Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content
Simple Summary
Abstract
1. Introduction
2. Influence of Pasture on Milk and Cheese Quality
2.1. Cows
2.1.1. Botanical Composition of Pasture and Milk Fatty Acid (FA) Profile
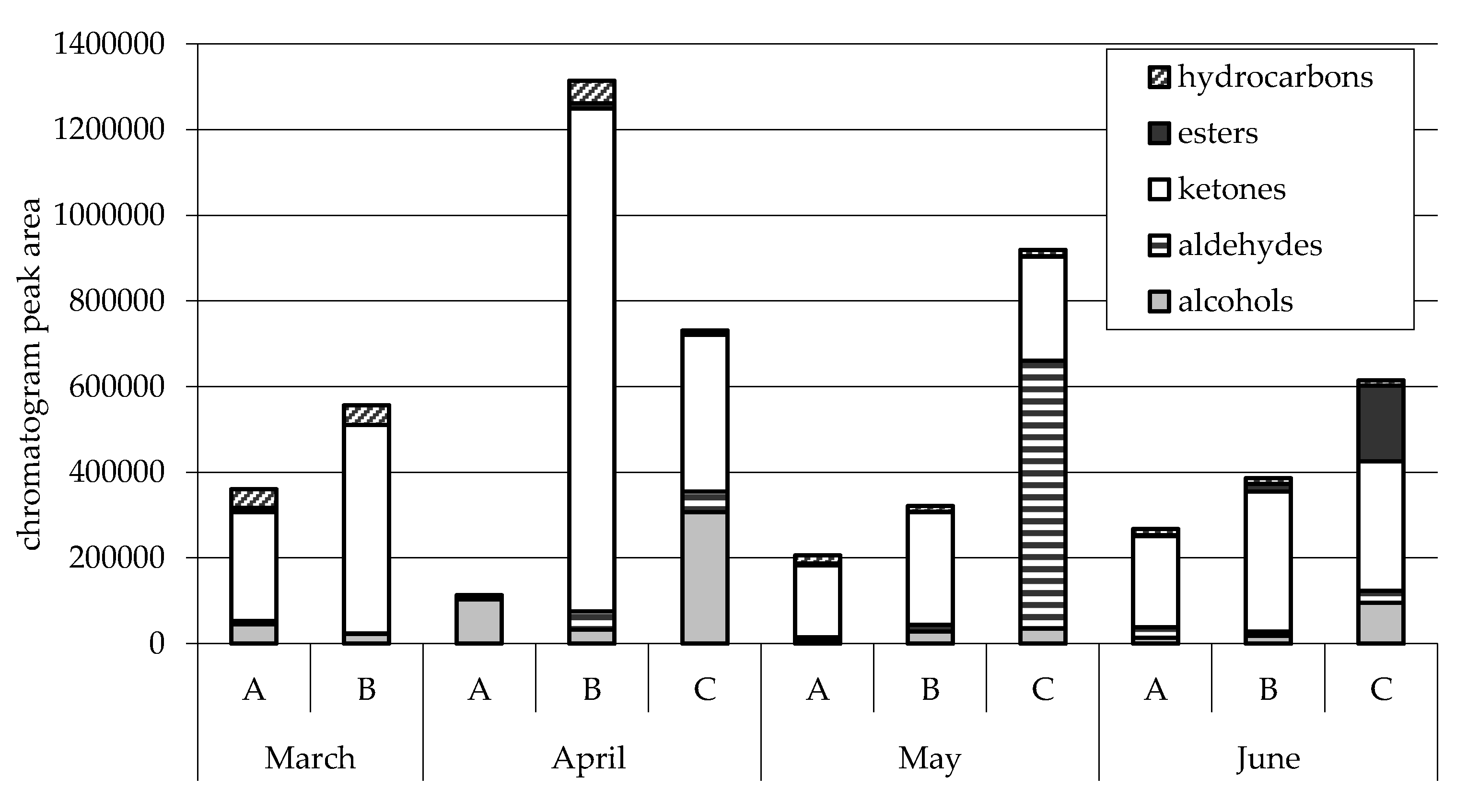
2.1.2. Minor Pasture Components and Cheese Flavor
2.1.3. Minor Pasture Components and Cheese Texture
2.1.4. Antioxidant Activity and Phenols Contents in Milk and Cheese
2.2. Sheep and Goat
2.2.1. Botanical Composition of Pasture and Milk Fatty Acid Profile
2.2.2. Minor Pasture Components and Cheese Flavor
2.3. Plant Biodiversity Secondary Metabolites and Their Role on Healthiness and Sensorial Features of Animal Products
2.4. Antioxidant Activity and Phenols Contents in Milk and Cheese
3. Traceability and Authenticity of Ruminant Products
4. Plant Biomarkers
4.1. Fatty Acids
4.2. Carotenoids
4.3. Terpens
4.4. Phenols
4.5. Redox Biomarkers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vannice, G.; Rasmussen, H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.; Muskiet, F.A. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Givens, D.I.; Shingfield, K.J. Optimizing dairy milk fatty acid composition. In Improving the Fat Content of Foods; Williams, C., Buttriss, J., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 252–280. [Google Scholar]
- Lee, M.R.F. Benefits of Forage Systems on Product Quality. Sustainable Food Trust on 13 February 2015 in Farming, Food Systems. Available online: https://sustainablefoodtrust.org/articles/role-for-grazing-livestock-red-meat/ (accessed on 10 September 2019).
- Glasser, F.; Ferlay, A.; Chilliard, Y. Oilseed lipid supplements and fatty acid composition of cow milk: A meta-analysis. J. Dairy Sci. 2008, 91, 4687–4703. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef] [PubMed]
- Moloney, A.P.; Monahan, F.J.; Schmidt, O. Quality and authenticity of grassland products. In EGF at 50: The Future of European Grasslands, Proceedings of the 25th General Meeting of the European Grassland Federation, Aberystwyth, Wales, UK, 7–11 September 2014; Hopkins, A., Collins, R.P., Fraser, M.D., King, V.R., Lloyd, D.C., Moorby, J.M., Robson, P.R.H., Eds.; Gomer Press Ltd.: Wales, UK, 2014; Volume 19, pp. 509–520. [Google Scholar]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. Eur. J. Lipid Sci. Technol. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M.; León-Ortiz, L.; Ramírez-Rodríguez, A.; Cabiddu, A.; Navarro-Ocaña, A.; Medina-Campo, O.N.; Pedraza-Chaverri, J. Goats’ feeding supplementation with Acacia farnesiana pods and their relationship with milk composition: Fatty acids, polyphenols, and antioxidant activity. Animals 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.C.; Mattioli, S.; Dal Bosco, A.; Piottoli, L.; Ranucci, D.; Branciari, R.; Cotozzolo, E.; Castellini, C. Rearing Romagnola geese in vineyard: Pasture and antioxidant intake, performance, carcass and meat quality. Ital. J. Anim. Sci. 2019, 18, 372–380. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, V.H.P. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Grappin, R.; Coulon, J.B. Terroir, lait et fromage: Elements de reflexion. Rencontres Autour Rech. Rumin. 1996, 3, 21–28. [Google Scholar]
- Bugaud, C.; Buchin, S.; Coulon, J.B.; Hauwuy, A.; Dupont, D. Influence of the nature of alpine pastures on plasmin activity, fatty acid and volatile compound composition of milk. Lait 2001, 81, 401–414. [Google Scholar] [CrossRef]
- Martin, B.; Ferlay, A.; Pradel, P.; Rock, E.; Grolier, P.; Dupont, D.; Gruffat, D.; Besle, J.M.; Ballot, N.; Chilliard, Y.; et al. Variabilité de la teneur des laits en constituants d’intérêt nutritionnel selon la nature des fourrages consommés par les vaches laitières. Rencontres Autour Rech. Rumin. 2002, 9, 347–350. [Google Scholar]
- Martin, B.; Fedele, V.; Ferlay, A.; Grolier, P.; Rock, E.; Gruffat, D.; Chilliard, Y. Effects of grass-based diets on the content of micronutrients and fatty acids in bovine and caprine dairy products. In Land use systems in grassland dominated regions, Proceedings of the 20th General Meeting of the European Grassland Federation, Luzern, Switzerland, 21–24 June 2004; Lüscher, A., Jeangros, B., Huguenin, O., Lobsiger, M., Millar, N., Suter, D., Eds.; VDF Hochschulverlag AG ETH Zentrum: Zürich, Switzerland, 2004; Volume 9, pp. 876–886. [Google Scholar]
- D’Urso, S.; Cutrignelli, M.I.; Calabrò, S.; Bovera, F.; Tudisco, R.; Piccolo, V.; Infascelli, F. Influence of pasture on fatty acid profile of goat milk. J. Anim. Phys. Anim. Nutr. 2008, 92, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Verdier-Metz, I.; Buchin, S.; Hurtaud, C.; Coulon, J.B. How do the nature of forages and pasture diversity influence the sensory quality of dairy livestock products? Anim. Sci. 2005, 81, 205–212. [Google Scholar] [CrossRef]
- Falchero, L.; Lombardi, G.; Gorlier, A.; Lonati, M.; Odoardi, M.; Cavallero, A. Variation in fatty acid composition of milk and cheese from cows grazed on two alpine pastures. Dairy Sci. Technol. 2010, 90, 657–672. [Google Scholar] [CrossRef]
- Tornambe, G.; Ferlay, A.; Farruggia, A.; Chilliard, Y.; Loiseau, P.; Pradel, P.; Graulet, B.; Chaveau-Duriot, B.; Martin, B. Influence of botanical diversity and development stage of mountain pastures on milk fatty acid composition, carotenoids, fat-soluble vitamins and sensory properties. In Grassland in a Changing World, Proceedings of the 23rd General Meeting of the European Grassland Federation, Kiel, Germany, 29 August–2 September 2010; Schnyder, H.H., Isselstein, J., Taube, E., Auerswald, K., Schellberg, J., Wachendorf, M., Herrmann, A., Gierus, M., Wrage, N., Hopkins, A., Eds.; Mecke Druck und Verlag: Duderstadt, Germany, 2010; Volume 15, pp. 589–591. [Google Scholar]
- Coppa, M.; Lonati, M.; Gorlier, A.; Falchero, L.; Cugno, D.; Lombardi, G.; Cavallero, A. Variation of fatty acid profile during the grazing season in cows’ milk from mountain permanent meadows. In Grassland in a Changing World, Proceedings of the 23rd General Meeting of the European Grassland Federation, Kiel, Germany, 29 August–2 September 2010; Schnyder, H., Isselstein, J., Taube, F., Auerswald, K., Schellberg, J., Wachendorf, M., Herrmann, A., Gierus, M., Wrage, N., Eds.; Mecke Druck und Verlag: Duderstadt, Germany, 2010; Volume 15, pp. 595–597. [Google Scholar]
- Gorlier, A.; Lonati, M.; Renna, M.; Lussiana, C.; Lombardi, G.; Battaglini, L.M. Changes in pasture and cow milk compositions during a summer transhumance in the western Italian Alps. J. Appl. Bot. Food Qual. 2012, 85, 216–223. [Google Scholar]
- Wyss, U.; Collomb, M. Fatty acid composition of different grassland species. In Grassland in a Changing World, Proceedings of the 23rd General Meeting of the European Grassland Federation, Kiel, Germany, 29 August–2 September 2010; Schnyder, H., Isselstein, J., Taube, F., Auerswald, K., Schellberg, J., Wachendorf, M., Herrmann, A., Gierus, M., Wrage, N., Eds.; Mecke Druck und Verlag: Duderstadt, Germany, 2010; Volume 15, pp. 631–633. [Google Scholar]
- Kälber, T.; Meier, J.S.; Kreuzer, M.; Leiber, F. Flowering catch crops used as forage plants for dairy cows: Influence on fatty acids and tocopherols in milk. J. Dairy Sci. 2011, 94, 1477–1489. [Google Scholar] [CrossRef]
- Carpino, S.; Mallia, S.; La Terra, S.; Melilli, C.; Licitra, G.; Acree, T.E.; Barbano, D.M.; Van Soest, P.J. Composition and aroma compounds of Ragusano cheese: Native pasture and total mixed rations. J. Dairy Sci. 2004, 87, 816–830. [Google Scholar] [CrossRef]
- Bonanno, A.; Tornambè, G.; Bellina, V.; De Pasquale, C.; Mazza, F.; Maniaci, G.; Di Grigoli, A. Effect of farming system and cheesemaking technology on the physicochemical characteristics, fatty acid profile, and sensory properties of Caciocavallo Palermitano cheese. J. Dairy Sci. 2013, 96, 710–724. [Google Scholar] [CrossRef]
- Buchin, S.; Martin, B.; Dupont, D.; Bornard, A.; Achilleos, C. Influence of the composition of Alpine highland pasture on the chemical, rheological and sensory properties of cheeses. J. Dairy Res. 1999, 66, 579–588. [Google Scholar] [CrossRef]
- Tornambé, G.; Martin, B.; Pradel, P.; Di Grigoli, A.; Bonanno, A.; Chilliard, Y.; Ferlay, A. The maturity stage of the grass affects milk fatty acids of cows grazing a mountain grassland. In Grassland in a Changing World, Proceedings of the 23rd General Meeting of the European Grassland Federation, Kiel, Germany, 29 August–2 September 2010; Schnyder, H., Isselstein, J., Taube, F., Auerswald, K., Schellberg, J., Wachendorf, M., Herrmann, A., Gierus, M., Wrage, N., Eds.; Mecke Druck und Verlag: Duderstadt, Germany, 2010; Volume 15, pp. 628–630. [Google Scholar]
- Lombardi, G.; Falchero, L.; Coppa, M.; Tava, A. Volatile compounds of Alpine vegetation as markers for the traceability of ‘grass-deriving’ dairy products. In Biodiversity and Animal Feed Future Challenges for Grassland Production, Proceedings of the 22nd General Meeting of the European Grassland Federation Uppsala, Sweden, 9–12 June 2008; Hopkins, A., Gustafsson, T., Bertilsson, J., Dalin, G., Nilsdotter-Linde, N., Spörndly, E., Eds.; SLU Repro: Uppsala, Sweden, 2008; Volume 13, pp. 400–402. [Google Scholar]
- Kilcawley, K.N. Impact of pasture feeding on the sensory aspects of milk and products. In Proceedings of the Grass-Fed Dairy Conference Naas, Kildare, Ireland, 25 October 2018. [Google Scholar]
- Veskoukis, A.; Kerasioti, E.; Priftis, A.; Kouka, P.; Spanidis, Y.; Makri, S.; Kouretas, D. A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: The biomarker issue. Curr. Opin. Toxicol. 2019, 13, 99–109. [Google Scholar] [CrossRef]
- Abdelguerfi, A.; Abdelguerfi-Laouar, M. Genetic resources of fodder and/or pastoral interest: Diversity, collection and valorization in Mediterranean region. Cah. Options Mediterr. 2004, 62, 29–41. [Google Scholar]
- Gałka, A.; Zarzycki, J.; Kopeć, M. Effect of different fertilization regimes on species composition and habitat in a long-term grassland experiment. In Integrating Efficient Grassland Farming and Biodiversity, Proceedings of the 13th International Occasional Symposium of the European Grassland Federation, Tartu, Estonia, 29–31 August 2005; Lillak, R., Viiralt, R., Linke, A., Geherman, V., Eds.; Greif printhouse: Tartu, Estonia, 2005; Volume 10, pp. 132–135. [Google Scholar]
- Cabiddu, A.; Decandia, M.; Addis, M.; Piredda, G.; Pirisi, A.; Molle, G. Managing Mediterranean pastures in order to enhance the level of beneficial fatty acids in sheep milk. Small Rumin. Res. 2005, 59, 169–180. [Google Scholar] [CrossRef]
- Addis, M.; Cabiddu, A.; Pinna, G.; Decandia, M.; Piredda, G.; Pirisi, A.; Molle, G. Milk and cheese fatty acid composition of sheep fed different Mediterranean forages with particular reference to CLA cis-9, trans-11. J. Dairy Sci. 2005, 88, 3443–3454. [Google Scholar] [CrossRef]
- Cabiddu, A.; Molle, G.; Decandia, M.; Spada, S.; Fiori, M.; Piredda, G.; Addis, M. Responses to condensed tannins of flowering sulla (Hedysarum coronarium L.) grazed by dairy sheep. Part 2: Effects on milk fatty acid profile. Livest. Sci. 2009, 123, 230–240. [Google Scholar] [CrossRef]
- Cabiddu, A.; Carta, G.; Molle, G.; Decandia, M.; Addis, M.; Piredda, G.; Delogu, A.; Pirisi, A.; Lai, V.; Cera, V.; et al. Relationship between feeding regimen and content of conjugated linoleic acid in sheep milk and cheese. In Proceedings of the First Joint Seminar of the FAOCIHEAM Sub-Networks on Sheep and Goat Nutrition and on Mountain and Mediterranean Pastures: “Sustainable Grazing, Nutritional Utilization and Quality of Sheep and Goat Products”, Granada, Spain, 2–4 October 2003. [Google Scholar]
- Addis, M.; Cabiddu, A.; Decandia, M.; Fiori, M.; Spada, S.; Bulleddu, C.; Cammelli, R.; Caria, A.; Lai, V.; Lutzoni, G.; et al. A survey on the milk fatty acid composition of forty dairy sheep flocks in Sardinia. Ital. J. Anim. Sci. 2007, 6, 532–534. [Google Scholar] [CrossRef]
- Biondi, L.; Valvo, M.A.; Di Gloria, M.; Tenghi, E.S.; Galofaro, V.; Priolo, A. Changes in ewe milk fatty acids following turning out to pasture. Small Rumin. Res. 2008, 75, 17–23. [Google Scholar] [CrossRef]
- Collomb, M.; Butikofer, U.; Maurer, J.; Sieber, R. Composition en acides gras du lait de brebis produit à diverses altitudes. Rev. Suisse d’Agriculture 2006, 38, 335–339. [Google Scholar]
- Cabiddu, A.; Addis, M.; Spada, S.; Sitzia, M.; Molle, G.; Piredda, G. The effect of different legumes-based pastures on the fatty acid composition of sheep milk with focus on CLA. In Land use systems in grassland dominated regions, Proceedings of the 20th General Meeting of the European Grassland Federation, Luzern, Switzerland, 21–24 June 2004; Lüscher, A., Jeangros, B., Huguenin, O., Lobsiger, M., Millar, N., Suter, D., Eds.; VDF Hochschulverlag AG ETH Zentrum: Zürich, Switzerland, 2004; Volume 9, pp. 1133–1135. [Google Scholar]
- Cabiddu, A.; Addis, M.; Pinna, G.; Spada, S.; Fiori, M.; Sitzia, M.; Pirisi, A.; Piredda, G.; Molle, G. The inclusion of a daisy plant (Chrysanthemum coronarium) in dairy sheep diet. 1: Effect on milk and cheese fatty acid composition with particular reference to C18:2 cis-9, trans-11. Livest. Sci. 2006, 101, 57–67. [Google Scholar] [CrossRef]
- Cabiddu, A.; Addis, M.; Decandia, M.; Piredda, G.; Spada, S.; Fiori, M.; Sitzia, M.; Fois, N.; Molle, G.; Landau, S.; et al. Effect of Chicory, Burr medic and Safflower based-pastures on milk fatty acid composition, especially conjugated linoleic acid cis 9, trans11. J. Anim. Sci. 2006, 84 (Suppl. 1), 376. [Google Scholar]
- Cabiddu, A.; Addis, M.; Fiori, M.; Spada, S.; Decandia, M.; Molle, G. Pros and cons of the supplementation with oilseed enriched concentrates on milk fatty acid profile of dairy sheep grazing Mediterranean pastures. Small Rumin. Res. 2017, 147, 63–72. [Google Scholar] [CrossRef]
- Pizzoferrato, L.; Manzi, P.; Marconi, S.; Fedele, V.; Claps, S.; Rubino, R. Degree of antioxidant protection: A parameter to trace the origin and quality of goat’s milk and cheese. J. Dairy Sci. 2007, 90, 4569–4574. [Google Scholar] [CrossRef] [PubMed]
- Cabiddu, A.; Decandia, M.; Scanu, G.; Molle, G.; Pirisi, A.; Piredda, G.; Addis, M.; Bertuzzi, T. Level of vitamins e and a and cholesterol in milk and cheese from goats fed with different feeding systems. In Proceedings of the 5th International Symposium on the Challenge to Sheep and Goats Milk Sectors, Sardinia, Italy, 18–20 April 2005. [Google Scholar]
- Cabiddu, A.; Molle, G.; Decandia, M. Influenza del sistema di allevamento caprino sulla composizione chimica del latte con particolare riferimento alla composizione acidica, vitaminica e fenolica. In Proceedings of the 5° Congresso Lattiero-Caseario AITeL Latte e Derivati: Ricerca, Innovazione e Valorizzazione Aula Magna di Agraria dell’Università di Bari, Bari, Italy, 9 September 2016. [Google Scholar]
- Addis, M.; Pinna, G.; Molle, G.; Fiori, M.; Spada, S.; Decandia, M.; Scintu, M.F.; Piredda, G.; Pirisi, A. The inclusion of a daisy plant (Chrysanthemum coronarium) in dairy sheep diet: 2. Effect on the volatile fraction of milk and cheese. Livest. Sci. 2006, 101, 68–80. [Google Scholar] [CrossRef]
- Fedele, F.; Rubino, R.; Claps, S.; Sepe, L.; Morone, G. Seasonal evolution of volatile compounds content and aromatic profile in milk and cheese from grazing goat. Small Rumin. Res. 2005, 59, 273–279. [Google Scholar] [CrossRef]
- Decandia, M.; Cabiddu, A.; Molle, G.; Branca, A.; Epifani, G.; Pintus, S.; Tavera, F.; Piredda, G.; Pinna, G.; Addis, M. Effect of different feeding systems on fatty acid composition and volatile compound content in goat milk. Options Méditerranéennes 2007, 74, 129–134. [Google Scholar]
- Scintu, M.F.; Fois, N.; Pes, M.; Del Caro, A.; Di Salvo, R. Bitternes evaluation in cheeses obtained from milk of chicory feed ewes. In Proceedings of the 8th Pangborn Sensory Science Symposium, Florence, Italy, 26–30 July 2009. [Google Scholar]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Lee, M.F.R. Forage polyphenol oxidase and ruminant livestock nutrition. Front. Plant Sci. 2014, 5, 694. [Google Scholar] [CrossRef]
- Cabiddu, A.; Lee, M.R.F.; Decandia, M.; Molle, G.; Salis, L.; Vargiu, M.; Winters, A.L. Characterization of polyphenol oxidase activity in a range of forage ecotypes with different phenol substrates. A new insight for PPO and protein bound phenol evaluation. Grass Forage Sci. 2014, 69, 678–692. [Google Scholar] [CrossRef]
- Monahan, F.J.; Moloney, A.P.; Downey, G.; Dunne, P.G.; Schmidt, O.; Harrison, S.M. Authenticity and traceability of grassland production and products. In Grassland in a Changing World, Proceedings of the 23rd General Meeting of the European Grassland Federation, Kiel, Germany, 29 August–2 September 2010; Schnyder, H., Isselstein, J., Taube, F., Auerswald, K., Schellberg, J., Wachendorf, M., Herrmann, A., Gierus, M., Wrage, N., Eds.; Mecke Druck und Verlag: Duderstadt, Germany, 2010; Volume 15, pp. 401–415. [Google Scholar]
- Prache, S.; Cornu, A.; Berdague, J.L.; Priolo, A. Traceability of animal feeding diet in the meat and milk of small ruminants. Small Rumin. Res. 2005, 59, 157–168. [Google Scholar] [CrossRef]
- Coppa, M.; Cabiddu, A.; Elsasser, M.; Hulin, S.; Lind, V.; Martin, B.; Mosquera-Losada, M.R.; Peeters, A.; Prache, S.; Van den Pol-van, D.A.; et al. Grassland-based products: Quality and authentication. In Grassland Resources for Extensive Farming Systems in Marginal Lands: Major Drivers and Future Scenarios; Porqueddu, C., Franca, A., Lombardi, G., Molle, G., Peratoner, G., Hopkins, A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; Volume 7, pp. 39–60. [Google Scholar]
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Building of prediction models by using Mid-Infrared spectroscopy and fatty acid profile to discriminate the geographical origin of sheep milk. LWT-Food Sci. Techbol. 2017, 75, 131–136. [Google Scholar] [CrossRef]
- Coppa, M.; Chassaing, C.; Ferlay, A.; Agabriel, C.; Laurent, C.; Borreani, G.; Barcarolo, R.; Baars, T.D.; Kusche, O.M.; Harstad, J.; et al. Potential of milk fatty acid composition to predict diet composition and authenticate feeding systems and altitude origin of European bulk milk. J. Dairy Sci. 2015, 98, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Acciaro, M.; Dimauro, C.; Decandia, M.; Sitzia, M.; Manca, C.; Giovanetti, V.; Cabiddu, A.; Addis, M.; Rassu, S.P.G.; Molle, G. Discriminant analysis to identify ruminant meat produced from pasture or stall-fed animals. In Grassland Resources for Extensive Farming Systems in Marginal Lands: Major Drivers and Future Scenarios; Porqueddu, C., Franca, A., Lombardi, G., Molle, G., Peratoner, G., Hopkins, A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; Volume 22, pp. 88–90. [Google Scholar]
- Noziere, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Anim. Feed Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Cabiddu, A.; Bertuzzi, T.; Decandia, M.; Scanu, G.; Sitzia, M.; Fois, N.; Addis, M.; Piredda, G.; Pirisi, A.; Molle, G. Effect of different feeding managements on vitamins, cholesterol and degree of antioxidant protection of milk and cheese from grazing sheep. In Proceedings of the 43° International Symposium “Nutripharm & Biosecurity” Porto Conte Ricerche, Alghero, Italy, 30 May 2008. [Google Scholar]
- Dumont, J.P.; Adda, J. Occurrence of sesquiterpenes in mountain cheese volatiles. J. Agric. Food Chem. 1978, 26, 36–367. [Google Scholar] [CrossRef]
- Moio, L.; Rillo, L.; Ledda, A.; Addeo, F. Odorous constituents of ovine milk in relationship to diet. J. Dairy Sci. 1996, 79, 1322–1331. [Google Scholar] [CrossRef]
- Viallon, C.; Verdier-Metz, I.; Denoyer, C.; Pradel, P.; Coulon, J.B.; Berdague, J.L. Desorbed terpenes and sesquiterpenes from forages and cheeses. J. Dairy Res. 1999, 66, 319–326. [Google Scholar] [CrossRef]
- Schlichtherle-Cerny, H.; Imhof, M.; Fernandez-Garcıa, E.; Bosset, J.O. Changes in Terpene Composition from Pasture to Cheese. Mitt. Lebensm. 2004, 95, 681–688. [Google Scholar] [CrossRef]
- Mariaca, R.G.; Berger, T.F.H.; Gauch, R.; Imhof, M.I.; Jeangros, B.; Bosset, J.O. Occurrence of volatile mono- and sesquiterpenoids in highland and lowland plant species as possible precursors for flavor compounds in milk and dairy products. J. Agric. Food Chem. 1997, 45, 4423–4434. [Google Scholar] [CrossRef]
- Bugaud, C.; Bornard, A.; Hauwuy, A.; Martin, B.; Salmon, J.C.; Tessier, L.; Buchin, S. Relation entre la composition botanique de végétations de montagne et leur composition en composés volatils. Fourrages 2000, 162, 141–155. [Google Scholar]
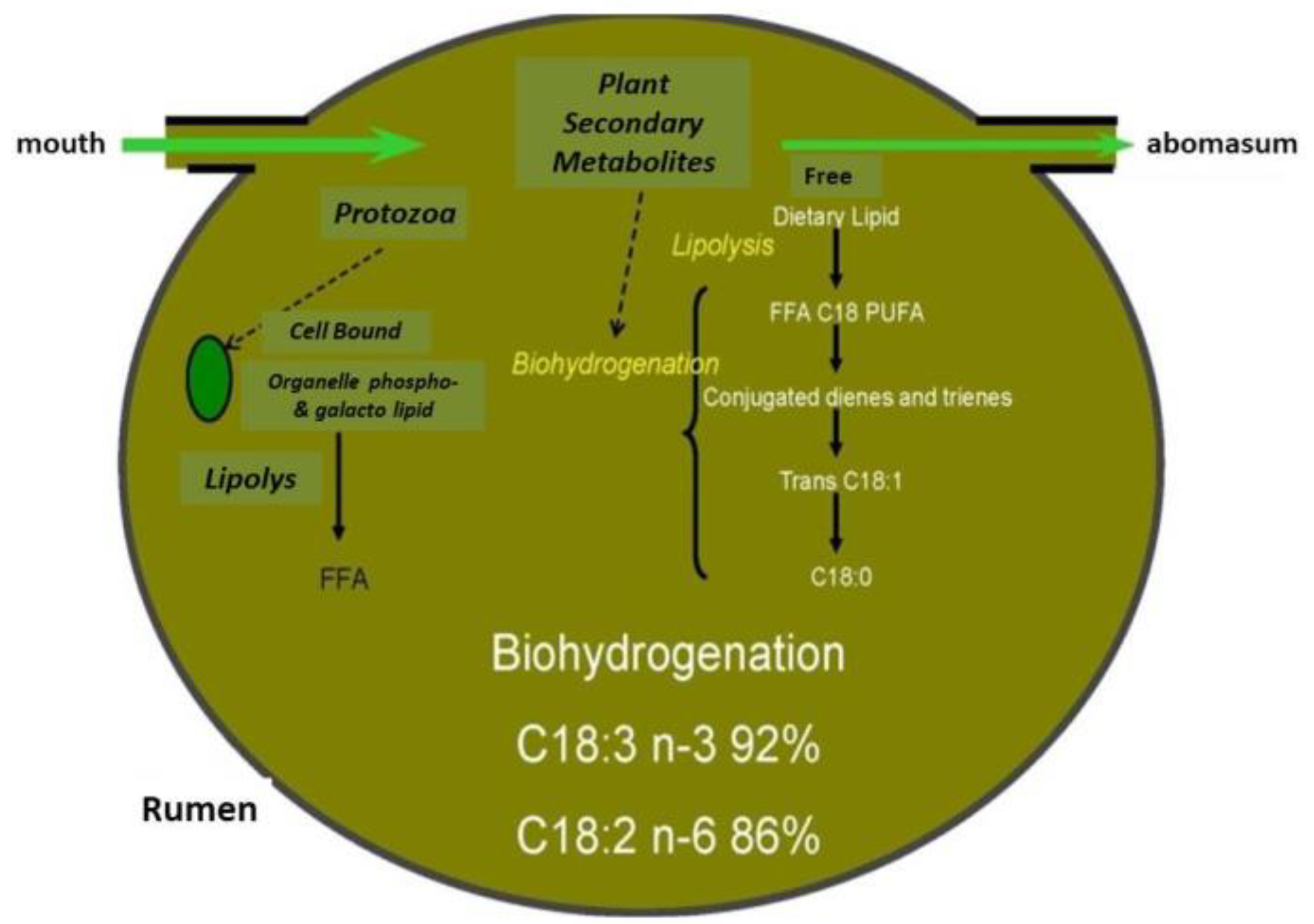
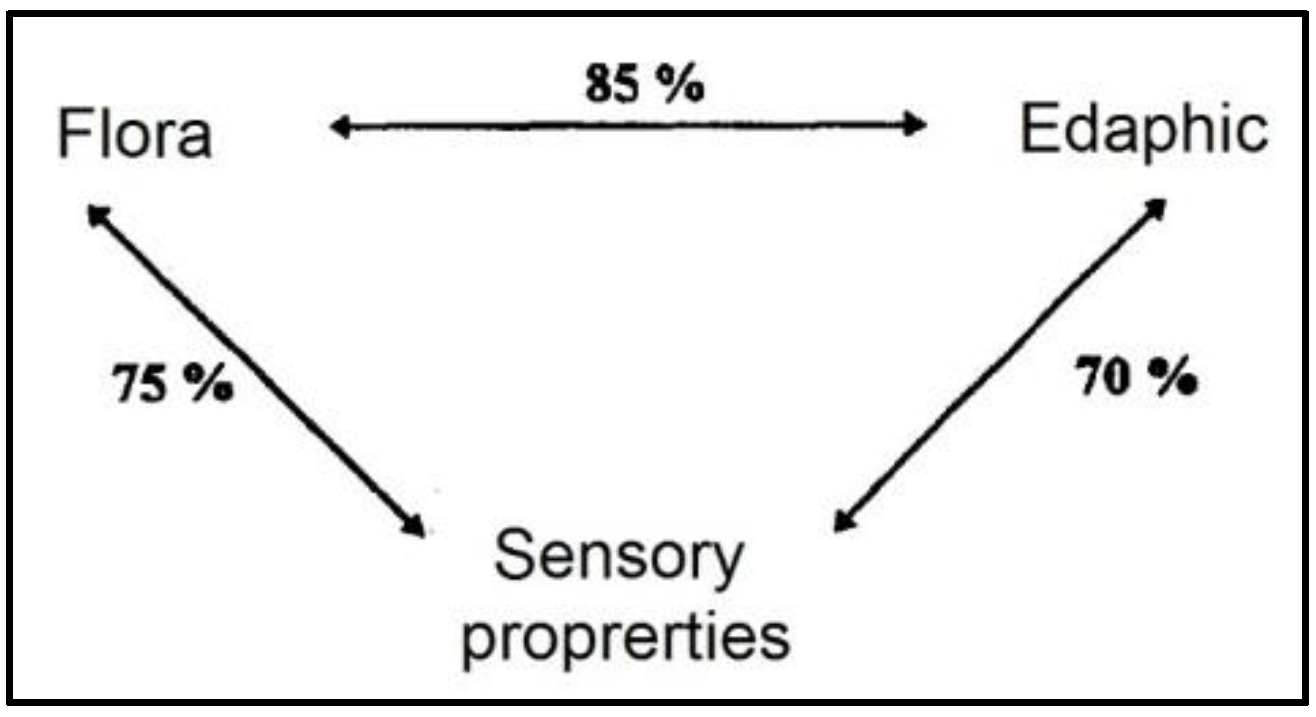
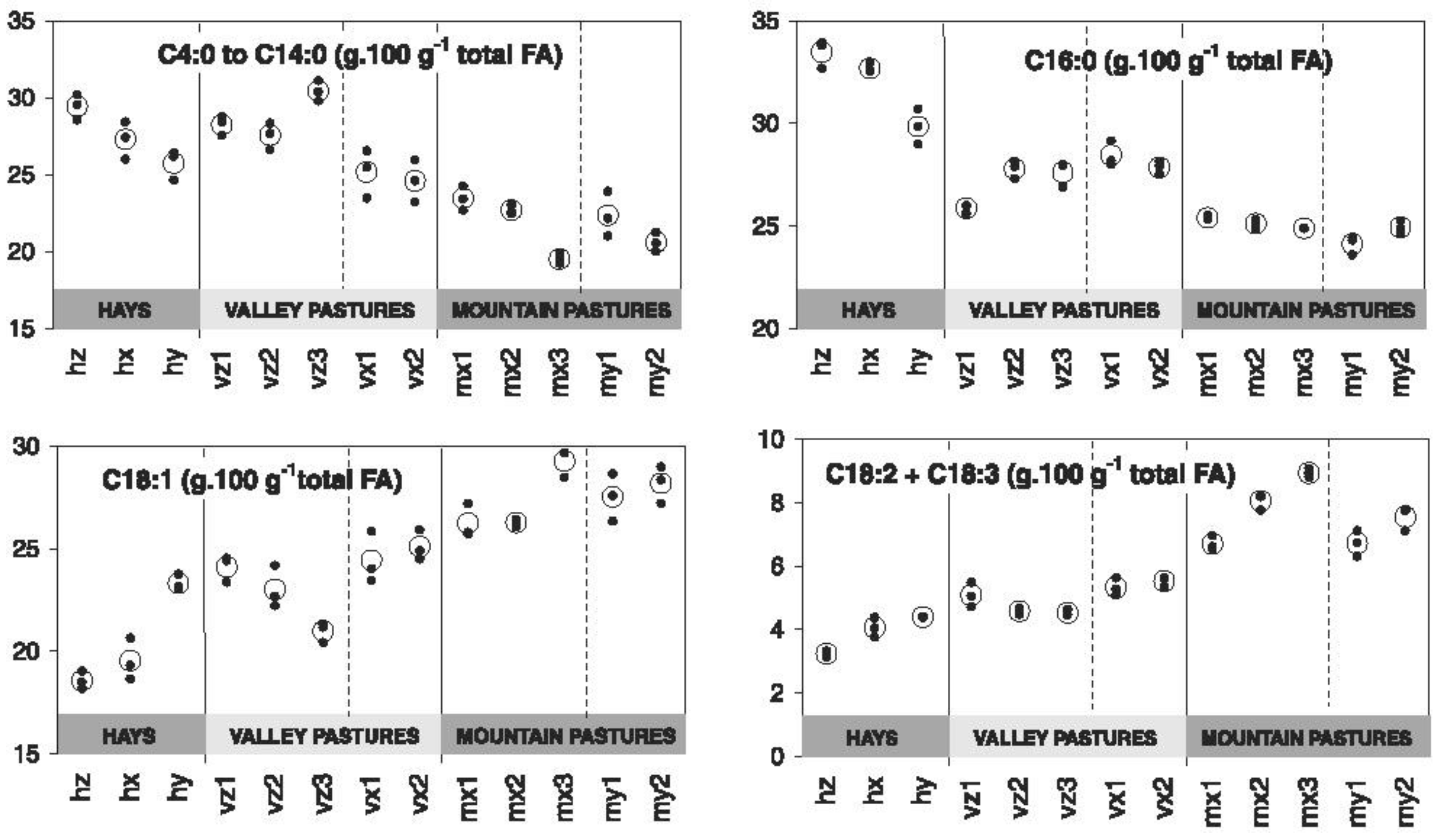
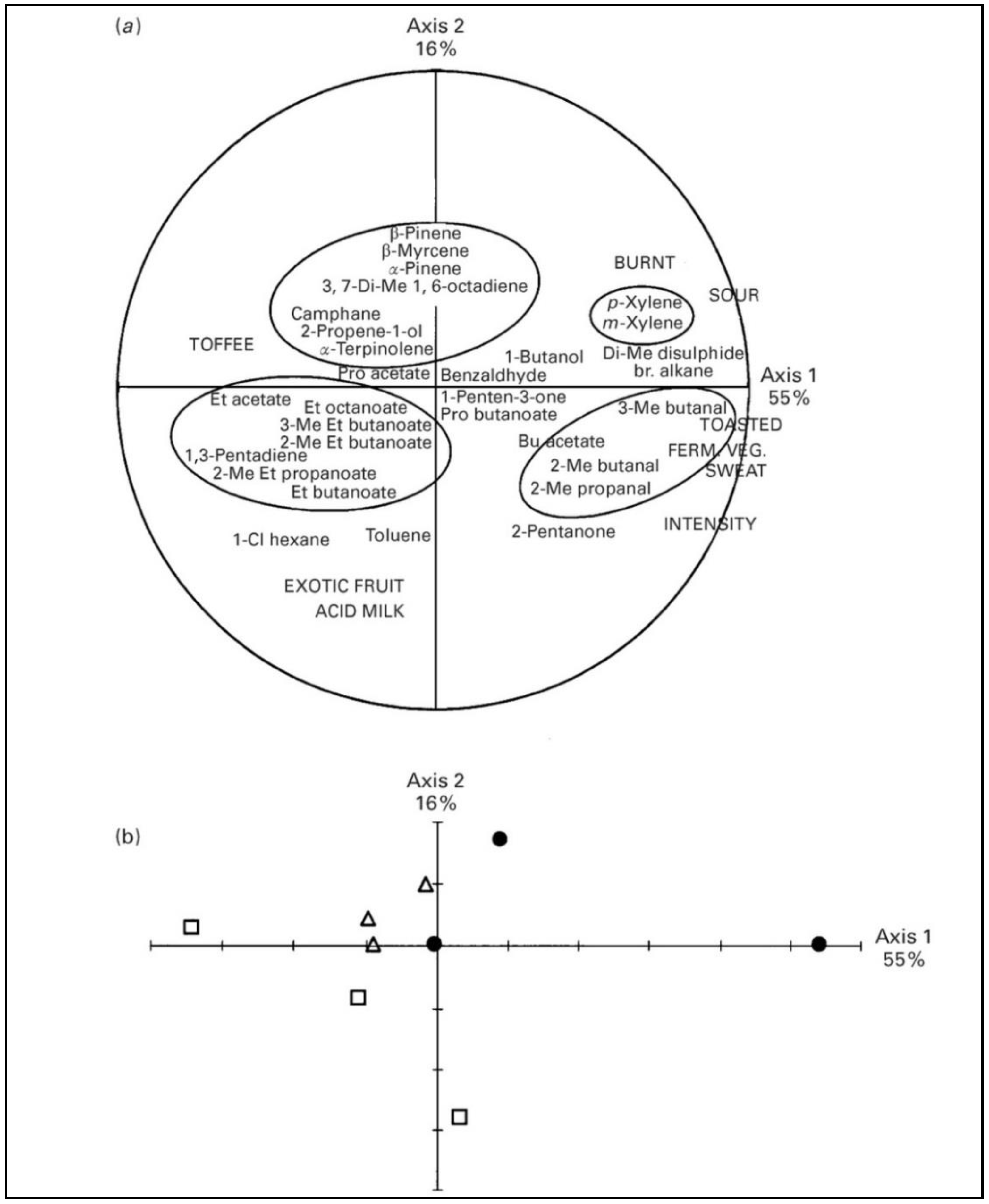
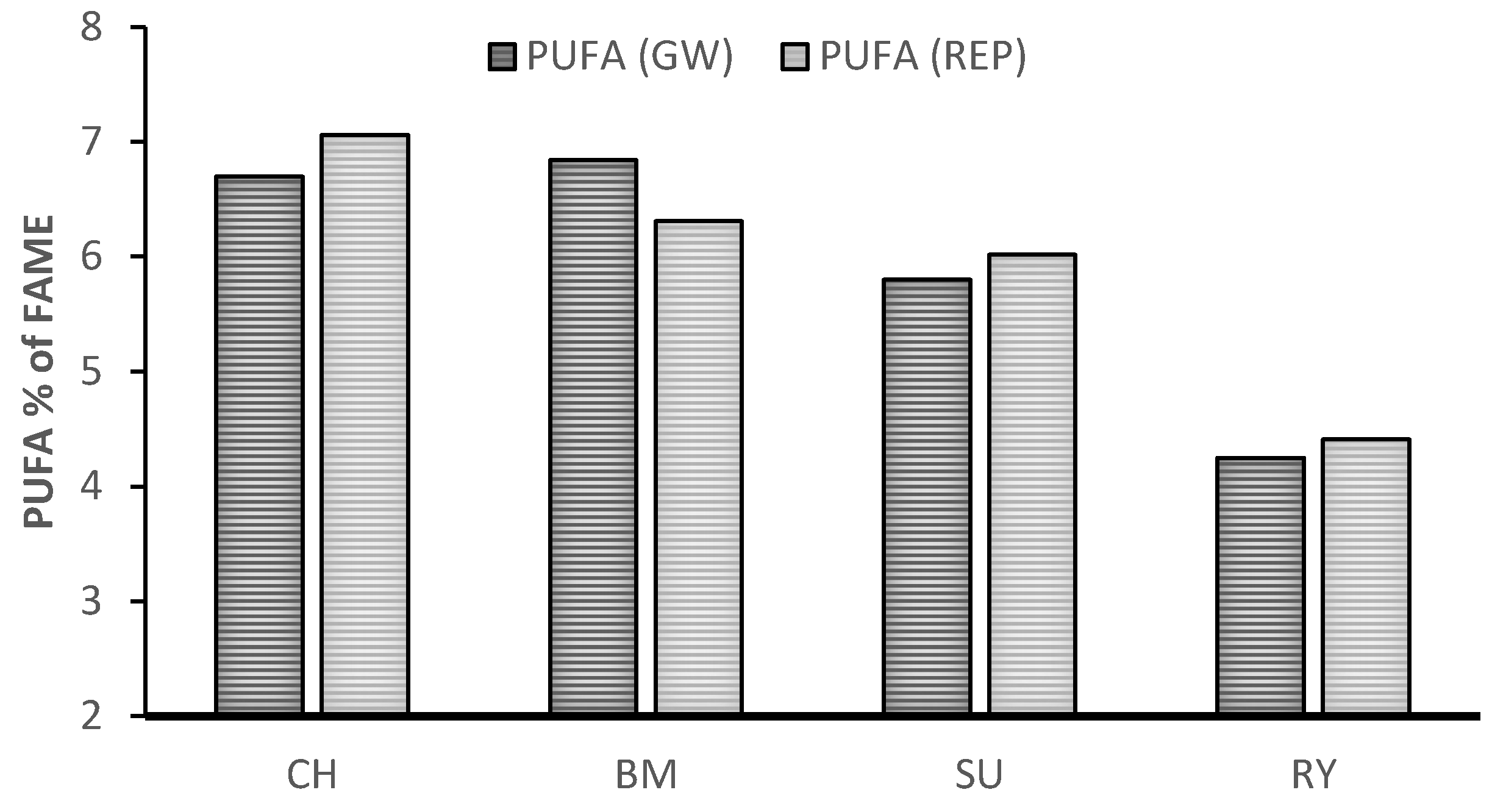
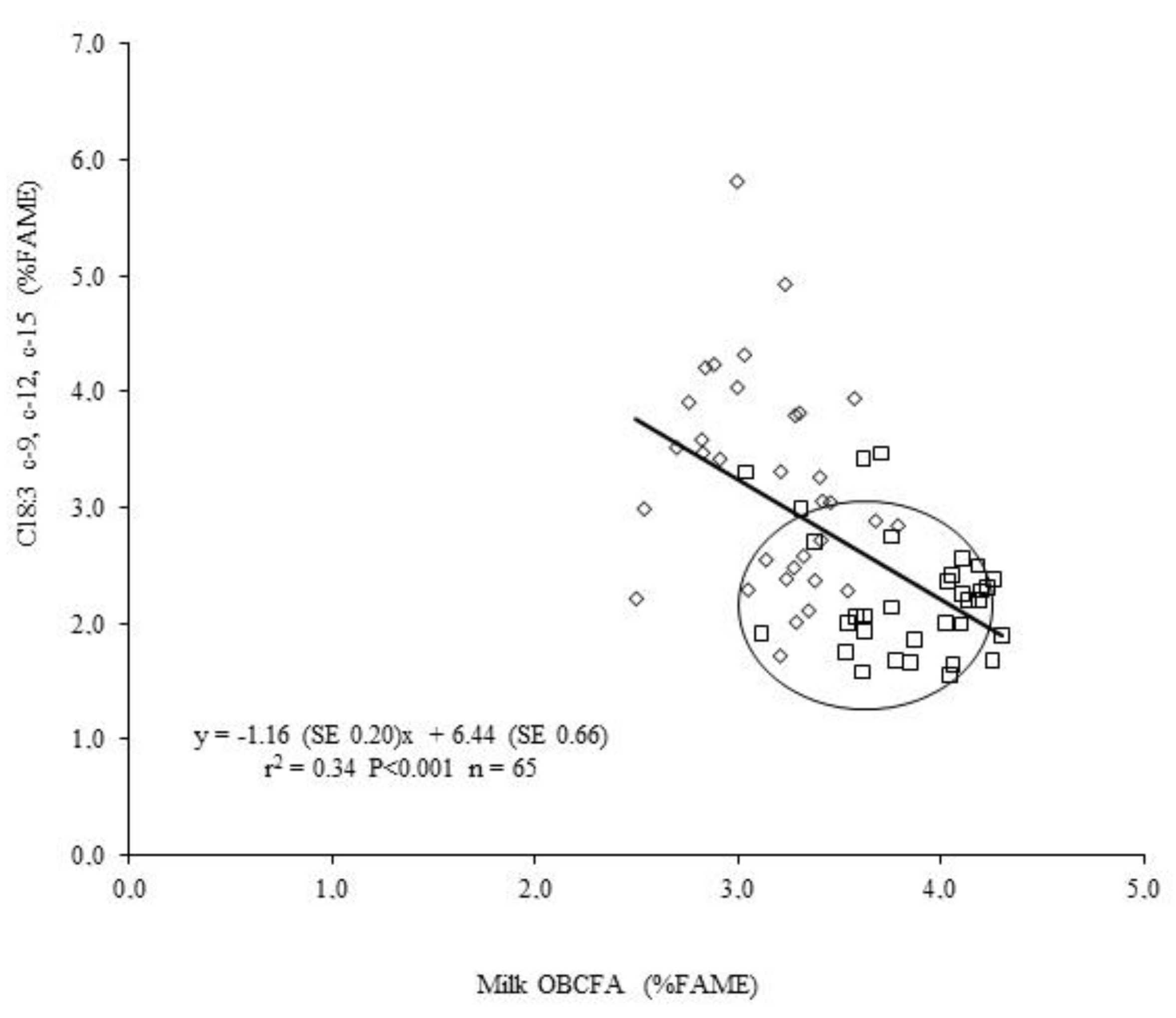
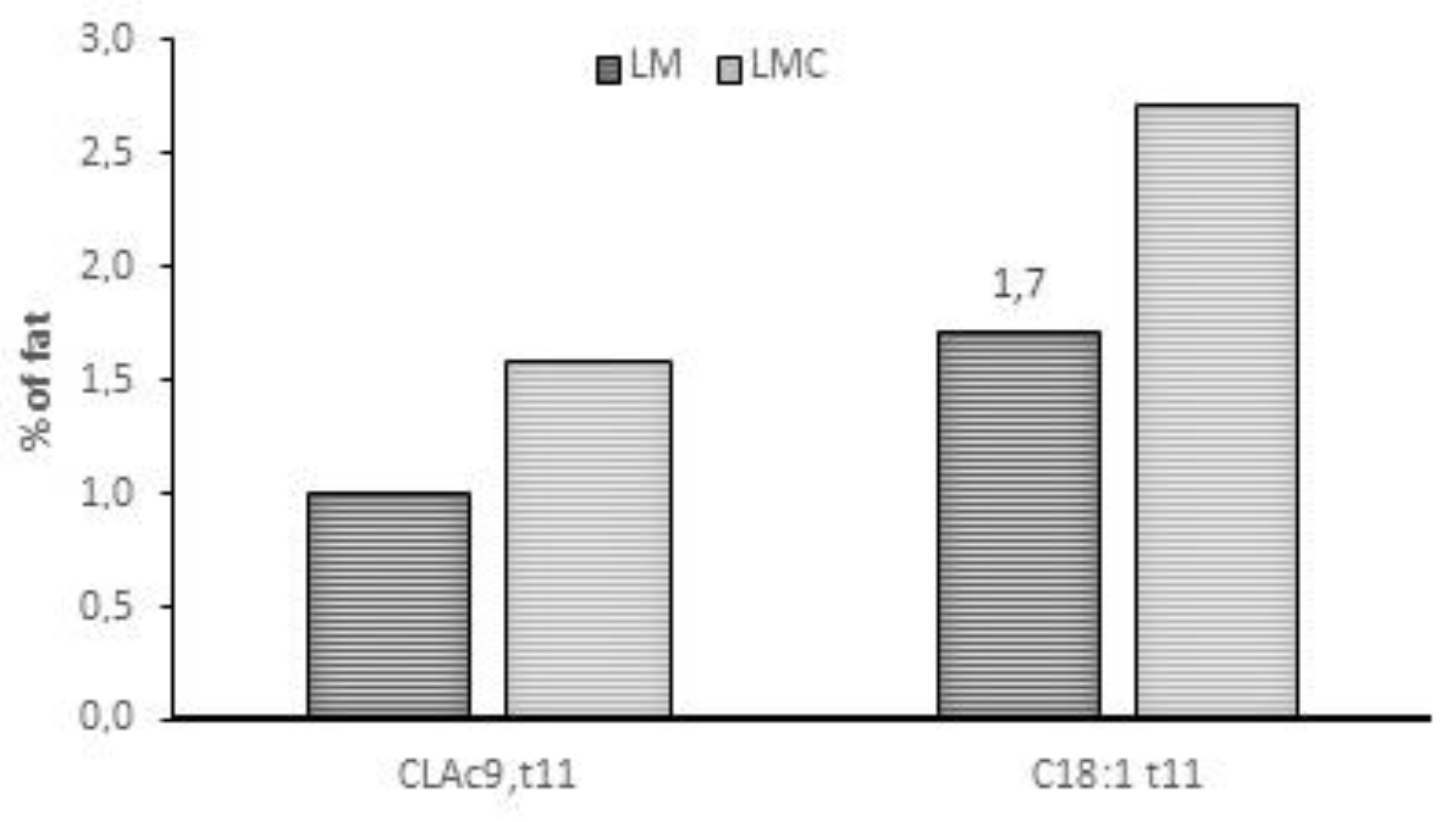
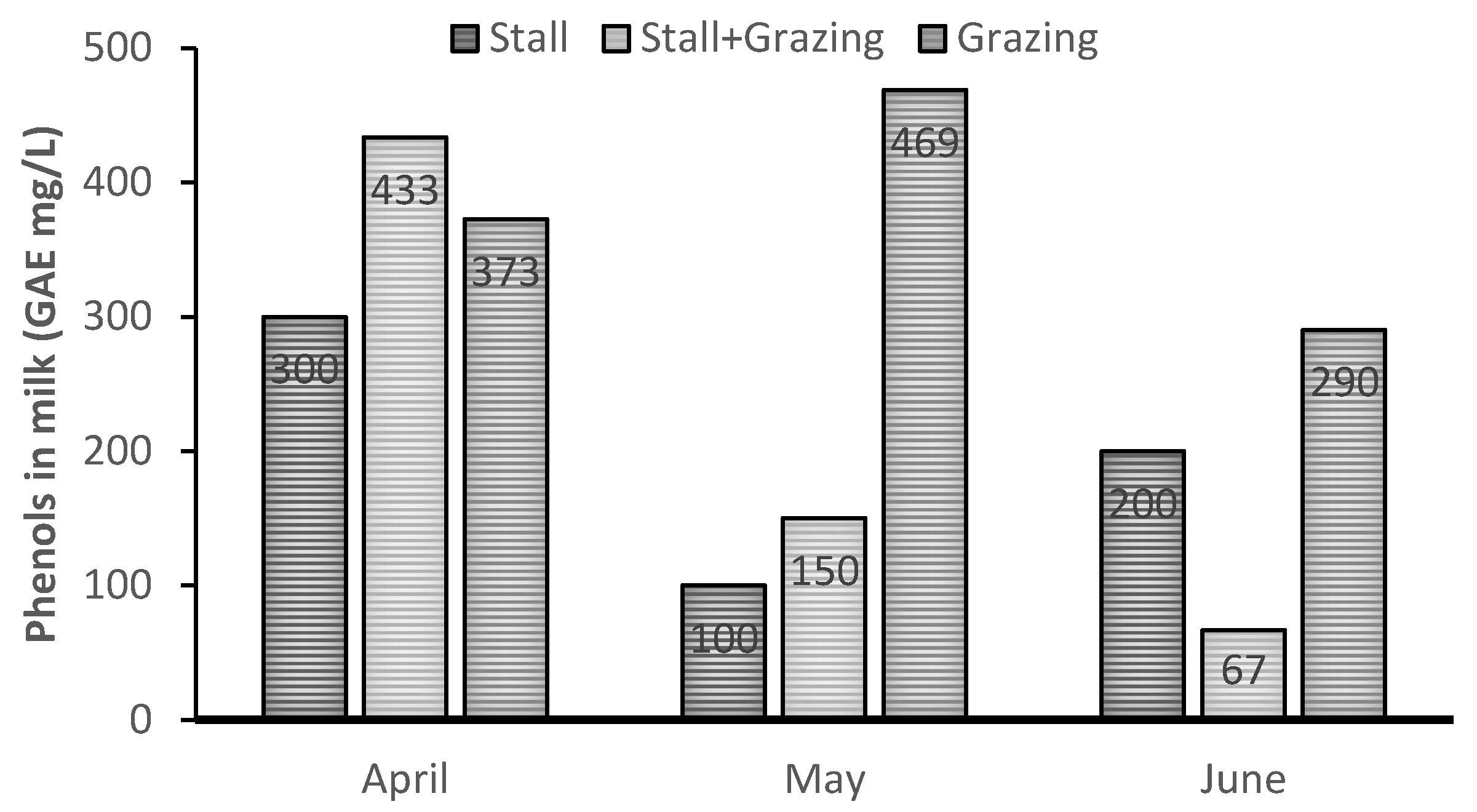
| Treatment | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|
| PAS | NFS | SLNA | SALA | ||||
| MUFA | % FAME | 24.67 ab | 22.27 b | 28.62 a | 28.81 a | 0.89 | 0.04 |
| PUFA | % FAME | 6.68 c | 6.89 bc | 9.84 a | 9.58 ab | 0.34 | 0.01 |
| SFA/UFA | ratio | 2.14 ab | 2.44 a | 1.66 b | 1.66 ab | 0.09 | 0.03 |
| AI (index) | ratio | 2.06 ab | 2.63 a | 1.87 b | 1.88 ab | 0.10 | 0.02 |
| n-3/n-6 | ratio | 2.08 a | 0.71 bc | 0.60 c | 0.90 b | 0.12 | 0.01 |
| I-Harris | % FAME | 0.13 | 0.10 | 0.10 | 0.12 | 0.01 | 0.06 |
| UTFA | % FAME | 0.30 b | 0.35 b | 0.60 a | 0.54 a | 0.03 | 0.01 |
| UTFA/CLA | ratio | 0.13 b | 0.19 a | 0.23 a | 0.19 a | 0.01 | 0.01 |
| Vit. A | Vit. E | Cholesterol | DAP(*1000) | ||
|---|---|---|---|---|---|
| Milk | C | 0.82a | 0.68a | 407a | 1.67 |
| HH | 0.97ab | 3.66b | 314b | 11.66 | |
| LH | 1.09b | 3.52b | 294b | 11.97 | |
| Cheese 24 h | C | 0.68a | 0.58a | 394a | 1.47 |
| HH | 0.92b | 3.74b | 316b | 11.84 | |
| LH | 1.06c | 3.76b | 309b | 12.17 | |
| Cheese 60 days | C | 0.66a | 0.65a | 390a | 1.67 |
| HH | 0.86b | 3.74b | 320b | 11.69 | |
| LH | 1.01c | 3.76b | 304b | 12.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabiddu, A.; Delgadillo-Puga, C.; Decandia, M.; Molle, G. Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals 2019, 9, 771. https://doi.org/10.3390/ani9100771
Cabiddu A, Delgadillo-Puga C, Decandia M, Molle G. Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals. 2019; 9(10):771. https://doi.org/10.3390/ani9100771
Chicago/Turabian StyleCabiddu, Andrea, Claudia Delgadillo-Puga, Mauro Decandia, and Giovanni Molle. 2019. "Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content" Animals 9, no. 10: 771. https://doi.org/10.3390/ani9100771
APA StyleCabiddu, A., Delgadillo-Puga, C., Decandia, M., & Molle, G. (2019). Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals, 9(10), 771. https://doi.org/10.3390/ani9100771








