentation, year | 5.6 ± 4.8 | 3 ± 3.3 | 6.9 ± 5 |
| 25(OH)D, nmol/l | 65 ± 24 | 93 ± 15 | 52 ± 15 |
| Platelets, G/L | 9.8 ± 10 | 6.9 ± 7.7 | 11 ± 11 |
| IBLS 1 | 4.7± 1.9 | 4.2 ± 2.1 | 4.9 ± 1.8 |
| SMOG 2 | 4.3 ± 1.5 | 3.6 ± 1.4 | 4.6 ± 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovic, D.; Benzon, B.; Batinic, M.; Culic, S.; Roganovic, J.; Markic, J. Hypovitaminosis D Influences the Clinical Presentation of Immune Thrombocytopenia in Children with Newly Diagnosed Disease. J. Clin. Med. 2019, 8, 1861. https://doi.org/10.3390/jcm8111861
Petrovic D, Benzon B, Batinic M, Culic S, Roganovic J, Markic J. Hypovitaminosis D Influences the Clinical Presentation of Immune Thrombocytopenia in Children with Newly Diagnosed Disease. Journal of Clinical Medicine. 2019; 8(11):1861. https://doi.org/10.3390/jcm8111861
Chicago/Turabian StylePetrovic, Davor, Benjamin Benzon, Marijan Batinic, Srđana Culic, Jelena Roganovic, and Josko Markic. 2019. "Hypovitaminosis D Influences the Clinical Presentation of Immune Thrombocytopenia in Children with Newly Diagnosed Disease" Journal of Clinical Medicine 8, no. 11: 1861. https://doi.org/10.3390/jcm8111861
APA StylePetrovic, D., Benzon, B., Batinic, M., Culic, S., Roganovic, J., & Markic, J. (2019). Hypovitaminosis D Influences the Clinical Presentation of Immune Thrombocytopenia in Children with Newly Diagnosed Disease. Journal of Clinical Medicine, 8(11), 1861. https://doi.org/10.3390/jcm8111861






