Gynostemma Pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of GPE
2.2. Animals
2.3. Experimental Design and Treatment
2.4. Biochemical Analyses of Serum
2.5. Histological Analysis
2.6. Quantitative Reverse Transcription PCR (RT-qPCR)
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of GPE on Body Weight and Fat Mass in HFD-Induced Obese C57BL/6N Mice
3.2. Effects of GPE on Serum Glucose, Lipid, ALT, and AST Levels
3.3. Effects of GPE on Adipose Tissue Accumulation
3.4. Effects of GPE on the Expression of Key Factors in Adipogenesis and Fat Oxidation
3.5. Effects of GPE on SIRT1 Expression and AMPK Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Baretic, M. Obesity drug therapy. Minerva Endocrinolog. 2013, 38, 245–254. [Google Scholar]
- Halford, J.C. Obesity drugs in clinical development. Curr. Opin. Investig. Drugs 2006, 7, 312–318. [Google Scholar] [PubMed]
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef]
- Matschinsky, F.M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 2009, 8, 399–416. [Google Scholar] [CrossRef]
- Li, Y.; Lin, W.; Huang, J.; Xie, Y.; Ma, W. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chin. Med. 2016, 11, 43. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.W.; Lee, C.; Jin, Q.; Lee, M.K.; Lee, C.K.; Hwang, B.Y. Flavonol glycosides from the aerial parts of Gynostemma pentaphyllum and their antioxidant activity. Arch. Pharmacal. Res. 2016, 39, 1232–1236. [Google Scholar] [CrossRef]
- Nookabkaew, S.; Rangkadilok, N.; Satayavivad, J. Determination of trace elements in herbal tea products and their infusions consumed in Thailand. J. Agric. Food Chem. 2006, 54, 6939–6944. [Google Scholar] [CrossRef]
- Yan, W.; Niu, Y.; Lv, J.; Xie, Z.; Jin, L.; Yao, W.; Gao, X.; Yu, L.L. Characterization of a heteropolysaccharide isolated from diploid Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2013, 92, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J. Composition analysis and dominance test of three kinds of raw variety of Gynostemma pentaphyllum. Zhongguo Zhongyao Zazhi 2004, 29, 317–319. [Google Scholar] [PubMed]
- Srichana, D.; Taengtip, R.; Kondo, S. Antimicrobial activity of Gynostemma pentaphyllum extracts against fungi producing aflatoxin and fumonisin and bacteria causing diarrheal disease. Southeast Asian J. Trop Med. Public Health 2011, 42, 704–710. [Google Scholar] [PubMed]
- Muller, C.; Gardemann, A.; Keilhoff, G.; Peter, D.; Wiswedel, I.; Schild, L. Prevention of free fatty acid-induced lipid accumulation, oxidative stress, and cell death in primary hepatocyte cultures by a Gynostemma pentaphyllum extract. Phytomedicine 2012, 19, 395–401. [Google Scholar] [CrossRef]
- Schild, L.; Roth, A.; Keilhoff, G.; Gardemann, A.; Brodemann, R. Protection of hippocampal slices against hypoxia/hypoglycemia injury by a Gynostemma pentaphyllum extract. Phytomedicine 2009, 16, 734–743. [Google Scholar] [CrossRef]
- Schild, L.; Chen, B.H.; Makarov, P.; Kattengell, K.; Heinitz, K.; Keilhoff, G. Selective induction of apoptosis in glioma tumour cells by a Gynostemma pentaphyllum extract. Phytomedicine 2010, 17, 589–597. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, W.; Huang, H.; Slavin, M.; Zhao, Y.; Whent, M.; Blackford, J.; Lutterodt, H.; Zhou, H.; Chen, P.; et al. Chemical composition of five commercial Gynostemma pentaphyllum samples and their radical scavenging, antiproliferative, and anti-inflammatory properties. J. Agric. Food Chem. 2010, 58, 11243–11249. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lee, M.M.; Chan, B.D.; Ma, V.W.; Zhang, W.; Yip, T.T.; Wong, W.T.; Tai, W.C. Gynostemma pentaphyllum saponins attenuate inflammation in vitro and in vivo by inhibition of NF-κB and STAT3 signaling. Oncotarget 2017, 8, 87401–87414. [Google Scholar] [CrossRef]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Ky, P.T.; Hoa, N.K.; Ostenson, C.G. Antidiabetic effects of add-on Gynostemma pentaphyllum extract therapy with sulfonylureas in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2012, 2012, 452313. [Google Scholar] [CrossRef]
- Wang, J.; Ha, T.K.Q.; Shi, Y.P.; Oh, W.K.; Yang, J.L. Hypoglycemic triterpenes from Gynostemma pentaphyllum. Phytochemistry 2018, 155, 171–181. [Google Scholar] [CrossRef]
- Yeo, J.; Kang, Y.J.; Jeon, S.M.; Jung, U.J.; Lee, M.K.; Song, H.; Choi, M.S. Potential hypoglycemic effect of an ethanol extract of Gynostemma pentaphyllum in C57BL/KsJ-db/db mice. J. Med. Food 2008, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]
- La Cour, B.; Molgaard, P.; Yi, Z. Traditional Chinese medicine in treatment of hyperlipidaemia. J. Ethnopharmacol. 1995, 46, 125–129. [Google Scholar] [CrossRef]
- Keilhoff, G.; Esser, T.; Titze, M.; Ebmeyer, U.; Schild, L. Gynostemma pentaphyllum is neuroprotective in a rat model of cardiopulmonary resuscitation. Exp. Ther. Med. 2017, 14, 6034–6046. [Google Scholar] [PubMed]
- Gauhar, R.; Hwang, S.L.; Jeong, S.S.; Kim, J.E.; Song, H.; Park, D.C.; Song, K.S.; Kim, T.Y.; Oh, W.K.; Huh, T.L. Heat-processed Gynostemma pentaphyllum extract improves obesity in ob/ob mice by activating AMP-activated protein kinase. Biotechnol. Lett. 2012, 34, 1607–1616. [Google Scholar] [CrossRef]
- Park, S.H.; Huh, T.L.; Kim, S.Y.; Oh, M.R.; Pichiah, P.B.T.; Chae, S.W.; Cha, Y.S. Antiobesity effect of Gynostemma pentaphyllum extract (actiponin): A randomized, double-blind, placebo-controlled trial. Obesity 2014, 22, 63–71. [Google Scholar] [CrossRef]
- Hu, Y.; Ip, F.C.; Fu, G.; Pang, H.; Ye, W.; Ip, N.Y. Dammarane saponins from Gynostemma pentaphyllum. Phytochemistry 2010, 71, 1149–1157. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, Y.N. Dammarane-type saponins from Gynostemma pentaphyllum. Phytochemistry 2011, 72, 1453–1459. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.L.; Zhou, P.P.; Meng, X.H.; Shi, Y.P. Further new gypenosides from jiaogulan (Gynostemma pentaphyllum). J. Agric. Food Chem. 2017, 65, 5926–5934. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, P.C.; Lin, J.M. Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum. Am. J. Chin. Med. 2000, 28, 87–96. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Ren, Y.; Gao, Y.; Kang, L.; Qiao, Q. Anticancer and immunoregulatory activity of Gynostemma pentaphyllum polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2014, 69, 1–4. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, M.; Qi, X.; Liu, Y.; Li, N.; Liu, Z.; Bian, Y. Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ-inducing diabetic rats. Arch. Pharmacal. Res. 2016, 39, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gou, S.H.; Huang, H.F.; Chen, X.Y.; Liu, J.; He, M.; Ma, Y.Y.; Zhao, X.N.; Zhang, Y.; Ni, J.M. Lipid-lowering, hepatoprotective, and atheroprotective effects of the mixture Hong-Qu and gypenosides in hyperlipidemia with NAFLD rats. J. Chin. Med. Assoc. 2016, 79, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Y.; Guo, X. Isolation, structures, and bioactivities of the polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: A review. BioMed. Res. Int. 2018, 2018, 6285134. [Google Scholar] [CrossRef] [PubMed]
- Attawish, A.; Chivapat, S.; Phadungpat, S.; Bansiddhi, J.; Techadamrongsin, Y.; Mitrijit, O.; Chaorai, B.; Chavalittumrong, P. Chronic toxicity of Gynostemma pentaphyllum. Fitoterapia 2004, 75, 539–551. [Google Scholar] [CrossRef]
- Chiranthanut, N.; Teekachunhatean, S.; Panthong, A.; Khonsung, P.; Kanjanapothi, D.; Lertprasertsuk, N. Toxicity evaluation of standardized extract of Gynostemma pentaphyllum Makino. J. Ethnopharmacol. 2013, 149, 228–234. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.M.; Lee, J.K.; Jo, S.K.; Kim, H.J.; Cha, K.M.; Lim, C.Y.; Moon, J.M.; Kim, T.Y.; Kim, E.J. Efficacy of Gynostemma pentaphyllum extract in anti-obesity therapy. Rec. Nat. Prod. 2019, in press. [Google Scholar]
- Cho, H.J.; Kim, W.K.; Kim, E.J.; Jung, K.C.; Park, S.; Lee, H.S.; Tyner, A.L.; Park, J.H. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G996–G1005. [Google Scholar] [CrossRef] [Green Version]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Zhi, S.; Feng, Z.; Lei, C.; Zhou, Y. Wide linearity range and highly sensitive MEMS-based micro-fluxgate sensor with double-layer magnetic core made of Fe-Co-B amorphous alloy. Micromachines 2017, 8, 352. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Liu, X.; Lu, W.; Jia, S.; Hong, T.; Li, R.; Zhang, H.; Peng, L.; Zhan, X. Anti-diabetic activity evaluation of a polysaccharide extracted from Gynostemma pentaphyllum. Int. J. Biol. Macromol. 2019, 126, 209–214. [Google Scholar] [CrossRef]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. 2006, 9, 281–291. [Google Scholar] [PubMed]
- Sahagun, D.O.; Marquez-Aguirre, A.L.; Quintero-Fabian, S.; Lopez-Roa, R.I.; Rojas-Mayorquin, A.E. Modulation of PPAR-gamma by nutraceutics as complementary treatment for obesity-related disorders and inflammatory diseases. PPAR Res. 2012, 2012, 318613. [Google Scholar]
- Kim, J.B.; Sarraf, P.; Wright, M.; Yao, K.M.; Mueller, E.; Solanes, G.; Lowell, B.B.; Spiegelman, B.M. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Investig. 1998, 101, 1–9. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Ann. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Latasa, M.J.; Griffin, M.J.; Moon, Y.S.; Kang, C.; Sul, H.S. Occupancy and function of the −150 sterol regulatory element and −65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. Mol. Cell. Biol. 2003, 23, 5896–5907. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Tuncman, G.; Gorgun, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007, 447, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K.; Cao, H.; Kono, K.; Gorgun, C.Z.; Furuhashi, M.; Uysal, K.T.; Cao, Q.; Atsumi, G.; Malone, H.; Krishnan, B.; et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005, 1, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Makowski, L.; Boord, J.B.; Maeda, K.; Babaev, V.R.; Uysal, K.T.; Morgan, M.A.; Parker, R.A.; Suttles, J.; Fazio, S.; Hotamisligil, G.S.; et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001, 7, 699–705. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.J. Hormone-sensitive lipase: Control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 2002, 43, 1585–1594. [Google Scholar] [CrossRef]
- Fogarty, S.; Hardie, D.G. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim. Biophys. Acta 2010, 1804, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32 (Suppl. 4), S7–S12. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Cool, B.; Zinker, B.; Chiou, W.; Kifle, L.; Cao, N.; Perham, M.; Dickinson, R.; Adler, A.; Gagne, G.; Iyengar, R.; et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Krakoff, J.; Clark, J.M.; Crandall, J.P.; Wilson, C.; Molitch, M.E.; Brancati, F.L.; Edelstein, S.L.; Knowler, W.C. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity 2010, 18, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Lee, H.; Kang, R.; Bae, S.; Yoon, Y. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/β-catenin pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar]
- Nguyen, P.H.; Gauhar, R.; Hwang, S.L.; Dao, T.T.; Park, D.C.; Kim, J.E.; Song, H.; Huh, T.L.; Oh, W.K. New dammarane-type glucosides as potential activators of AMP-activated protein kinase (AMPK) from Gynostemma pentaphyllum. Bioorg. Med. Chem. 2011, 19, 6254–6260. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; de Oliveira, R.M.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
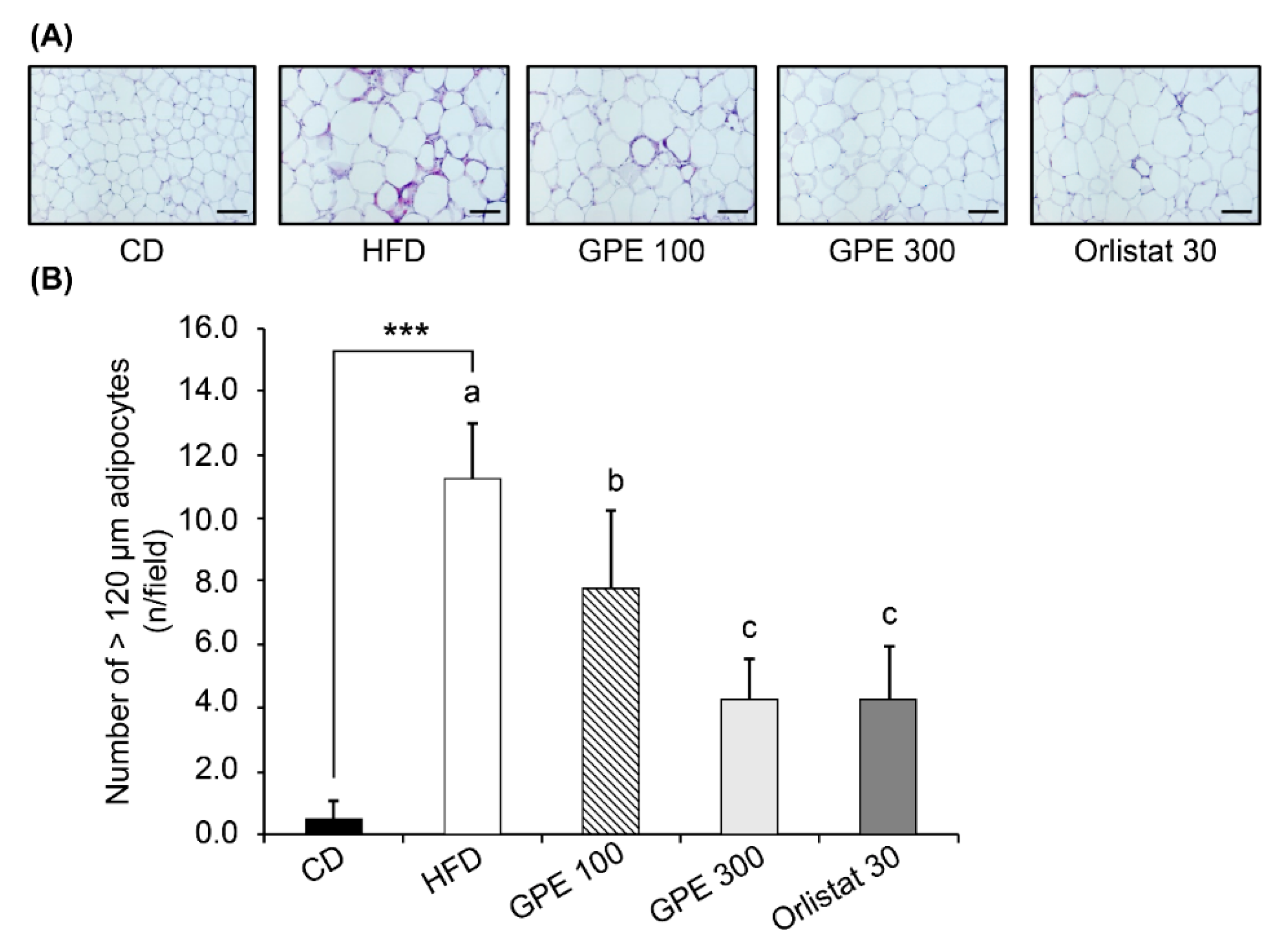

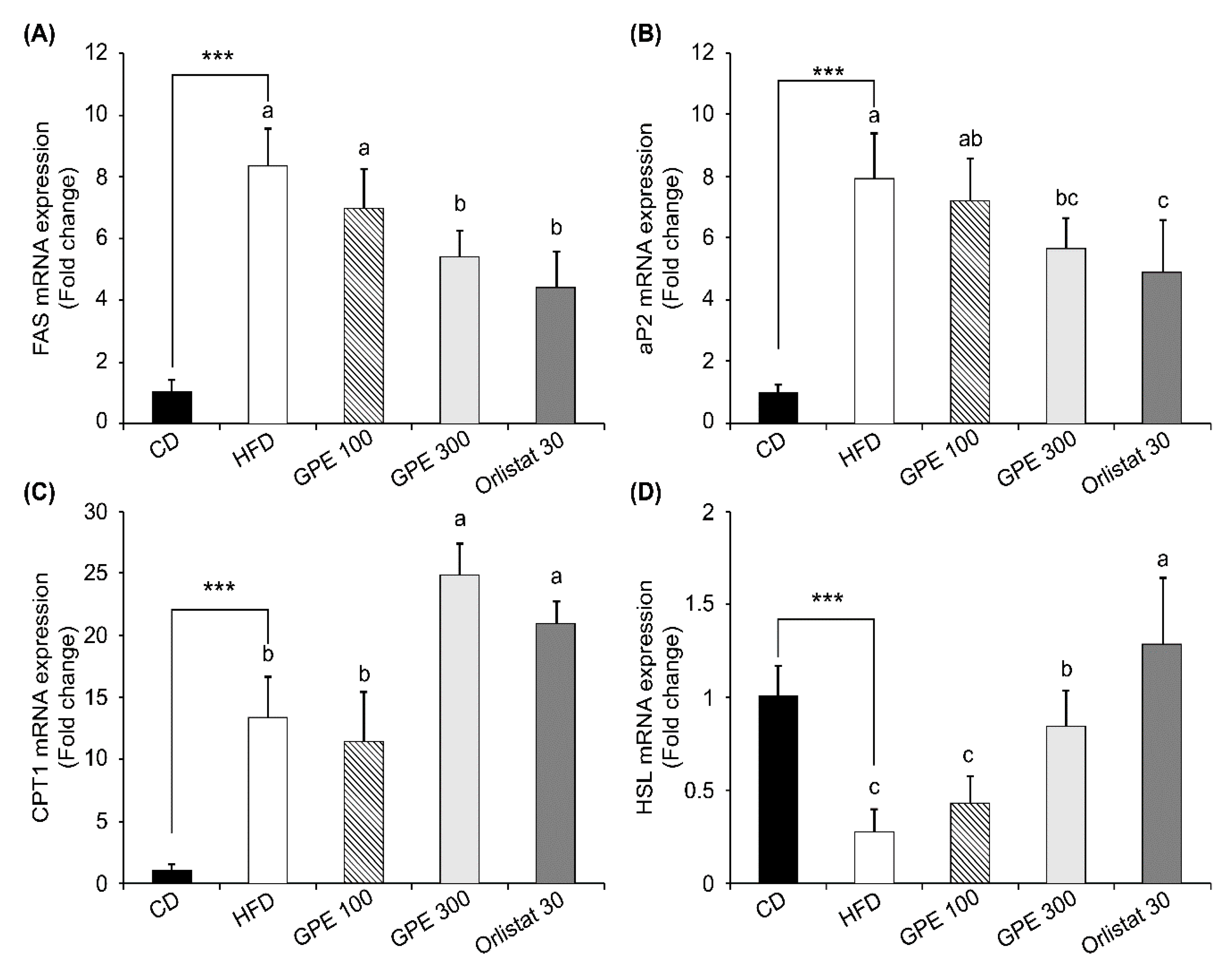
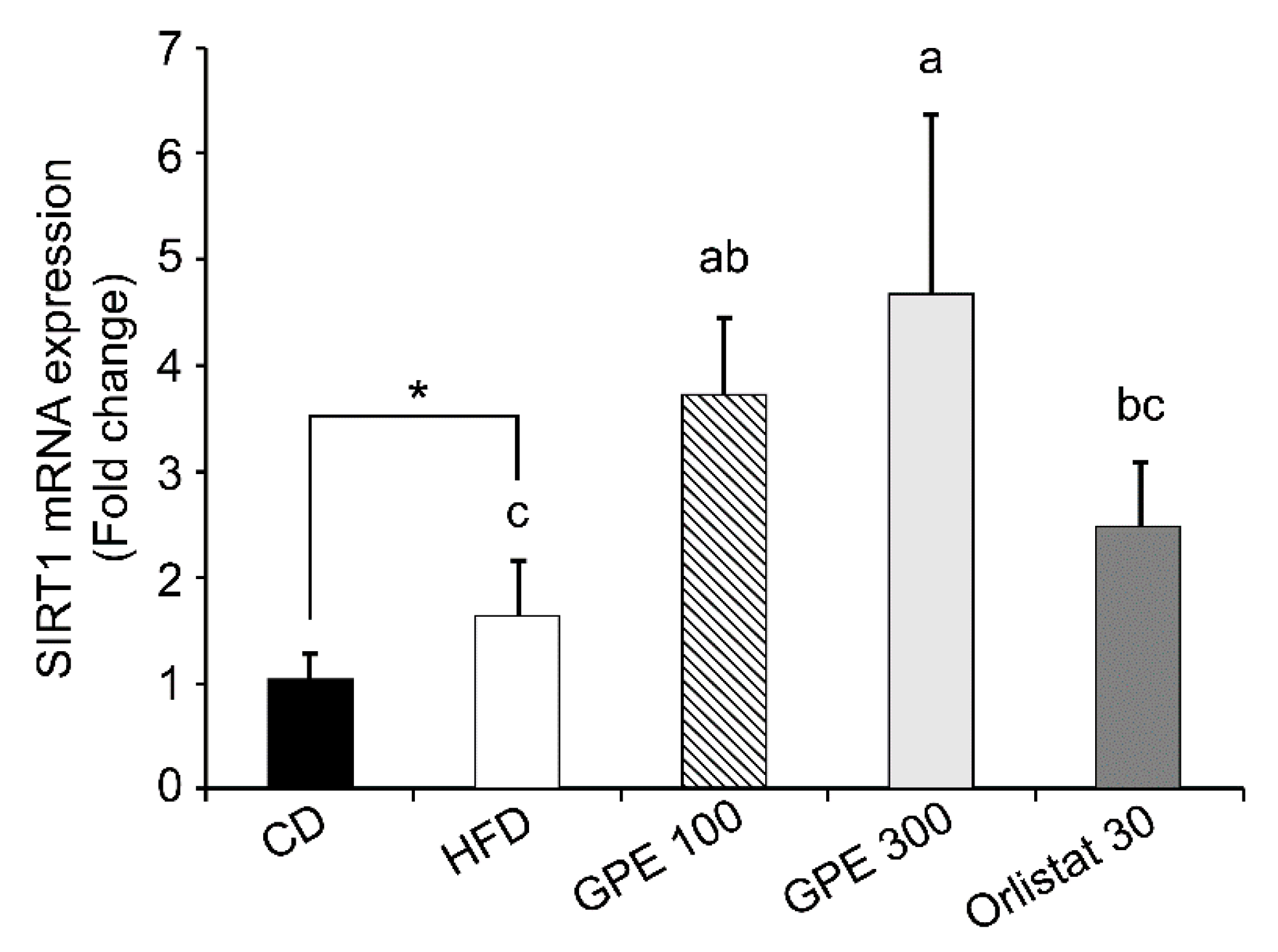
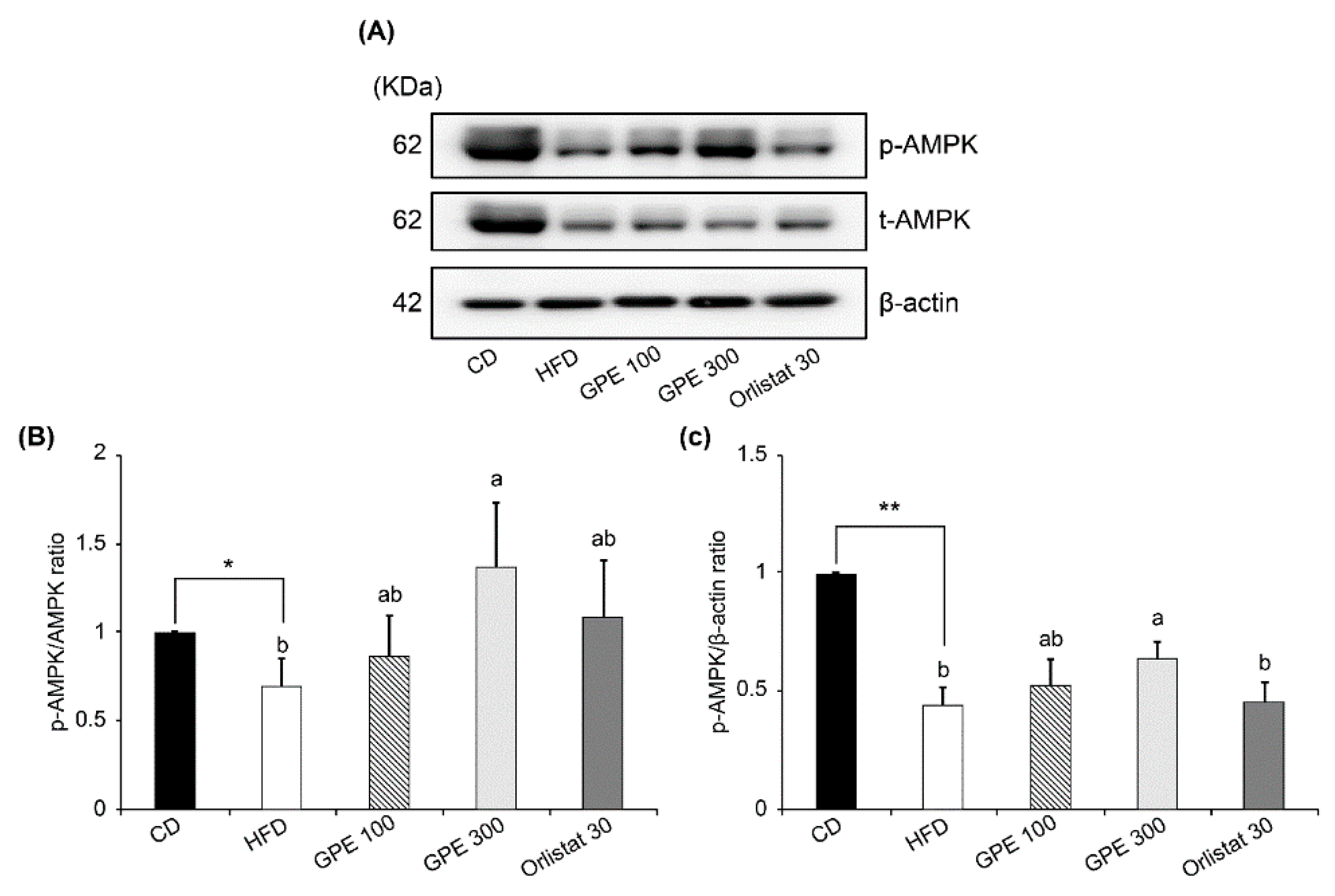
| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Ap2 | GGATTTGGTCACCATCCGGT | TTCACCTTCCTGTCGTCTGC |
| Cebpa | TGGACAAGAACAGCAACGAGTAC | TGGACAAGAACAGCAACGAGTAC |
| Cpt1 | GTGCTGGAGGTGGCTTTGGT | TGCTTGACGGATGTGGTTCC |
| Fas | AGGGGTCGACCTGGTCCTCA | GCCATGCCCAGAGGGTGGTT |
| Hsl | CCGTTCCTGCAGACTCTCTC | CCACGCAACTCTGGGTCTAT |
| Pparg | CAAAACACCAGTGTGAATTA | ACCATGGTAATTTCTTGTGA |
| Sirt1 | GCAACAGCATCTTGCCTGAT | GTGCTACTGGTCTCACTT |
| Srebp1c | CACTTCTGGAGACATCGCAAAC | ATGGTAGACAACAGCCGCATC |
| Gapdh | AGGTTGTCTCCTGCGACT | TGCTGTAGCCGTATTCATTGTCA |
| CD | HFD | GPE 100 | GPE 300 | Orlistat 30 | |
|---|---|---|---|---|---|
| Initial body weight (g) | 21.4 ± 0.8 | 21.5 ± 0.8 | 21.7 ± 0.9 | 21.5 ± 0.7 | 21.7 ± 0.7 |
| Final body weight (g) | 30.1 ± 1.7 | 45.6 ± 1.8 ***,a | 39.8 ± 1.3 b | 37.3 ± 2.3 c | 41.0 ± 1.8 b |
| Body weight gain (g) | 8.7 ± 1.1 | 24.1 ± 1.3 ***,a | 18.1 ± 1.4 b | 15.8 ± 2.2 c | 19.3 ± 1.9 b |
| Lean mass percentage (%) | 72.6 ± 5.3 | 56.6 ± 0.8 ***,b | 57.9 ± 2.0 a,b | 59.2 ± 1.4 a | 58.2 ± 1.4 a |
| Fat mass percentage (%) | 27.4 ± 5.4 | 43.3 ± 0.8 ***,a | 42.1 ± 2.0 a,b | 40.8 ± 1.3 b | 41.8 ± 1.4 b |
| Food intake (g/day) | 2.69 ± 0.04 | 2.33 ± 0.08 ***,a | 2.11 ± 0.05 c | 2.07 ± 0.07 c | 2.17 ± 0.02 b |
| Food efficiency ratio 1 | 0.058 ± 0.007 | 0.184 ± 0.010 ***,a | 0.153 ± 0.011 b | 0.137 ± 0.020 c | 0.159 ± 0.016 b |
| CD | HFD | GPE 100 | GPE 300 | Orlistat 30 | |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 101.2 ± 25.6 | 166.1 ± 40.7 ***,a | 137.0 ± 17.1 b | 106.3 ± 13.5 c | 135.3 ± 18.1 b |
| Triglyceride (mg/dL) | 35.7 ± 4.6 | 71.9 ± 9.6 ***,a | 49.2 ± 9.0 b | 50.4 ± 9.7 b | 57.9 ± 13.6 b |
| Total cholesterol (mg/dL) | 94.3 ± 16.5 | 126.5 ± 29.1 **,a | 125.7 ± 19.8 a | 100.8 ± 15.4 b | 139.3 ± 27.9 a |
| LDL-cholesterol (mg/dL) | 26.2 ± 7.0 | 37.9 ± 6.6 **,a | 28.6 ± 3.8 b | 23.2 ± 4.7 c | 28.7 ± 4.4 b |
| HDL-cholesterol (mg/dL) | 63.2 ± 11.0 | 88.6 ± 34.8 * | 79.8 ± 25.7 | 79.2 ± 9.6 | 89.2 ± 23.9 |
| ALT (U/L) | 80.8 ± 24.6 | 275.7 ± 103.2 ***,a | 127.3 ± 40.0 b | 105.1 ± 30.4 b | 91.3 ± 19.5 b |
| AST (U/L) | 150.0 ± 51.5 | 247.8 ± 77.3 **,a | 184.9 ± 49.1 b | 175.5 ± 43.9 b | 154.6 ± 32.7 b |
| CD | HFD | GPE 100 | GPE 300 | Orlistat 30 | |
|---|---|---|---|---|---|
| Epididymal fat (g) | 0.99 ± 0.32 | 2.47 ± 0.30 ***,a | 2.40 ± 0.24 a | 2.10 ± 0.24 b | 2.35 ± 0.22 a |
| Retroperitoneal fat (g) | 0.58 ± 0.16 | 1.59 ± 0.22 ***,a | 1.27 ± 0.12 b | 1.08 ± 0.10 c | 1.28 ± 0.09 b |
| Mesenteric fat (g) | 0.69 ± 0.18 | 1.87 ± 0.26 ***,a | 1.18 ± 0.07 b,c | 1.01 ± 0.21 c | 1.23 ± 0.15 b |
| Inguinal fat (g) | 0.33 ± 0.08 | 0.58 ± 0.08 ***,a | 0.36 ± 0.16 b | 0.33 ± 0.09 b | 0.36 ± 0.15 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.S.; Lim, S.-M.; Jung, J.I.; Kim, S.M.; Lee, J.K.; Kim, Y.H.; Cha, K.M.; Oh, T.K.; Moon, J.M.; Kim, T.Y.; et al. Gynostemma Pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1. Nutrients 2019, 11, 2475. https://doi.org/10.3390/nu11102475
Lee HS, Lim S-M, Jung JI, Kim SM, Lee JK, Kim YH, Cha KM, Oh TK, Moon JM, Kim TY, et al. Gynostemma Pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1. Nutrients. 2019; 11(10):2475. https://doi.org/10.3390/nu11102475
Chicago/Turabian StyleLee, Hyun Sook, Su-Min Lim, Jae In Jung, So Mi Kim, Jae Kyoung Lee, Yoon Hee Kim, Kyu Min Cha, Tae Kyu Oh, Joo Myung Moon, Tae Young Kim, and et al. 2019. "Gynostemma Pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1" Nutrients 11, no. 10: 2475. https://doi.org/10.3390/nu11102475





