1. Introduction
The capsaicinoids family (for example capsaicin, nordihydrocapsaicin, dihydrocapsaicin, homodihydrocapsaicin, homocapsaicin) leads to intense spicy flavor of hot peppers, chilies, and some pungent foods [
1]. The main components of the majority of capsicum species are capsaicin and dihydrocapsaicin [
2,
3]. The Food and Drug Administration (FDA) and European Medicines Agency (EMA) have approved capsaicin as a topical treatment for neuropathic pain, and pointed out that its analgesic effect depends on the dose [
4]. At present, capsaicin also has some cases of application of topical analgesics for painful clinical diseases [
5,
6,
7]. Oral capsaicin or direct consumption of pepper also has important pharmacological effects on human health. Moreover, natural capsaicin is also exploited owning to its anti-inflammation [
8], anticancer [
9,
10,
11], antibacterial, and antioxidant [
12,
13] properties. Furthermore, it is available for cardiovascular and gastrointestinal diseases [
14]. The preparation technology of high-purity capsaicin has attract a strong interest of pharmaceutical chemists due to its wide biological activities.
A variety of techniques, such as aqueous enzymatic extraction [
15], solvent extraction [
16], and microwave- or ultrasound-assisted extraction [
17,
18] can be used for the extraction of capsaicin. However, these processes have obvious drawbacks, including large amounts of organic solvents, low recovery and purity, lengthy processes, etc. Furthermore, a purification step must complement to obtain a high-purity capsaicin product. Currently, the capsaicinoids extract can be purified by simulated moving bed chromatography [
19], high-performance liquid chromatographic techniques [
20], sequential centrifugal partition chromatography (SCPC) [
21], and high-speed counter current chromatography (HSCCC) [
22]. However, these purification technologies often represent the bottleneck of the whole production process because of high cost and sophisticated of those equipment.
For the above-mentioned reasons, aqueous two phase extraction technology combined with chromatography was established by our research group for preparation of high-purity capsaicin [
23,
24]. An aqueous two-phase system (ATPS) has been widely employed for purifying biological materials in biotechnology and biochemistry in the past decades. The high concentration of water (between 65% and 90% in mass) in these systems provides a mild and biocompatible environment for the extraction target molecules [
25]. However, the low interfacial tension, small density differences, and high viscosities of the ATPS will result in long time consumption on phase separation [
26,
27]. Thus, phase separation might become a decisive procedure. In order to accelerate phase separation, the auxiliary equipment and extra energy are necessary [
28]. Recently, the new ‘tunable aqueous polymer-phase impregnated resins’ (TAPPIR
®)-technology, which was prepared by immobilizing one phase of ATPS in porous particles solved these challenges. In 2012, Schembecker et al. were approved the relevant patent (NO.-US 20140228549 A1) for this technology [
29]. Recently, the researchers have conducted a preliminary discussion on the TAPPIR technology and obtained some scientific achievements [
30,
31,
32,
33,
34,
35]. The results demonstrated that the multistage processing of TAPPIR was excellent as a downstream processing technique. The polymer/salt ATPS is generally adopted and the polymer-rich phase is impregnated into porous solids due to its viscosity characteristics [
31,
32,
33]. It is generally accepted that the PEG/citrate ATPSs are more beneficial to the extraction of bioactive substances [
36]. Thus, the PEG/sodium citrate ATPSs were commonly used in the TAPPIR extraction step. According to the literature [
37], the capsaicin can be extracted more efficiently from fresh chili by the pure 1-ethyl-3-methyl imidazolium hydrogen sulfate ([Emim] [HSO
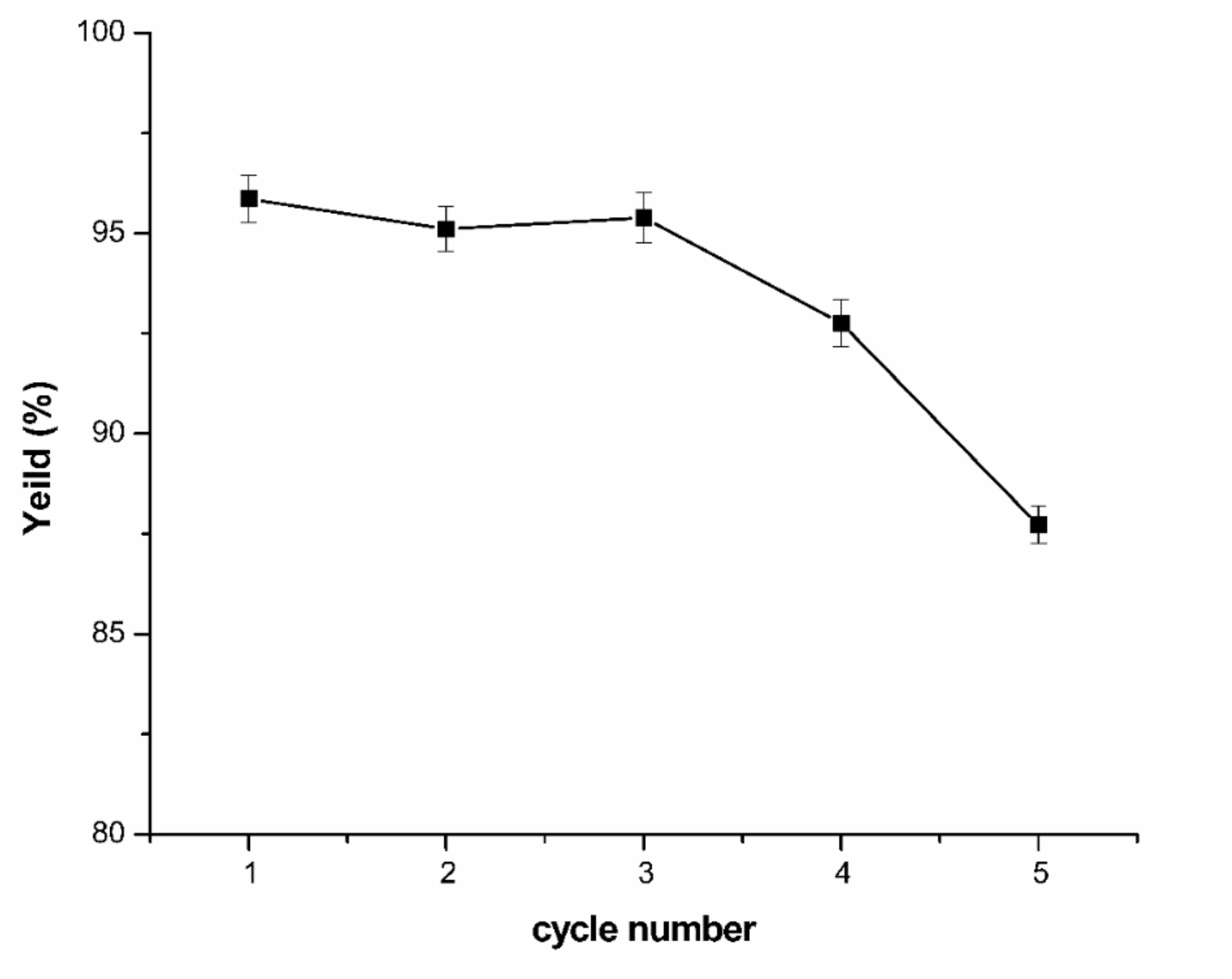
4]) and 1-ethyl-3-methylimidazolium acetate ([Emim] [OAc]). However, the high price and viscosity of the two ionic liquids (ILs) hinders the application of this technology at present. Therefore, a low quantity of two ILs was introduced in this work for their superior extraction ability to enhance the yield of the capsaicin. In addition to the extraction efficiency, the reusability of impregnated solids in multiple cycles also determines the competitiveness of TAPPIR [
34,
35].
In addition to the disadvantage of ATPS, the recovery of capsaicin was also reduced due to two steps chromatographic purification in our previous studies [
23,
24]. The further optimization of the extraction and purification method of capsaicin is very necessary. Practice shows that an inexpensive and novel reverse-phase resin (SKP-10-4300, Jinan, Shandong, China ) has an obvious advantage in the capsaicin purification process [
24]. Hence, the combination TAPPIR and one-step chromatography technology was investigated and SKP-10-4300 was applied in this work.
The purpose of this work was to construct an efficient method for the separation and purification of capsaicin from capsicum oleoresin by combination of TAPPIR and chromatography. The effect of the relevant parameters such as the type impregnated resins and molecular weight of polymer, system pH, the amount of sample loading and addition of ionic liquid the capsaicin were investigated in the TAPPIR extraction step. Subsequently, the purification process of capsaicin was implemented using reverse phase chromatography. Adsorption and desorption properties of SKP-10-4300 was explored and a high recovery and purity of capsaicin were obtained.
3. Materials and Methods
3.1. Materials
Capsicum oleoresin was provided by Yingchaolabeier Co., Ltd. (Shandong, China). The capsaicin standard was purchased from Sigma (St. Louis, MO, USA). Macroporous adsorption resins (MARs) D101-1, 201 × 7, HZ202, D301, D301G, D201, and ADS-17 were provided by Hebei Huazhong Chemical Industries Co., Ltd. (Hebei, China). HZ816, HZ835, and HZ915MARs were purchased from Shanghai Huazhen Technology Co., Ltd. (Shanghai, China). The reverse-phase resin (SKP-10-4300) was obtained from Jinan Bona Biological (Jinan, Shandong, China). [Emim] [HSO4] and [Emim] [OAc] were prepared by Lanzhou Greenchem ILS, LICP. CAS. China (Lanzhou, Gansu, China). Polyethylene glycol (PEG1000, PEG2000, PEG4000, PEG6000) was obtained from Shanghai Lingfeng. Sodium citrate were acquired from Tianjin Guangcheng Chemical Company (Tianjin, China). Methanol (MeOH) of HPLC grade, ethyl acetate, and ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The others reagents were analytical grade. Deionized water was employed throughout in the experiment.
3.2. Methods
3.2.1. Static Adsorption and Desorption Tests of the MARs
All the MARs require pretreatment to remove impurities trapped inside the pores. The resins were soaked in ethanol for 48 h and washed by deionized water until the wash was clear. Then the resins were soaked in 4–5% (w/w) HCl solution for 4 h, and washed by deionized water till the washes was neutral. Then the resins were immersed in 4–5% (w/w) NaOH solution for 4 h, and washed by deionized water till the washing liquid was neutral. Then, the MARs were preserved in deionized water and removed from the deionized water by vacuum drawing and filtering system for static adsorption and desorption test. A total of 0.2 g of various resins was accurately weighed and mixed with 50 mL capsaicin standard liquid (44.2 μg/mL) in a conical flask. The experiment was carried out at 30 °C in constant temperature oscillator with 100 r/min rotational speed for 24 h. Then, the resins which have adsorbed the capsaicin were rinsed twice by deionized water. Furthermore, 50 mL of ethyl acetate was added to desorb the capsaicin. The original alternative resins were estimated by the ratio of adsorption and desorption.
3.2.2. The Procedure of the TAPPIR® Extraction
In this work, the experimental procedure for TAPPIR extraction was implemented as described by Winssen et al. [
31]. Firstly, the ATPSs were prepared and centrifuged at 1500 r/min at temperature for 10 min. The pH value was adjusted by adding B-R buffer which was prepared by adding amount of 0.20 M NaOH to the solution including 0.04 H
3PO
4, 0.04 M CH
3COOH, and 0.04 M H
3BO
3. Then, the selected resin was impregnated into the top phase of the ATPS to construct impregnation resin. Impregnation took place in an ultrasonic bath for 1h. After impregnation, the particles were separated from the dispersion by filtration and sipped up the surface with sterile filter paper. Subsequently, 3 g of these impregnated particles were dispersed in 10 mL bottom phase of the ATPS with capsicum oleoresin. This dispersion was shaken at 25 °C in constant temperature oscillator with 180 r/min rotational speed for 1 h. After this, the impregnated resin containing capsaicinoids was removed and the back extraction was carried out using ethyl acetate to accurately determine the amount of capsaicin. During the back extraction, the system was shaken at 25 °C in a constant temperature oscillator with a 180 r/min rotational speed for 30 min. The capsaicin content of the ethyl acetate phase was determined using HPLC. The capsaicin yield is defined by Equation (2) as follows:
where
Cethyl acetate,
Vethyl acetate,
Madded, and
Cadded are represent the capsaicin concentration in ethyl acetate phase, the ethyl acetate phase volume, the added quality of capsaicin oleoresin, and the capsaicin concentration in capsaicin oleoresin, respectively.
3.2.3. Response Surface Methodology (RSM)
Design Expert version 8.0.6.1 (Stat-Ease Inc., Minneapolis, MN, USA) software was employed in the process of experiment design, analysis, and modeling.
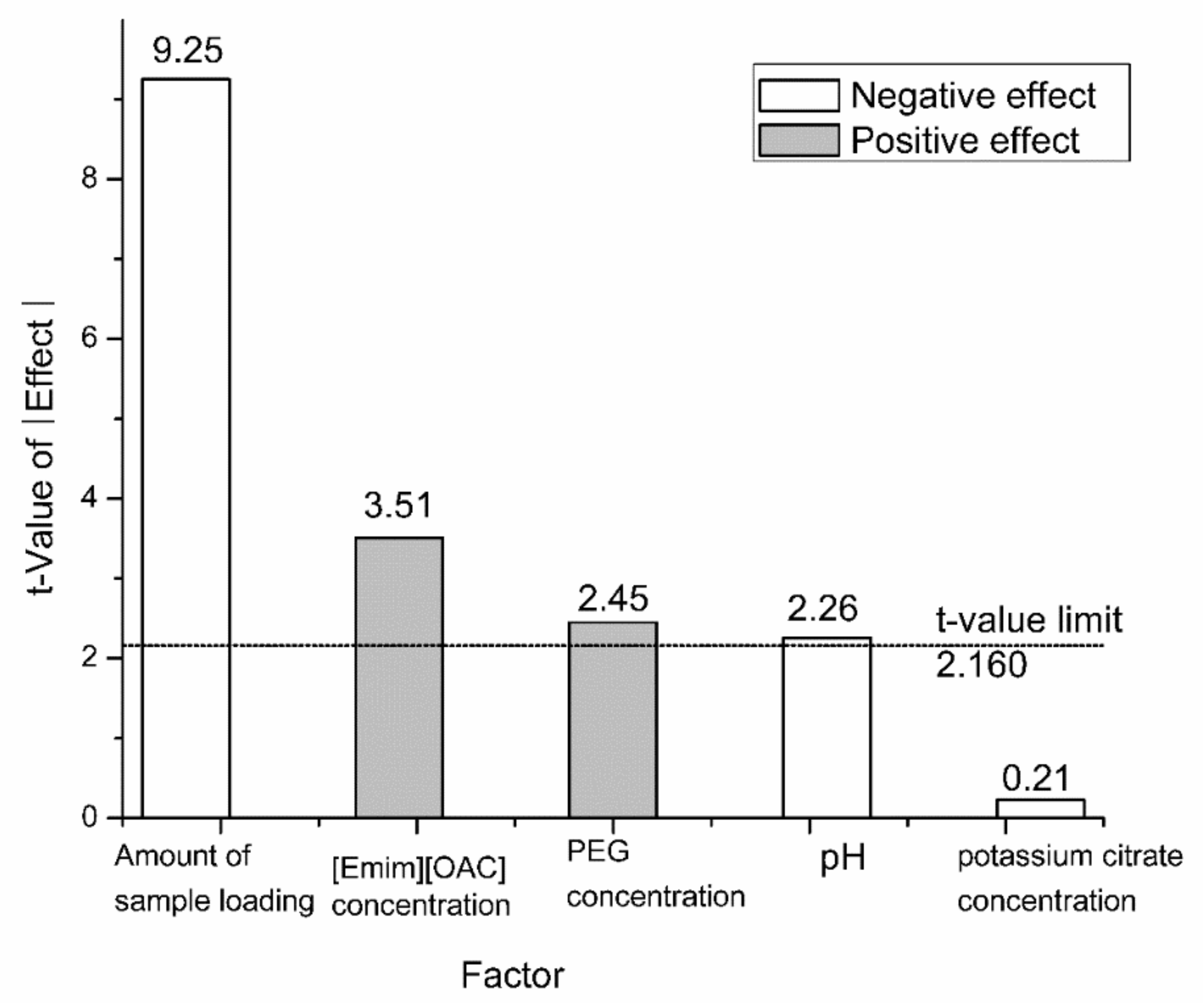
Screening experiment was initially performed through fractional factorial designs. The concentration of PEG6000, concentration of potassium citrate, concentration of [Emim] [OAC], pH, and amount of sample loading were taken into account in this step. The capsaicin yield (Y) was set as the response variable.
In order to obtain an optimized extraction system and evaluate the interaction between various factors, the response surface methodology (RMS) was introduced. The optimum conditions can be predicted by the second-order model as follows:
Among them, Y is the response variable (in percentage), β0 is the fixed response value of the experimental center point, βi, βij, and βii were the linear, cross product coefficients, and quadratic, respectively, and Xi and Xj were independent variables of the code.
3.2.4. Preparation of the Reversed Chromatographic Column
The reversed resins should be soaked in ethanol for 12 h to make the resin full swelling. The preparative reversed phase column (10 mm × 800 mm) was filled with SKP-10-4300 reverse-phase resin (43 mL) with a length of 55 cm. Then, the glass chromatography column was sequentially leached with ethanol, 50% (v/v) ethanol, 25% (v/v) ethanol, and 10% (v/v) ethanol over 1 h. The flow rate of the eluent was 2 bed volume (BV) per hour.
3.2.5. Preparation of the Reversed Chromatographic Loading Solution
After TAPPIR extraction, the impregnated resin containing capsaicinoids was removed. The back-extraction step was employed as in
Section 3.2.2 in this paper. Then, the ethyl acetate phase was separated and concentrated under reduced pressure at 40–50 °C using a rotary evaporator. Subsequently, the concentrate was diluted to appropriate concentration of capsaicin solution by adding 10% ethanol solution with different pH. The pH of ethanol solution was adjusted by 4–5% (
w/
w) HCl or NaOH solution.
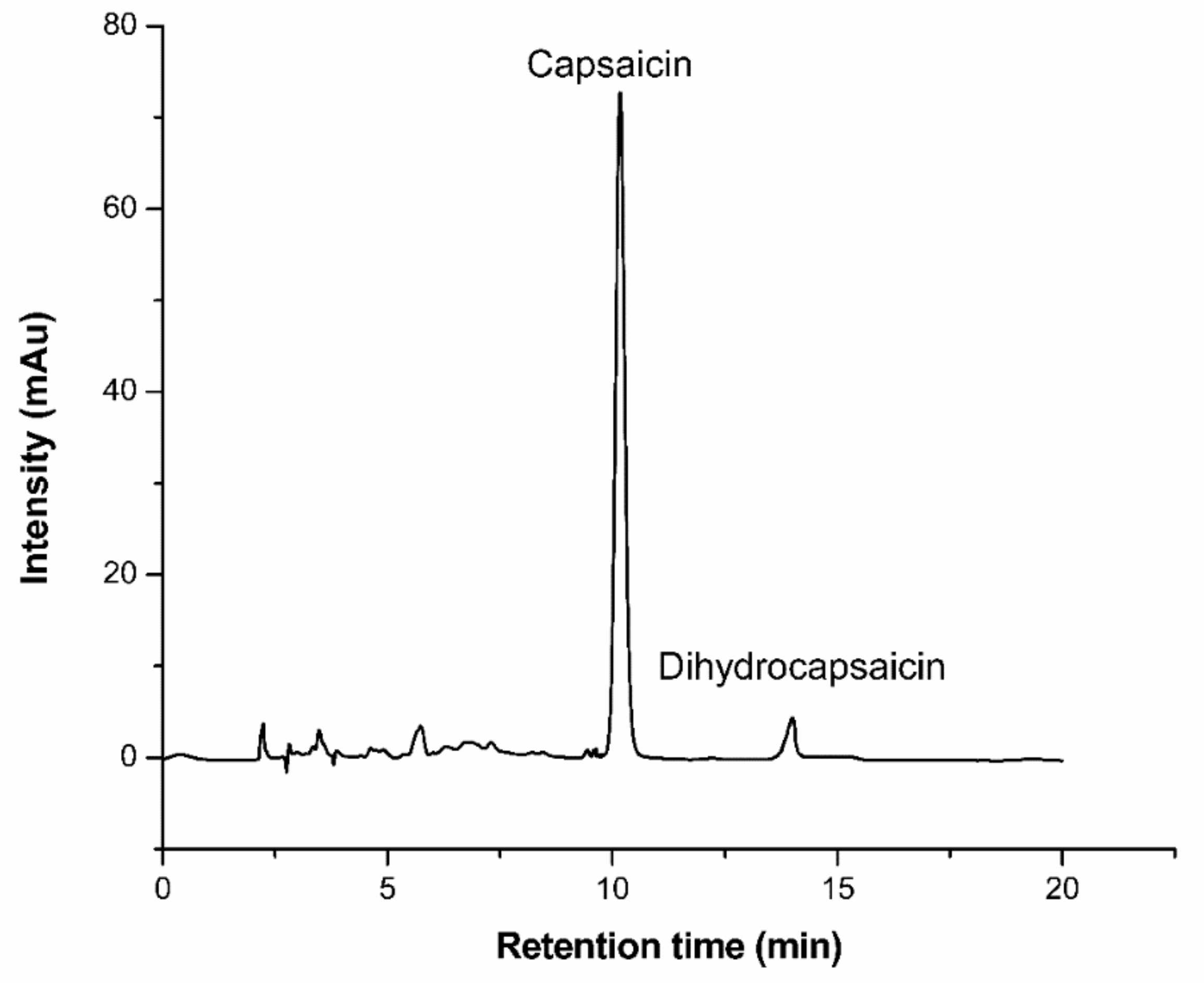
3.2.6. HPLC Analyses
The capsaicin concentration of samples were determined by HPLC on an Agilent 1260 infinity LC system at 35 °C. The EC-C18 column (Agilent Poroshell 120, 4.6 × 150 mm, 5 μm) (made in USA) was employed in the analysis test. The detection wavelength of the DAD detector was set at 280 nm. The mobile phase was a mixture (Vmethanol:Vwater = 70:30) with a flow rate of 0.5 mL/min.