Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis of Garlic Extract
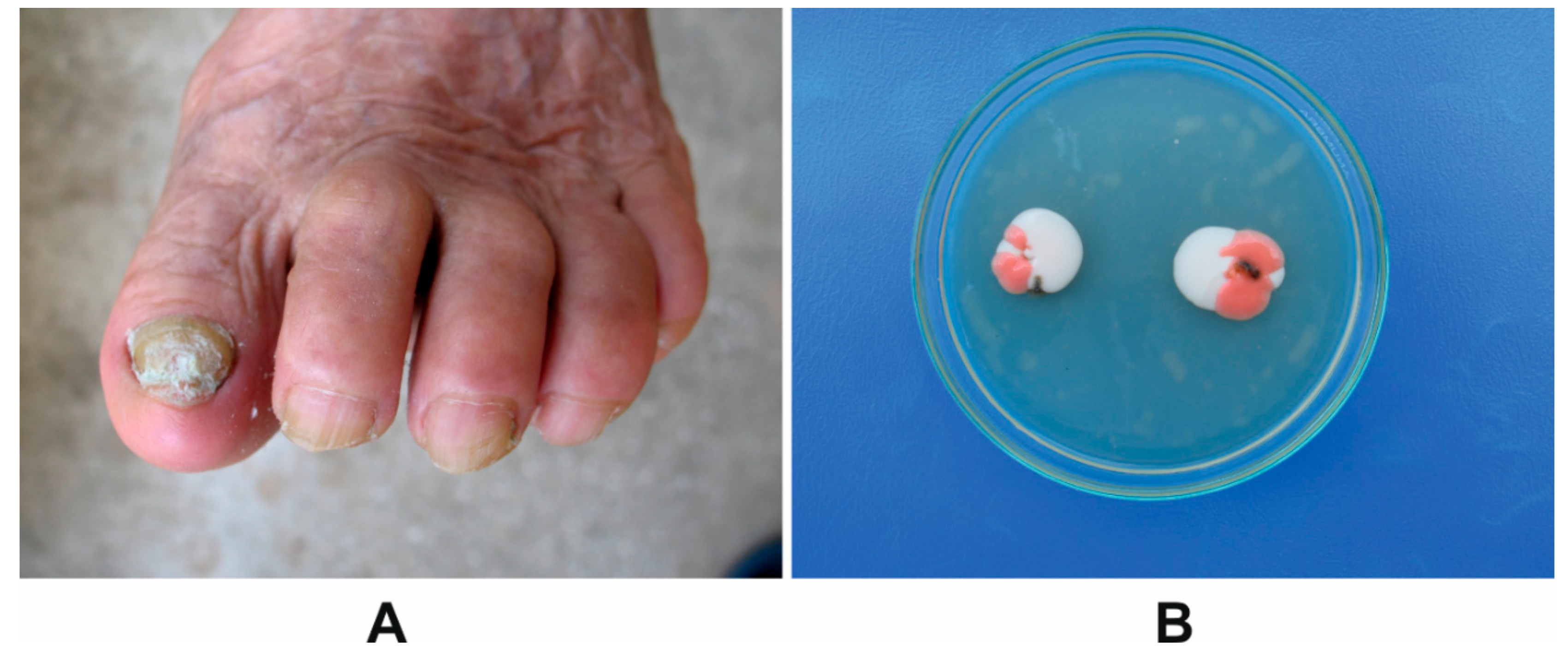
2.2. Onychomycosis Characteristics
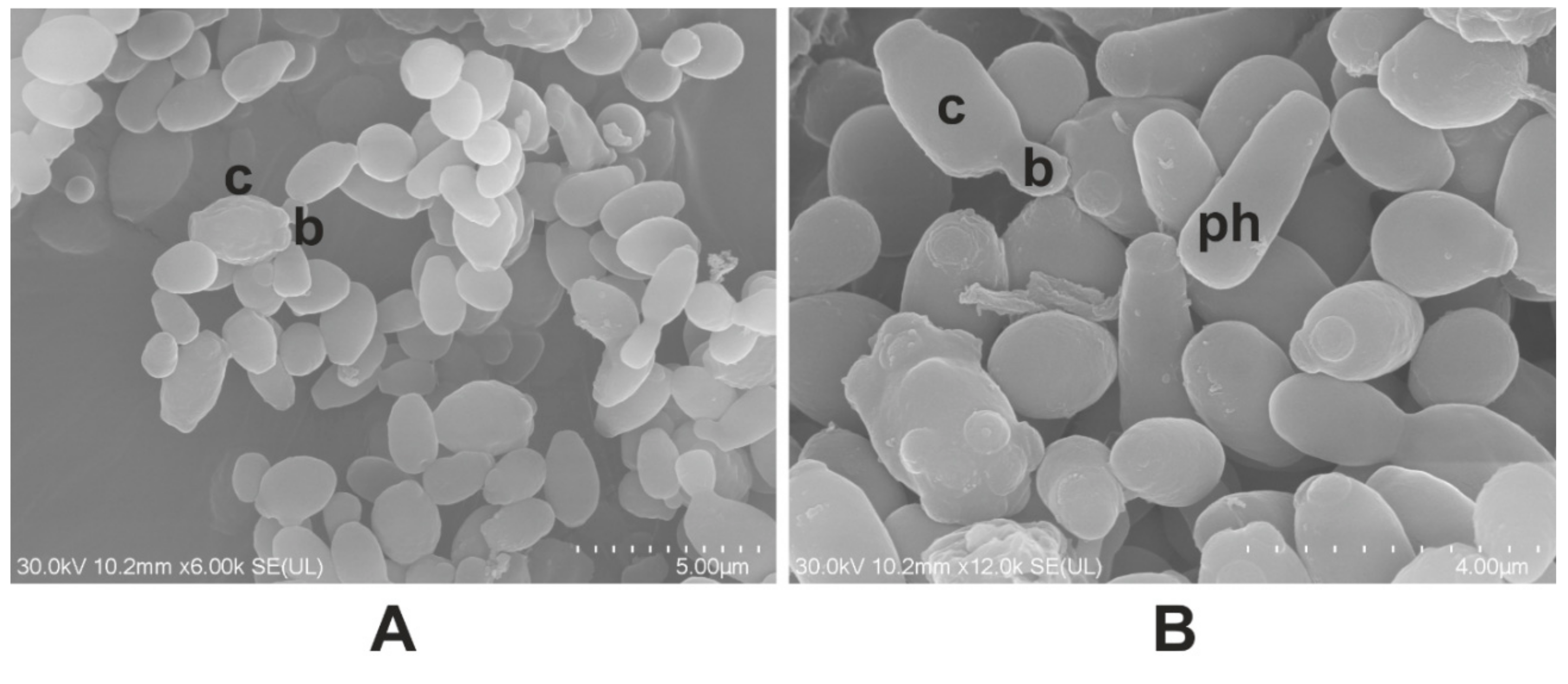
2.3. Molecular Confirmation
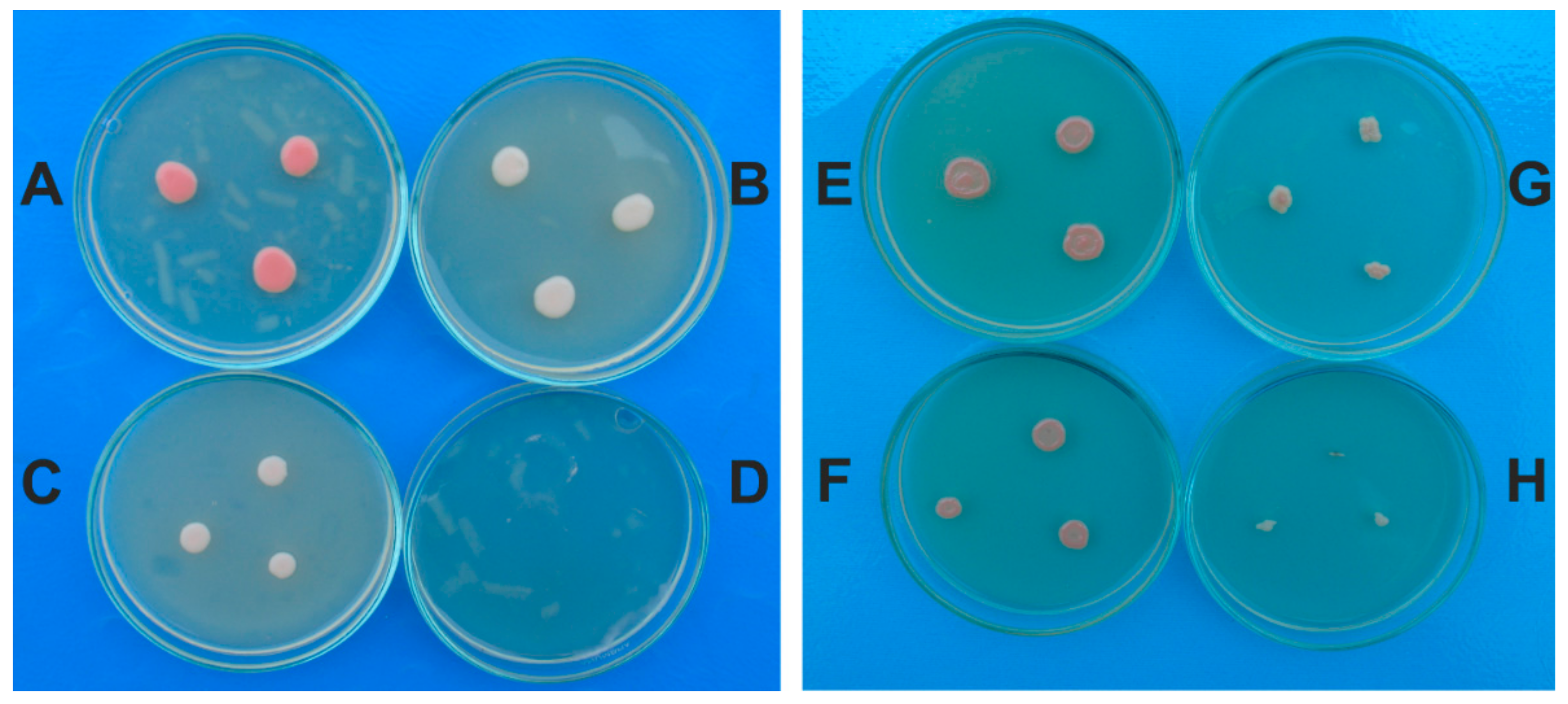
2.4. The Antifungal Effect of A. sativum Extract
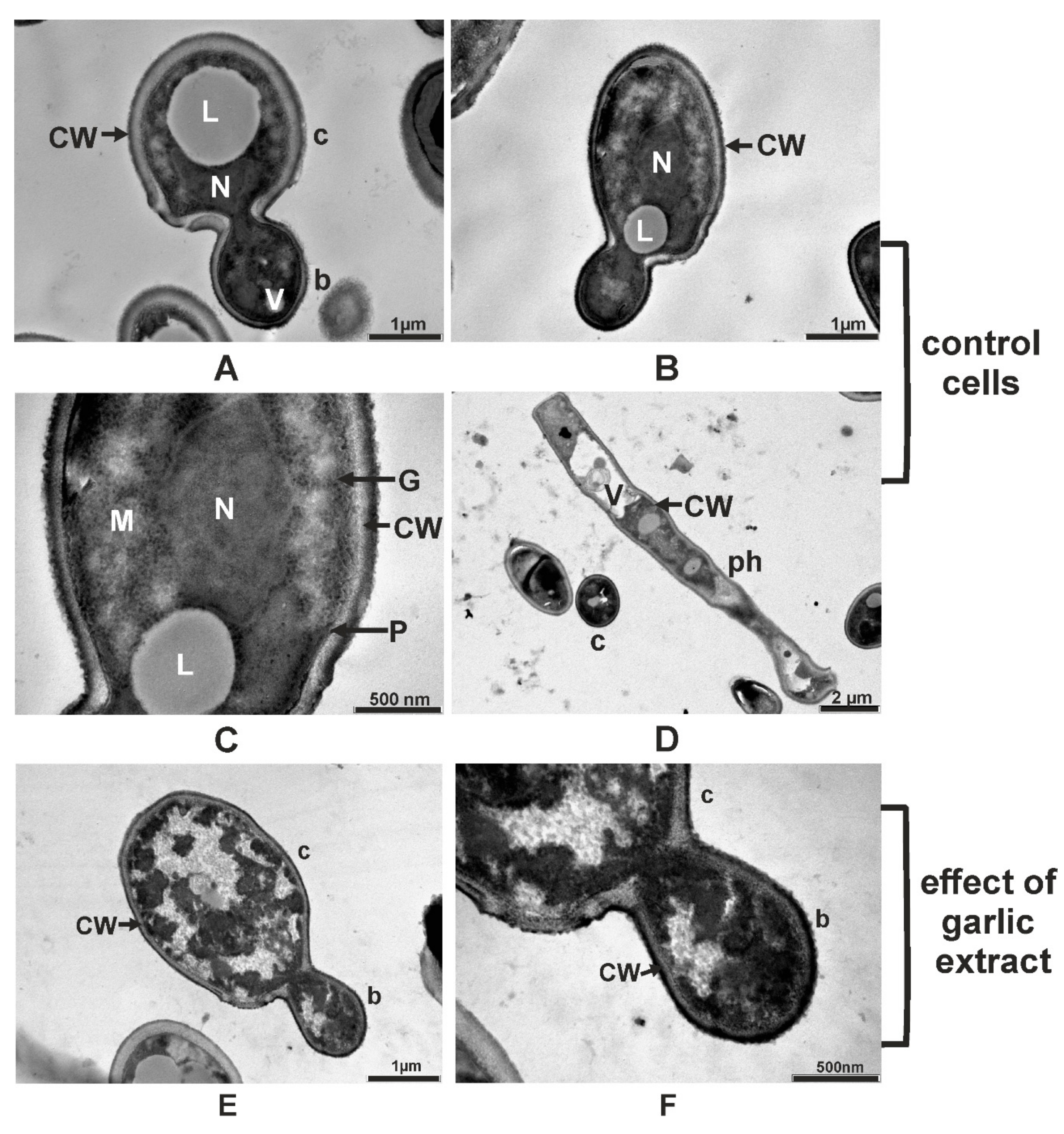
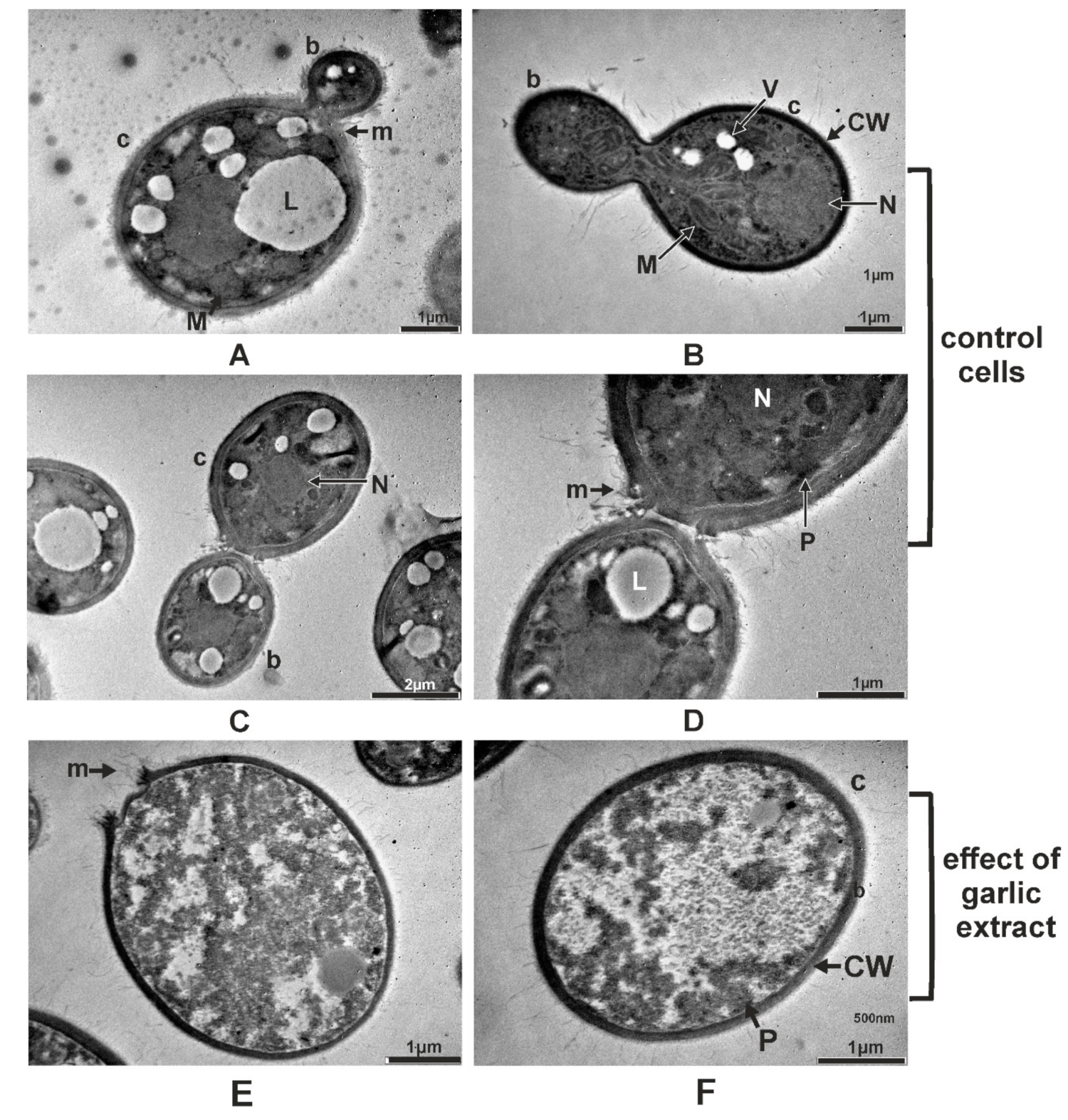
2.5. Ultrastructural Changes Produced by A. sativum Extract
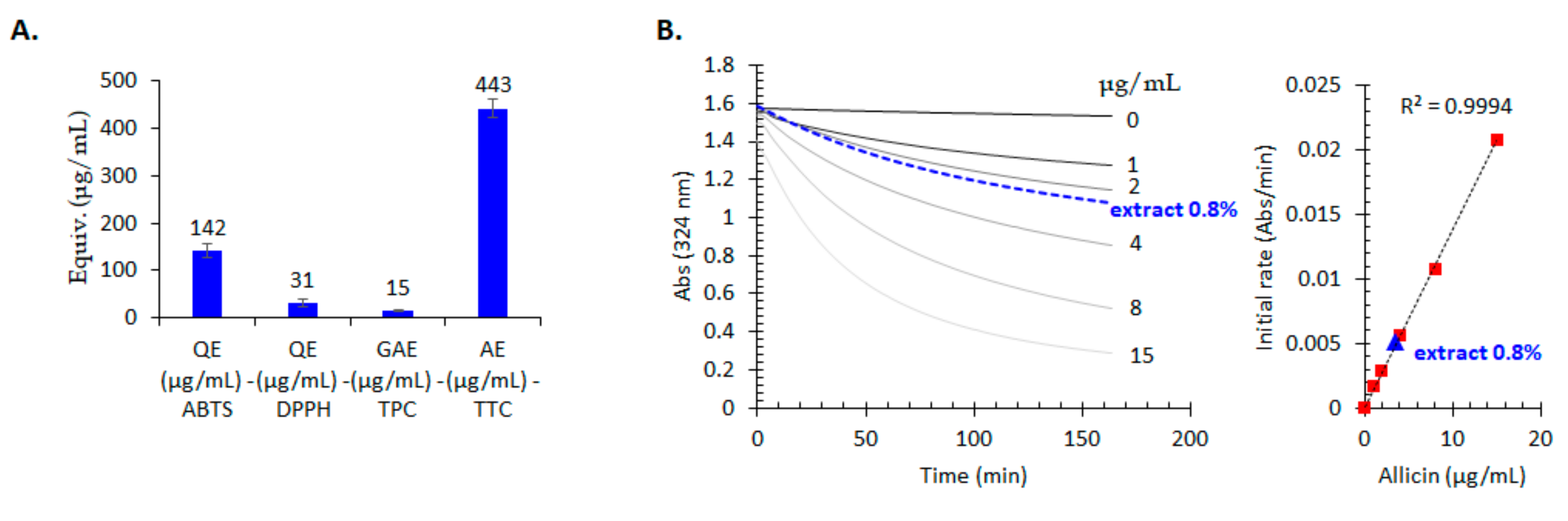
2.6. Antioxidant Properties Evaluation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Allium sativum Extract
4.3. Phytochemical Analysis
4.4. Fungal Strain Isolation and Growth Conditions
4.5. Molecular Confirmation of the Fungi
4.6. The Antifungal Activity Evaluation
4.7. Electron Microscopy
4.8. In Vitro Antioxidant Effect
4.9. In Vivo Antioxidant Effect
4.10. Systemic Oxidative Stress Markers Determination
4.11. Statistical Analysis
5. Conclusions
Data Availability
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Westerberg, D.P.; Voyack, M.J. Onychomycosis: Current trends in diagnosis and treatment. Am. Fam. Physician 2013, 88, 762–770. [Google Scholar] [PubMed]
- Welsh, O.; Vera-Cabrera, L.; Welsh, E. Onychomycosis. Clin. Derm. 2010, 28, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, E.O.; Arroyo-Camarena, S.; Tejada-García, D.L.; Porras-López, C.F.; Arenas, R. Onychomycosis due to opportunistic molds. An. Bras. Derm. 2015, 90, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Shi, Q.-S.; Dai, H.-Q.; Liang, Q.; Xie, X.-B.; Huang, X.-M.; Zhao, G.-Z.; Zhang, L.-X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef]
- Nogueira, M.; Istel, F.; Jenull, S.; Walker, L.; Gow, N.; Lion, T. Quantitative Analysis of Candida Cell Wall Components by Flow Cytometrywith Triple-Fluorescence Staining. J. Microbiol. Mod. Tech. 2017. [Google Scholar]
- Pfaller, M.; Diekema, D.; Mendez, M.; Kibbler, C.; Erzsebet, P.; Chang, S.-C.; Gibbs, D.; Newell, V.; Group, G.A.S. Candida guilliermondii, an opportunistic fungal pathogen with decreased susceptibility to fluconazole: Geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program. J. Clin. Microbiol. 2006, 44, 3551–3556. [Google Scholar] [CrossRef]
- Schwarzmüller, T.; Ma, B.; Hiller, E.; Istel, F.; Tscherner, M.; Brunke, S.; Ames, L.; Firon, A.; Green, B.; Cabral, V.; et al. Systematic Phenotyping of a Large-Scale Candida glabrata Deletion Collection Reveals Novel Antifungal Tolerance Genes. Plos Pathog. 2014, 10. [Google Scholar] [CrossRef]
- Ellis, D.; Davis, S.; Alexiou, H.; Handke, R.; Bartley, R. Descriptions of medical fungi, mycology unit, women’s and children’s hospital Adelaide, Australia, 2007.
- Cebeci Güler, N.; Tosun, I.; Aydin, F. The identification of Meyerozyma guilliermondii from blood cultures and surveillance samples in a university hospital in Northeast Turkey: A ten-year survey. J. Mycol. Med. 2017, 27, 506–513. [Google Scholar] [CrossRef]
- Mügge, C.; Haustein, U.F.; Nenoff, P. Causative agents of onychomycosis—A retrospective study. J. Dtsch. Derm. Ges 2006, 4, 218–228. [Google Scholar] [CrossRef]
- Da Cunha, M.M.; Dos Santos, L.P.; Dornelas-Ribeiro, M.; Vermelho, A.B.; Rozental, S. Identification, antifungal susceptibility and scanning electron microscopy of a keratinolytic strain of Rhodotorula mucilaginosa: A primary causative agent of onychomycosis. Fems. Immunol. Med. Microbiol. 2009, 55, 396–403. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, M.; Chen, H.; Pan, W.; Liao, W. Rhodotorula minuta as onychomycosis agent in a Chinese patient: First report and literature review. Mycoses 2014, 57, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Vimal, V.; Devaki, T. Hepatoprotective effect of allicin on tissue defense system in galactosamine/endotoxin challenged rats. J. Ethnopharmacol. 2004, 90, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Tech. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Shi, D.; Lu, G.; Mei, H.; De Hoog, G.S.; Zheng, H.; Liang, G.; Shen, Y.; Li, T.; Liu, W. Onychomycosis due to Chaetomium globosum with yellowish black discoloration and periungual inflammation. Med. Mycol. Case Rep. 2016, 13, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Duhard, É. Les paronychies. La Presse Médicale 2014, 43, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Sinikumpu, S.-P.; Huilaja, L.; Auvinen, J.; Jokelainen, J.; Puukka, K.; Ruokonen, A.; Timonen, M.; Tasanen, K. The Association Between Low Grade Systemic Inflammation and Skin Diseases: A Cross-sectional Survey in the Northern Finland Birth Cohort 1966. Acta Derm. Venereol. 2018, 98, 65–69. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Pârvu, M. Polyphenolic Compounds, Antioxidant, and Cardioprotective Effects of Pomace Extracts from Fetească Neagră Cultivar. Oxid. Med. Cell Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Andreicuţ, A.-D.; Parvu, A.E.; Moț, A.C.; Parvu, M.; Fischer-Fodor, E.; Feldrihan, V.; Cătoi, A.F.; Cecan, M.; Irimie, A. Anti-inflammatory and antioxidant effects of Mahonia aquifolium leaves and bark extracts. Farmacia 2018, 66. [Google Scholar]
- Gupta, A.K.; Versteeg, S.G.; Shear, N.H. Onychomycosis in the 21st century: An update on diagnosis, epidemiology, and treatment. J. Cutan. Med. Surg. 2017, 21, 525–539. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucl. Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Miron, T.; Shin, I.; Feigenblat, G.; Weiner, L.; Mirelman, D.; Wilchek, M.; Rabinkov, A. A spectrophotometric assay for allicin, alliin, and alliinase (alliin lyase) with a chromogenic thiol: Reaction of 4-mercaptopyridine with thiosulfinates. Anal. Biochem. 2002, 307, 76–83. [Google Scholar] [CrossRef]
- Hay, R.J.; Baran, R. Onychomycosis: A proposed revision of the clinical classification. J. Am. Acad. Dermatol. 2011, 65, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Parvu, M.; Parvu, E.; Toiu, A. Chemical constituents of three Allium species from Romania. Molecules 2013, 18, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Głód, D.; Żebrowska, M.E.; Sznitowska, M.; Krauze-Baranowska, M. TLC determination of flavonoids from different cultivars of Allium cepa and Allium ascalonicum. Acta Pharm. 2016, 66, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Activity of phenolic compounds from plant origin against Candida species. Ind. Crop. Prod. 2015, 74, 648–670. [Google Scholar] [CrossRef]
- Kanoun, K.; Abbouni, B.; Boudissa, S.; Bouhafs, N.; Seddiki, M. Étude de l’activité des extraits de feuilles de Punica granatum Linn sur Candida albicans et Rhodotorula spp. Phytothérapie 2016, 14, 5–16. [Google Scholar] [CrossRef]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef]
- Moț, A.-C.; Damian, G.; Sarbu, C.; Silaghi-Dumitrescu, R. Redox reactivity in propolis: Direct detection of free radicals in basic medium and interaction with hemoglobin. Redox Rep. 2009, 14, 267–274. [Google Scholar] [CrossRef]
- Moț, A.C.; Pârvu, M.; Pârvu, A.E.; Roşca-Casian, O.; Dina, N.E.; Leopold, N.; Silaghi-Dumitrescu, R.; Mircea, C. Reversible naftifine-induced carotenoid depigmentation in Rhodotorula mucilaginosa (A. Jörg.) FC Harrison causing onychomycosis. Sci. Rep. 2017, 7, 11125. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Perez, G.; Abraira, C.; Oster, J.R.; Lespier, L.; Vaamonde, C.A. The Effect of Dexamethasone on Urinary Acidification. Proc. Soc. Exp. Biol. Med. 1975, 150, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Buendía, A.S.A.; González, M.T.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. Immunomodulatory effects of the nutraceutical garlic derivative allicin in the progression of diabetic nephropathy. Int. J. Mol. Sci. 2018, 19, 1–13. [Google Scholar]
- Pârvu, A.E.; Pârvu, M.; Vlase., L.; Miclea., P.; Mot, A.C.; Silaghi-Dumitrescu, R. Anti-inflammatory effects of Allium schoenoprasum L. leaves. J. Physiol. Pharm. 2014, 65, 309–315. [Google Scholar]
- Başkol, M.; Dolbun Seçkin, K.; Başkol, G. Advanced oxidation protein products, total thiol levels and total oxidant/antioxidant status in patients with nash. Turk. J. Gastroenterol. 2014, 25, 32–37. [Google Scholar] [CrossRef]
Sample Availability: Samples of the studied compounds (garlic extract, fungal isolates, and electron microscopic images) are available from the authors. |


| Garlic Extract Concentration (%) | Colony a Diameter (mm) | P a | Naftifine Solution Concentration (%) | Colony b Diameter (mm) | P b |
|---|---|---|---|---|---|
| C | 11.66 ± 0.81 | 0 | C | 11.66 ± 0.81 | 0 |
| 2 | 11.16 ± 0.40 | 4.28 ± 0.40 | 0.5 | 10.0 ± 0.63 | 14.23 ± 0.63 |
| 4 | 9.66 ± 0.51 | 17.15 ± 0.51 | 1 | 8.33 ± 0.51 | 28.55 ± 0.51 |
| 6 | 6.66 ± 0.51 | 42.88 ± 0.51 | 2 | 6.83 ± 0.83 | 41.42 ± 0.83 |
| 8 | 3.50 ± 0.54 | 69.98 ± 0.54 | 3 | 5.16 ± 0.40 | 55.74 ± 0.40 |
| 10 | 1.33 ± 0.51 | 88.59 ± 0.51 | 4 | 5.16 ± 0.40 | 55.74 ± 0.40 |
| 12 | 0 | 100 | 5 | 5.16 ± 0.40 | 55.74 ± 0.40 |
| Garlic Extract Concentration (%) | Colony a Diameter (mm) | P a | Naftifine Solution Concentration (%) | Colony b Diameter (mm) | P b |
|---|---|---|---|---|---|
| C | 13.33 ± 0.51 | 0 | C | 13.33 ± 0.51 | 0 |
| 2 | 12.16 ± 0.75 | 8.77 ± 0.75 | 0.1 | 12.83 ± 0.40 | 3.75 ± 0.40 |
| 4 | 10.33 ± 0.51 | 22.50 ± 0.51 | 0.5 | 10.5 ± 0.54 | 21.23 ± 0.54 |
| 6 | 9.0 ± 0.63 | 32.48 ± 0.63 | 1.0 | 7.83 ± 0.40 | 41.26 ± 0.40 |
| 8 | 7.33 ± 0.57 | 45.01 ± 0.57 | 1.5 | 6.33 ± 0.51 | 52.51 ± 0.51 |
| 10 | 4.0 ± 0.63 | 69.99 ± 0.63 | 2 | 3.66 ± 0.81 | 72.54 ± 0.81 |
| 12 | 1.33 ± 0.51 | 90.02 ± 0.51 | 2.5 | 1.33 ± 0.51 | 90.02 ± 0.51 |
| 14 | 100 | 3.0 | 0 | 100 |
| TOS (μmol H2O2 equiv/L) | TAR (mmol trolox equiv/L) | OSI | NOx (μmol/L) | MDA (nmol/mL) | SH mmol (TSH/mL) | |
|---|---|---|---|---|---|---|
| Control | 38.22 ± 4.77 a*** | 1.0897 ± 0.0014 a** | 35.04 ± 4.39 a*** | 52.88 ± 2.60 a*** | 5.82 ± 0.56 a*** | 0.67 ± 0.07 a*** |
| Inflammation | 66.35 ± 8.49 b**, c* | 1.0891 ± 0.0009 b*, c* | 60.90 ± 7.81 b**, c* | 82.42 ± 0.27 b***, c** | 7.50 ± 0.77 b***, c* | 0.50 ± 0.08 b**, c* |
| Diclofenac | 48.65 ± 8.36 a**, b* | 1.0970 ± 0.0017 b* | 44.47 ± 7.59 a***, b* | 58.71 ± 5.29 a**, b* | 5.75 ± 0.80 a**, b* | 0.71 ± 0.11 a**, b* |
| Allicin | 33.76 ± 4.82 a**, c* | 1.0884 ± 0.0005 a*, c* | 31.02 ± 4.43 a**, c* | 40.30 ± 6.12 a***, c* | 5.57 ± 0.48 a**, c* | 0.75 ± 0.13 a**, c* |
| A. sativum 100% | 33.13 ± 8.06 a**, b*, c* | 1.0894 ± 0.0009 a*, b*, c* | 30.43 ± 7.39 a**, b*, c* | 38.12 ± 7.57 a***, b*, c** | 5.54 ± 0.32 a**, b*, c* | 0.60 ± 0.09 a**, b*, c* |
| A. sativum 50% | 31.88 ± 6.91 a**, b*, c* | 1.0884 ± 0.0009 a*, b*, c* | 29.27 ± 6.34 a**, b*, c* | 38.15 ± 8.10 a***, b*, c** | 5.48 ± 0.63 a**, b*, c* | 0.81 ± 0.29 a***, b*, c* |
| A. sativum 25% | 32.25 ± 3.75 a**, b*, c* | 1.0884 ± 0.0005 a*, b*, c* | 29.63 ± 3.43 a**, b*, c* | 53.91 ± 8.83 a**,b*, c* | 5.79 ± 0.51 a**, b*, c* | 0.62 ± 0.15 a**, b*, c* |
| Group | 7 Days Treatment by Gavage: 1mL/Animal | i.m. 0.6 mL/kg bw | |
|---|---|---|---|
| Control | tap water | saline solution | Blood collection under general anesthesia |
| Inflammation | tap water | turpentine oil | |
| Diclofenac | 10 mg/kg bw | turpentine oil | |
| Allicin | 450 μg/mL | turpentine oil | |
| A. sativum 100% | 1mL/animal | turpentine oil | |
| A. sativum 50% | 1mL/animal | turpentine oil | |
| A. sativum 25% | 1mL/animal | turpentine oil |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pârvu, M.; Moţ, C.A.; Pârvu, A.E.; Mircea, C.; Stoeber, L.; Roşca-Casian, O.; Ţigu, A.B. Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis. Molecules 2019, 24, 3958. https://doi.org/10.3390/molecules24213958
Pârvu M, Moţ CA, Pârvu AE, Mircea C, Stoeber L, Roşca-Casian O, Ţigu AB. Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis. Molecules. 2019; 24(21):3958. https://doi.org/10.3390/molecules24213958
Chicago/Turabian StylePârvu, Marcel, Cătălin A. Moţ, Alina E. Pârvu, Cristina Mircea, Leander Stoeber, Oana Roşca-Casian, and Adrian B. Ţigu. 2019. "Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis" Molecules 24, no. 21: 3958. https://doi.org/10.3390/molecules24213958
APA StylePârvu, M., Moţ, C. A., Pârvu, A. E., Mircea, C., Stoeber, L., Roşca-Casian, O., & Ţigu, A. B. (2019). Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis. Molecules, 24(21), 3958. https://doi.org/10.3390/molecules24213958






