Associations of Anti-Aquaporin 5 Autoantibodies with Serologic and Histopathological Features of Sjögren’s Syndrome
Abstract
1. Introduction
2. Experimental Section
2.1. Serum Samples and Clinical Data
2.2. Cell-Based Immunofluorescence Cytochemistry (CB-IFC)
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
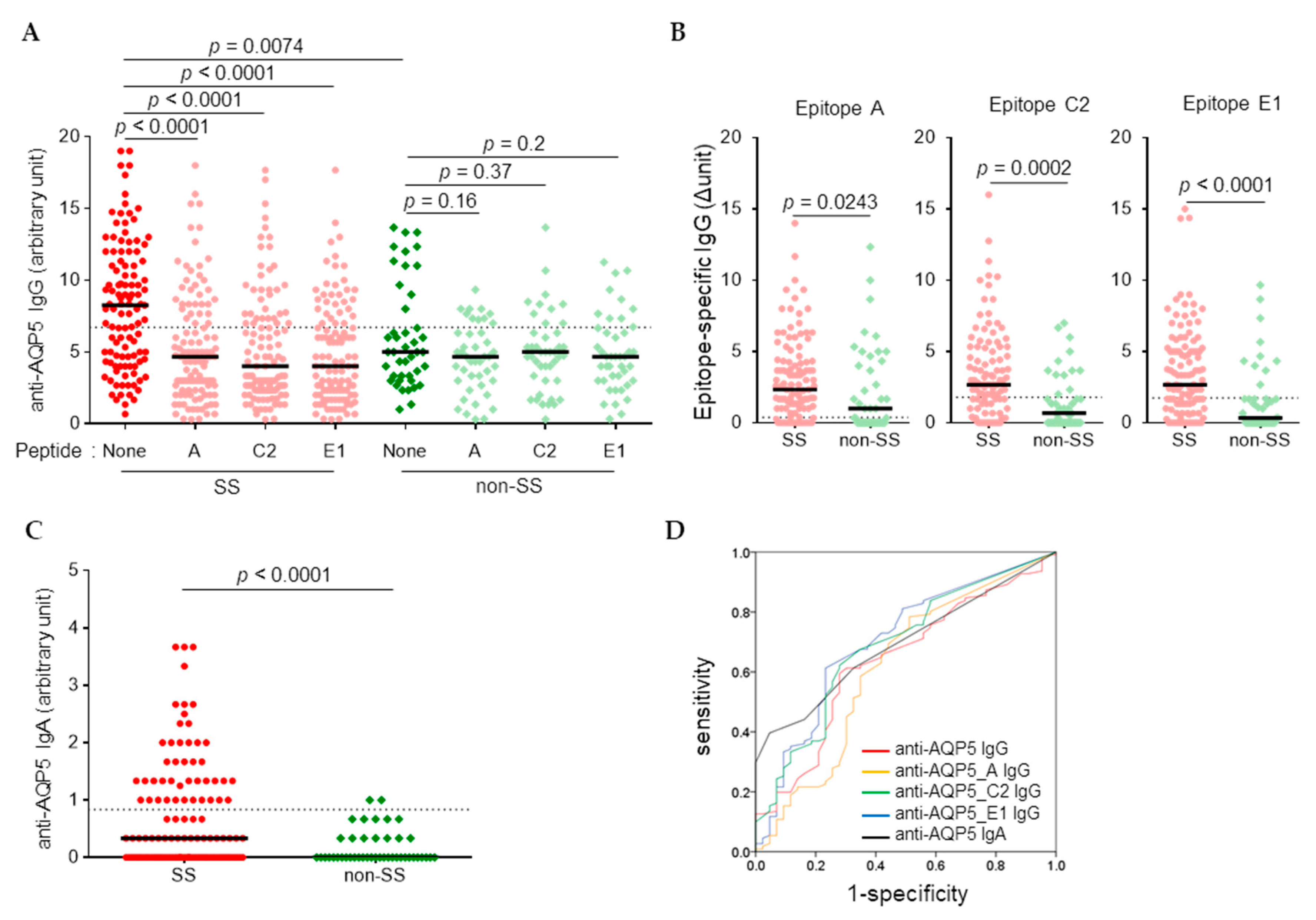
3.1. Higher Levels of Anti-AQP5 IgG and IgA Were Detected in SS than in Non-SS by CB-IFC
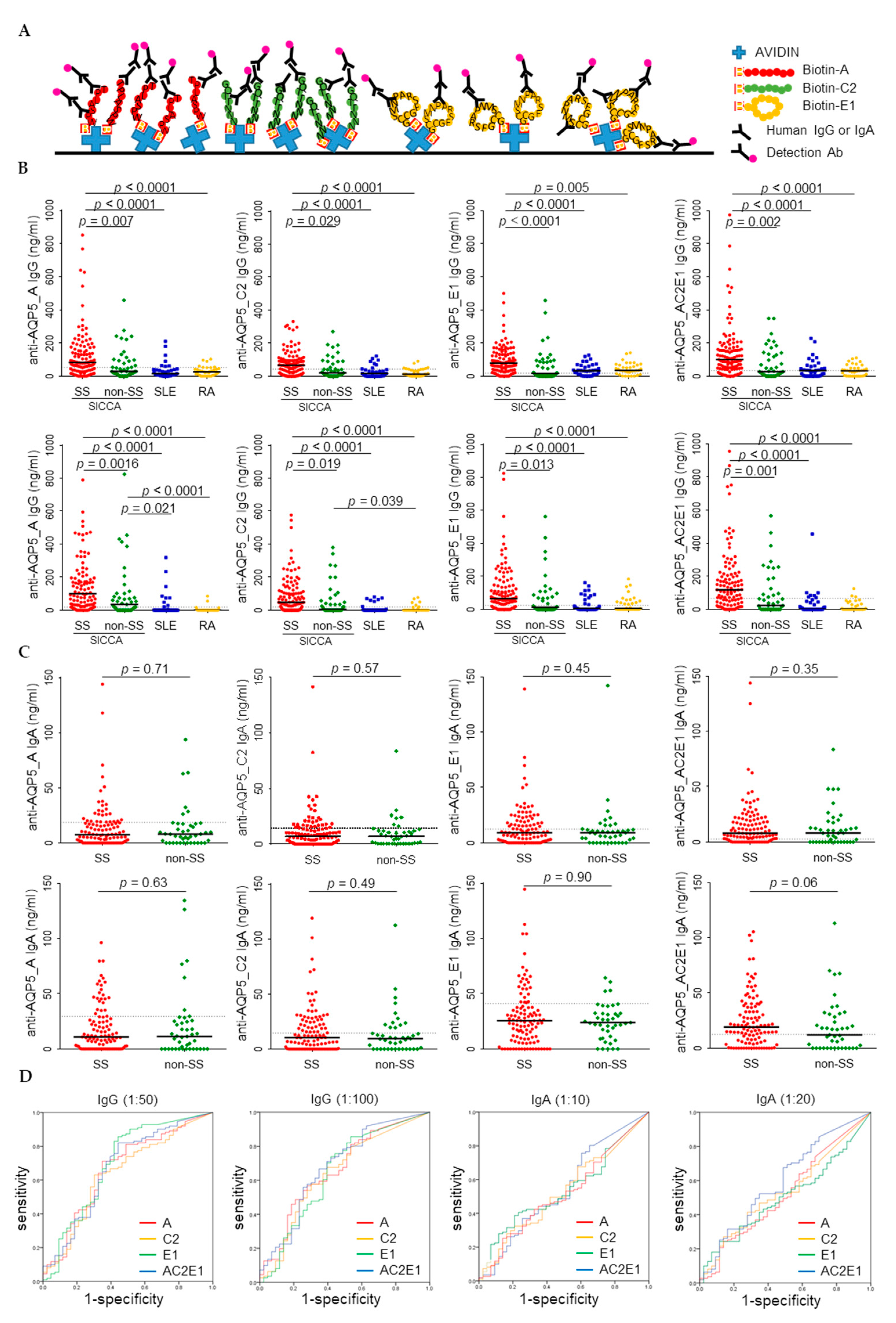
3.2. Higher Levels of Anti-AQP5 IgG but Not IgA were Detected in the SS Sera by ELISA
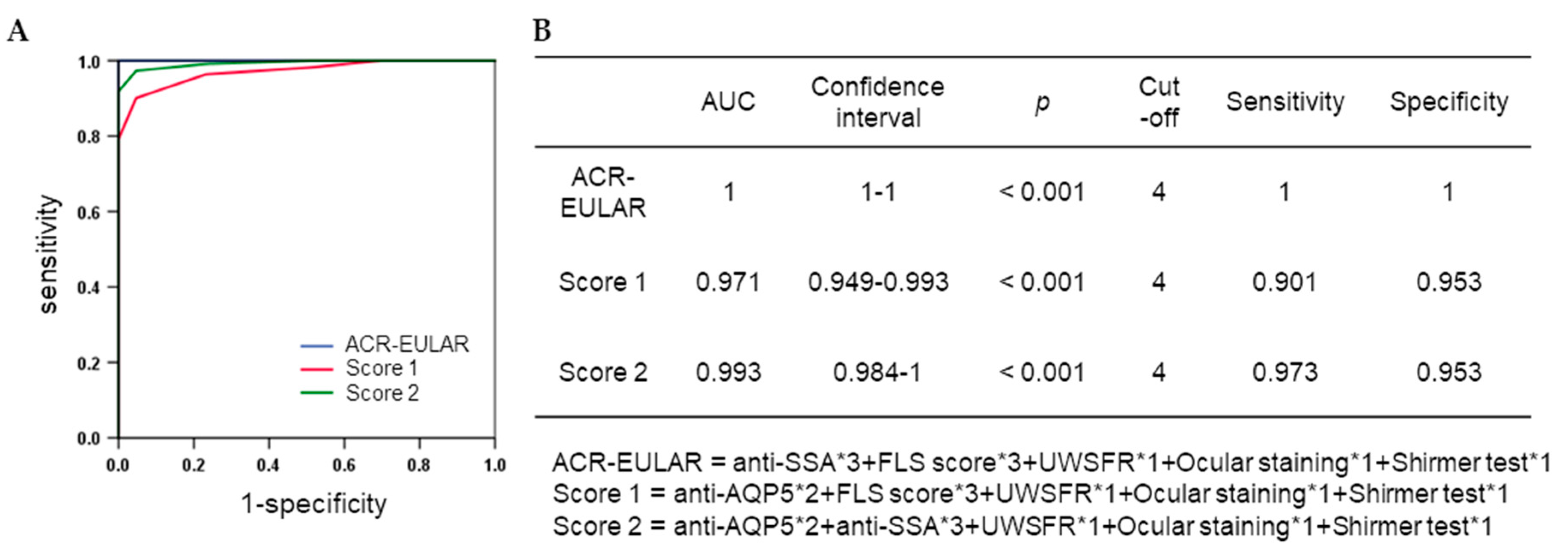
3.3. Association between Anti-AQP5 Autoantibodies and Disease Criteria for SS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borchers, A.T.; Naguwa, S.M.; Keen, C.L.; Gershwin, M.E. Immunopathogenesis of Sjogren’s syndrome. Clin. Rev. Allergy Immunol. 2003, 25, 89–104. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koh, J.H.; Kim, J.W.; Sung, Y.K.; Lee, S.S.; Choe, J.Y.; Shim, S.C.; Kim, H.S.; Kim, H.R.; Kim, J.M.; et al. Performance of the 2016 ACR-EULAR classification criteria for primary Sjogren’s syndrome in a Korean cohort. Rheumatol. Int. 2018, 38, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, H.; Hagiwara, S.; Asashima, H.; Takahashi, H.; Hirota, T.; Noma, H.; Umehara, H.; Kawakami, A.; Nakamura, H.; Sano, H.; et al. Comparison of performance of the 2016 ACR-EULAR classification criteria for primary Sjögren’s syndrome with other sets of criteria in Japanese patients. Ann. Rheum. Dis. 2017, 76, 1980–1985. [Google Scholar] [CrossRef]
- Fox, R.I. Sjogren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Retamozo, S.; Ramos-Casals, M. Phenotyping Sjögren’s syndrome: Towards a personalised management of the disease. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 198–209. [Google Scholar]
- Gliozzi, M.; Greenwell-Wild, T.; Jin, W.; Moutsopoulos, N.M.; Kapsogeorgou, E.; Moutsopoulos, H.M.; Wahl, S.M. A link between interferon and augmented plasmin generation in exocrine gland damage in Sjögren’s syndrome. J. Autoimmun. 2013, 40, 122–133. [Google Scholar] [CrossRef]
- Yoshimura, S.; Nakamura, H.; Horai, Y.; Nakajima, H.; Shiraishi, H.; Hayashi, T.; Takahashi, T.; Kawakami, A. Abnormal distribution of AQP5 in labial salivary glands is associated with poor saliva secretion in patients with Sjögren’s syndrome including neuromyelitis optica complicated patients. Mod. Rheumatol. 2016, 26, 384–390. [Google Scholar] [CrossRef]
- Dawson, L.J.; Stanbury, J.; Venn, N.; Hasdimir, B.; Rogers, S.N.; Smith, P.M. Antimuscarinic antibodies in primary Sjögren’s syndrome reversibly inhibit the mechanism of fluid secretion by human submandibular salivary acinar cells. Arthritis Rheum. 2006, 54, 1165–1173. [Google Scholar] [CrossRef]
- Alam, J.; Koh, J.H.; Kim, N.; Kwok, S.K.; Park, S.H.; Song, Y.W.; Park, K.; Choi, Y. Detection of autoantibodies against aquaporin-5 in the sera of patients with primary Sjögren’s syndrome. Immunol. Res. 2016, 64, 848–856. [Google Scholar] [CrossRef]
- Alam, J.; Koh, J.H.; Kwok, S.K.; Park, S.H.; Park, K.; Choi, Y. Functional Epitopes for Anti-Aquaporin 5 Antibodies in Sjögren Syndrome. J. Dent. Res. 2017, 96, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Soyfoo, M.S.; Chivasso, C.; Perret, J.; Delporte, C. Involvement of Aquaporins in the Pathogenesis, Diagnosis and Treatment of Sjögren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 3392. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.M.; Uh, S.T. Treatment of connective tissue disease-associated interstitial lung disease: The pulmonologist’s point of view. Korean J. Intern. Med. 2017, 32, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.W.; Jung, K.H.; Lee, Y.S.; Kim, H.J.; Yoon, D.Y.; Lee, S.H.; Hann, H.J.; Kim, K.H.; Han, S.; Kim, Y.; et al. Incidence, prevalence, mortality and causes of death in systemic sclerosis in Korea: A nationwide population-based study. Br. J. Dermatol. 2018, 178, e37–e39. [Google Scholar] [CrossRef]
- Alam, J.; Jeon, S.; Choi, Y. Determination of Anti-aquaporin 5 Autoantibodies by Immunofluorescence Cytochemistry. Methods Mol. Biol. 2019, 1901, 79–87. [Google Scholar]
- Bubb, M.O.; Green, F.; Conradie, J.D.; Tchernyshev, B.; Bayer, E.A.; Wilchek, M. Natural antibodies to avidin in human serum. Immunol. Lett. 1993, 35, 277–280. [Google Scholar] [CrossRef]
- Waters, P.J.; Pittock, S.J.; Bennett, J.L.; Jarius, S.; Weinshenker, B.G.; Wingerchuk, D.M. Evaluation of aquaporin-4 antibody assays. Clin. Exp. Neuroimmunol. 2014, 5, 290–303. [Google Scholar] [CrossRef]
- Theander, E.; Jonsson, R.; Sjöström, B.; Brokstad, K.; Olsson, P.; Henriksson, G. Prediction of Sjögren’s Syndrome Years Before Diagnosis and Identification of Patients with Early Onset and Severe Disease Course by Autoantibody Profiling. Arthritis Rheumatol. 2015, 67, 2427–2436. [Google Scholar] [CrossRef]
- Direito, I.; Madeira, A.; Brito, M.A.; Soveral, G. Aquaporin-5: From structure to function and dysfunction in cancer. Cell. Mol. Life Sci. 2016, 73, 1623–1640. [Google Scholar] [CrossRef]
- Xu, M.; Xiao, M.; Li, S.; Yang, B. Aquaporins in Nervous System. Adv. Exp. Med. Biol. 2017, 969, 81–103. [Google Scholar] [PubMed]
- Kassan, S.S.; Moutsopoulos, H.M. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch. Intern. Med. 2004, 164, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
| SICCA Registry | Seoul St. Mary’s Hospital | ||||
|---|---|---|---|---|---|
| SS (n = 111) | Non-SS (n = 43) | SLE (n = 35) | RA (n = 35) | ||
| Age (years), mean ± SD | 50.4 ± 13.6 | 53.1 ± 14.9 | 30.5 ± 9.4* | 57.0 ± 12.4 | |
| anti-SSA+, n (%) | 102 (91.9) | 0 (0) | 20 (69.0), 29† | ND | |
| anti-SSB+, n (%) | 61 (55.0) | 0 (0) | 6 (20.7), 29† | ND | |
| RF+, n (%) | 75 (67.6) | 0 (0) | 0 (0), 22† | 27 (77.1) | |
| ANA+, n (%) | 75 (67.6) | 0 (0) | 34 (97.1) | 14 (45.2), 31† | |
| UWSFR ≤0.1 mL/min, n (%) | 84 (75.7) | 15 (34.9) | ND | ND | |
| Labial salivary gland biopsy results | FLS score ≥1, n (%) | 80 (80), 100† | 0 (0) | ND | ND |
| FLS score 0 << 1, n (%) | 7 (7), 100† | 8 (18.6) | ND | ND | |
| FLS score = 0, n (%) | 13 (13), 100† | 35 (81.4) | ND | ND | |
| N/SCS | 13 (13), 100† | 34 (79.0) | ND | ND | |
| Schirmer’s test ≤5 mm in 5 min, n (%) | 63 (57.3), 110† | 11 (25.6) | ND | ND | |
| Ocular staining score ≥3, n (%) | 105 (95.5), 110† | 0 (0) | ND | ND | |
| Method | Ab | Dilution of Sera | AUC | Confidence Interval | p | Cut-off Value | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| CB-IFC | anti-AQP5_IgG | 1:100 | 0.638 | 0.542–0.735 | 0.008 | 6.71 | 0.595 | 0.721 | 0.658 |
| anti-AQP5_A IgG | 1:100 | 0.616 | 0.510–0.721 | 0.026 | 0.38 | 0.784 | 0.488 | 0.636 | |
| anti-AQP5_C2 IgG | 1:100 | 0.688 | 0.595–0.781 | <0.001 | 1.79 | 0.622 | 0.721 | 0.672 | |
| anti-AQP5_E1 IgG | 1:100 | 0.703 | 0.609–0.796 | <0.001 | 1.75 | 0.613 | 0.767 | 0.690 | |
| anti-AQP5 IgA | 1:10 | 0.694 | 0.612–0.777 | <0.001 | 0.83 | 0.396 | 0.953 | 0.675 | |
| ELISA | anti-AQP5_A IgG | 1:50 | 0.665 | 0.565–0.764 | 0.002 | 54.1 | 0.712 | 0.651 | 0.681 |
| anti-AQP5_C2 IgG | 1:50 | 0.640 | 0.540–0.740 | 0.007 | 44.3 | 0.631 | 0.698 | 0.664 | |
| anti-AQP5_E1 IgG | 1:50 | 0.695 | 0.593–0.798 | <0.001 | 19.3 | 0.856 | 0.558 | 0.707 | |
| anti-AQP5_ACE IgG | 1:50 | 0.671 | 0.570–0.772 | 0.001 | 34.9 | 0.820 | 0.558 | 0.689 | |
| anti-AQP5_A IgG | 1:100 | 0.664 | 0.567–0.762 | 0.002 | 20.3 | 0.811 | 0.465 | 0.638 | |
| anti-AQP5_C2 IgG | 1:100 | 0.645 | 0.544–0.746 | 0.005 | 21.6 | 0.676 | 0.605 | 0.640 | |
| anti-AQP5_E1 IgG | 1:100 | 0.649 | 0.544–0.753 | 0.004 | 26.0 | 0.739 | 0.581 | 0.660 | |
| anti-AQP5_ACE IgG | 1:100 | 0.682 | 0.583–0.781 | <0.001 | 66.0 | 0.667 | 0.651 | 0.659 |
| Disease Criteria for Sjogren’s Syndrome | n | Anti-AQP5_E1 IgG by CB-IFC | Anti-AQP5_E1 IgG by ELISA | |||||
|---|---|---|---|---|---|---|---|---|
| ≥1.75 (au) | <1.75 (au) | p* | ≥19.3 (ng/mL) | <19.3 (ng/mL) | p* | |||
| anti-SSA | Positive | 102 | 62 (60.8%) | 40 (39.2%) | 0.001 | 84 (82.4%) | 18 (17.6%) | <0.001 |
| Negative | 52 | 17 (32.7%) | 35 (67.3%) | 27 (51.9%) | 25 (48.1%) | |||
| anti-SSB | Positive | 61 | 35 (57.4%) | 26 (42.6%) | 0.222 | 50 (82.0%) | 11 (18.0%) | 0.027 |
| Negative | 93 | 44 (47.3%) | 49 (52.7%) | 61 (65.6%) | 32 (34.4%) | |||
| RF | Positive | 75 | 45 (60.0%) | 30 (40.0%) | 0.035 | 63 (84.0%) | 12 (16.0%) | 0.001 |
| Negative | 79 | 34 (43.0%) | 45 (57.0%) | 48 (60.8%) | 31 (39.2%) | |||
| ANA | Positive | 75 | 46 (61.3%) | 29 (38.7%) | 0.015 | 67 (89.3%) | 8 (10.7%) | <0.001 |
| Negative | 79 | 33 (41.8%) | 46 (58.2%) | 44 (55.7%) | 35 (44.3%) | |||
| UWSFR | ≤0.1 ml/min | 99 | 56 (56.6%) | 43 (43.4%) | 0.079 | 74 (74.7%) | 25 (25.3%) | 0.322 |
| >0.1 ml/min | 55 | 23 (41.8%) | 32 (58.2%) | 37 (67.3%) | 18 (32.7%) | |||
| FLS score | ≥1 | 80 | 50 (62.5%) | 30 (37.5%) | 0.006 | 70 (87.5%) | 10 (12.5%) | <0.001 |
| 0 << 1 | 15 | 7 (46.7%) | 8 (53.3%) | 10 (66.7%) | 5 (33.3%) | |||
| =0 | 48 | 16 (33.3%) | 32 (66.7%) | 24 (50.0%) | 24 (50.0%) | |||
| Schirmer’s test | ≤5 mm in 5 min | 74 | 36 (48.6%) | 38 (51.4%) | 0.618 | 58 (78.4%) | 16 (21.6%) | 0.391 |
| >5 mm in 5 min | 79 | 39 (49.4%) | 40 (50.6%) | 55 (69.6%) | 24 (30.4%) | |||
| Ocular staining score | ≥3 | 105 | 66 (62.9%) | 39 (37.1%) | <0.001 | 86 (81.9%) | 19 (18.1%) | <0.001 |
| <3 | 49 | 13 (26.5%) | 36 (73.5%) | 25 (51.0%) | 24 (49.0%) | |||
| Disease Criteria for Sjogren’s Syndrome | IgA by CB-IFC (au) | IgG by CB-IFC (au) | IgG by ELISA (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AQP5 | A | C2 | E1 | AQP5 | A | C2 | E1 | AC2E1 | ||
| anti-SSA | Positive | 0.3 (3.7) | 2.3 (16.0) | 2.4 (16.0) | 2.5 (15.0) | 8.2 (18.3) | 67 (852) | 53 (332) | 66 (500) | 85 (975) |
| Negative | 0 (3.7) | 1.5 (12.3) | 1.0 (10.3) | 1.0 (9.7) | 5.3 (12.7) | 39 (458) | 31 (271) | 35 (457) | 46 (349) | |
| P† | < 0.001 | 0.099 | 0.008 | 0.002 | 0.019 | 0.005 | 0.016 | 0.002 | 0.004 | |
| anti-SSB | Positive | 0.3 (3.7) | 2.3 (14.0) | 2.3 (12.8) | 2.3 (14.3) | 7.3 (18.3) | 74 (852) | 52 (332) | 78 (500) | 86 (975) |
| Negative | 0.3 (3.7) | 1.8 (12.3) | 1.7 (16.0) | 1.7 (15.0) | 6.8 (18.0) | 55 (459) | 41 (298) | 52 (457) | 64 (351) | |
| P† | 0.183 | 0.356 | 0.243 | 0.406 | 0.688 | 0.253 | 0.635 | 0.052 | 0.208 | |
| RF | Positive | 0.3 (3.7) | 2.3 (14.0) | 2.4 (16.0) | 2.7 (14.3) | 8.3 (18.3) | 65 (852) | 45 (332) | 67 (500) | 71 (975) |
| Negative | 0.0 (3.7) | 1.7 (12.3) | 1.7 (9.7) | 1.5 (15.0) | 6.3 (17.0) | 43 (768) | 30 (309) | 46 (457) | 42 (787) | |
| P† | 0.099 | 0.155 | 0.035 | 0.080 | 0.247 | 0.007 | 0.254 | 0.003 | 0.002 | |
| ANA | Positive | 0.3 (3.7) | 2.0 (14.0) | 2.5 (12.8) | 2.3 (15.0) | 8.3 (18.3) | 54 (852) | 44 (332) | 61 (500) | 66 (975) |
| Negative | 0.0 (3.7) | 1.7 (12.3) | 1.3 (16.0) | 1.5 (14.4) | 6.0 (18.0) | 43 (628) | 25 (301) | 34 (457) | 46 (546) | |
| P† | < 0.001 | 0.157 | 0.024 | 0.055 | 0.409 | 0.093 | 0.004 | < 0.001 | 0.012 | |
| UWSFR | ≤ 0.1 ml/min | 0.3 (3.7) | 1.8 (14.0) | 2.3 (16.0) | 2.3 (15.0) | 6.8 (18.3) | 66 (852) | 47 (332) | 66 (500) | 82 (975) |
| > 0.1 ml/min | 0.0 (3.7) | 1.8 (10.0) | 1.7 (10.3) | 1.7 (14.4) | 8.0 (16.7) | 91 (768) | 66 (309) | 64 (457) | 91 (787) | |
| P† | 0.327 | 0.944 | 0.329 | 0.259 | 0.420 | 0.381 | 0.240 | 0.992 | 0.166 | |
| FLS score | ≥ 1 | 0.3 (3.7) | 2.1 (19.3) | 2.5 (21.4) | 2.5 (20.7) | 8.0 (18.3) | 84 (852) | 69 (332) | 88 (500) | 102 (975) |
| 0 << 1 | 0.3 (3.7) | 0.0 (11.3) | 1.0 (19.0) | 1.3 (15.7) | 6.0 (18.0) | 101 (768) | 31 (309) | 37 (457) | 129 (787) | |
| = 0 | 0.0 (3.3) | 1.7 (16.0) | 1.2 (9.2) | 1.0 (13.7) | 6.0 (11.7) | 38 (279) | 23 (271) | 25 (383) | 30 (349) | |
| P‡ | 0.010 | 0.017 | 0.016 | 0.045 | 0.295 | 0.010 | 0.031 | < 0.001 | 0.001 | |
| Schirmer’s test | < 5mm in 5min | 0.3 (3.3) | 1.8 (19.3) | 2.3 (15.8) | 1.9 (19.0) | 7.5 (18.3) | 78 (852) | 53 (332) | 80 (500) | 87 (975) |
| ≥ 5mm in 5min | 0 (3.7) | 1.8 (15.7) | 1.7 (24.7) | 2 (20.7) | 6.3 (18.0) | 65 (768) | 44 (309) | 61 (457) | 87 (787) | |
| P† | 0.388 | 0.652 | 0.498 | 0.894 | 0.975 | 0.756 | 0.942 | 0.170 | 0.660 | |
| Ocular | ≥ 3 | 0.3 (3.7) | 2.6 (14.0) | 2.7 (16.0) | 3.0 (15.0) | 8.3 (18.3) | 82 (852) | 67 (332) | 80 (500) | 101 (975) |
| staining score | < 3 | 0.0 (2.7) | 1.0 (12.3) | 1.0 (7.0) | 0.3 (9.7) | 5.0 (13.0) | 48 (640) | 33 (271) | 22 (457) | 46 (647) |
| P† | < 0.001 | 0.017 | < 0.001 | < 0.001 | 0.007 | 0.041 | 0.049 | 0.001 | 0.013 | |
| Adverse Prognostic Markers | Anti-AQP5_E1 IgG by ELISA | |||||||
|---|---|---|---|---|---|---|---|---|
| Positivity | High Titer (> median) | |||||||
| n | ≥19.3 (ng/mL) | <19.3 (ng/mL) | p* | >79.8 (ng/mL) | ≤79.8 (ng/mL) | p* | ||
| FLS | ≥3 | 41 | 35 (85.4%) | 6 (14.6%) | 0.932 | 27 (65.9%) | 14 (34.1%) | 0.013 |
| <3 | 59 | 50 (84.7%) | 9 (15.3%) | 24 (40.7%) | 35 (59.3%) | |||
| GC | Positive | 8 | 6 (75.0%) | 2 (25.0%) | 0.330 | 4 (50.0%) | 4 (50.0%) | 0.876 |
| Negative | 87 | 76 (87.4%) | 11 (12.6%) | 46 (52.9%) | 41 (47.1%) | |||
| RF | Positive | 75 | 63 (84.0%) | 12 (16.0%) | 0.652 | 40 (53.3%) | 35 (46.7%) | 0.250 |
| Negative | 36 | 29 (80.6%) | 7 (19.4%) | 15 (41.7%) | 21 (58.3%) | |||
| IgG | >1.445 mg/dL | 79 | 66 (83.5%) | 13 (16.5%) | 0.771 | 40 (50.6%) | 39 (49.4%) | 0.720 |
| ≤1.445 mg/dL | 32 | 26 (72.2%) | 6 (18.8%) | 15 (46.9%) | 17 (53.1%) | |||
| C3 | <67 mg/dL | 6 | 5 (83.3%) | 1 (16.7%) | 0.976 | 3 (50%) | 3 (50%) | 0.982 |
| ≥67 mg/dL | 105 | 87 (82.9%) | 18 (17.1%) | 52 (49.5%) | 53 (50.5%) | |||
| C4 | <16 mg/dL | 21 | 19 (90.5%) | 2 (9.5%) | 0.305 | 11 (52.4%) | 10 (47.6%) | 0.773 |
| ≥16 mg/dL | 90 | 73 (81.1%) | 17 (18.9%) | 44 (48.9%) | 46 (51.1%) | |||
| Salivary gland enlargement | Positive | 12 | 6 (50%) | 6 (50%) | 0.974 | 6 (50.0%) | 6 (50.0%) | 0.974 |
| Negative | 99 | 49 (49.5%) | 50 (50.5%) | 49 (49.5%) | 50 (50.5%) | |||
| Systemic disease | Positive | 20 | 16 (80.0%) | 4 (20%) | 0.705 | 9 (45.0%) | 11 (55.0%) | 0.653 |
| Negative | 91 | 76 (83.5%) | 15 (16.5%) | 46 (50.5%) | 45 (49.5%) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Lee, J.; Park, S.-H.; Kim, H.-D.; Choi, Y. Associations of Anti-Aquaporin 5 Autoantibodies with Serologic and Histopathological Features of Sjögren’s Syndrome. J. Clin. Med. 2019, 8, 1863. https://doi.org/10.3390/jcm8111863
Jeon S, Lee J, Park S-H, Kim H-D, Choi Y. Associations of Anti-Aquaporin 5 Autoantibodies with Serologic and Histopathological Features of Sjögren’s Syndrome. Journal of Clinical Medicine. 2019; 8(11):1863. https://doi.org/10.3390/jcm8111863
Chicago/Turabian StyleJeon, Sumin, Jennifer Lee, Sung-Hwan Park, Hyun-Duck Kim, and Youngnim Choi. 2019. "Associations of Anti-Aquaporin 5 Autoantibodies with Serologic and Histopathological Features of Sjögren’s Syndrome" Journal of Clinical Medicine 8, no. 11: 1863. https://doi.org/10.3390/jcm8111863
APA StyleJeon, S., Lee, J., Park, S.-H., Kim, H.-D., & Choi, Y. (2019). Associations of Anti-Aquaporin 5 Autoantibodies with Serologic and Histopathological Features of Sjögren’s Syndrome. Journal of Clinical Medicine, 8(11), 1863. https://doi.org/10.3390/jcm8111863




