Synthesis of CuO/ZnO Nanocomposites and Their Application in Photodegradation of Toxic Textile Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of CuO/ZnO
2.3. Characterization
2.4. Evaluation of Photocatalytic Activity
2.5. Detection of Active Species
3. Results and Discussion
3.1. XRD Patterns Study
3.2. SEM and EDX Study
3.3. FTIR Spectral Study
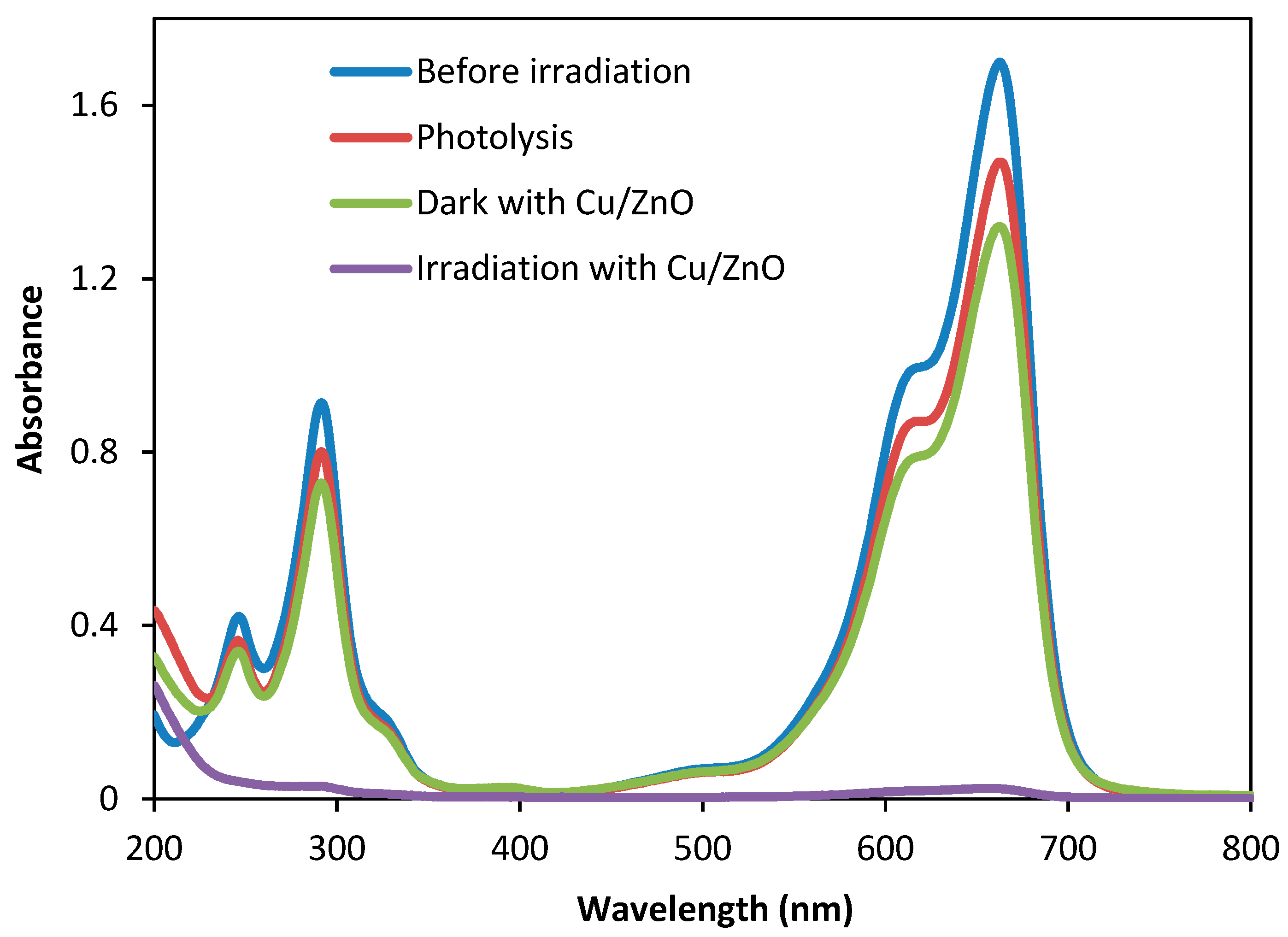
3.4. UV-VIS Spectral Changes
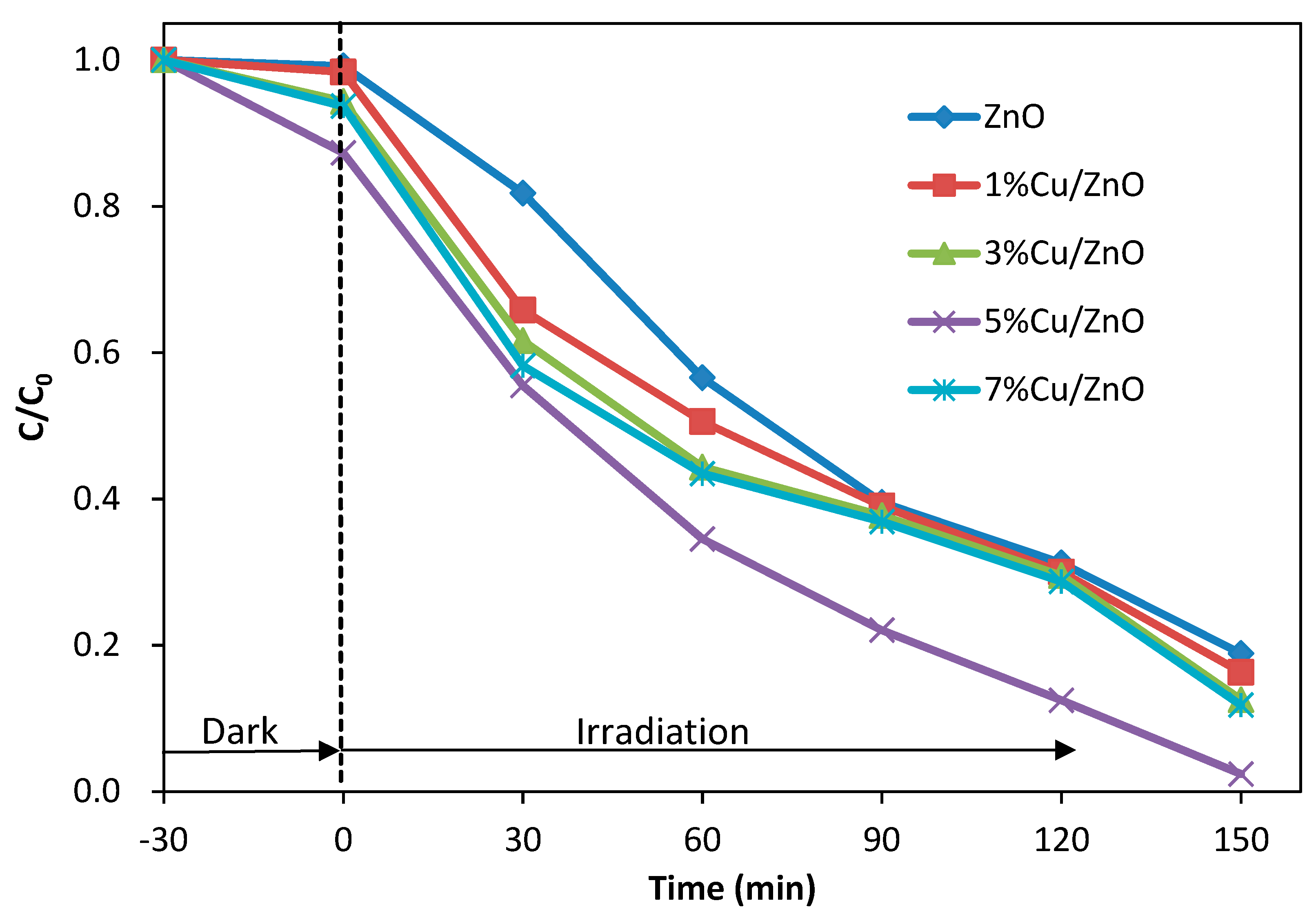
3.5. Photocatalytic Dye Degradation
3.6. Effect of Initial Dye Concentration
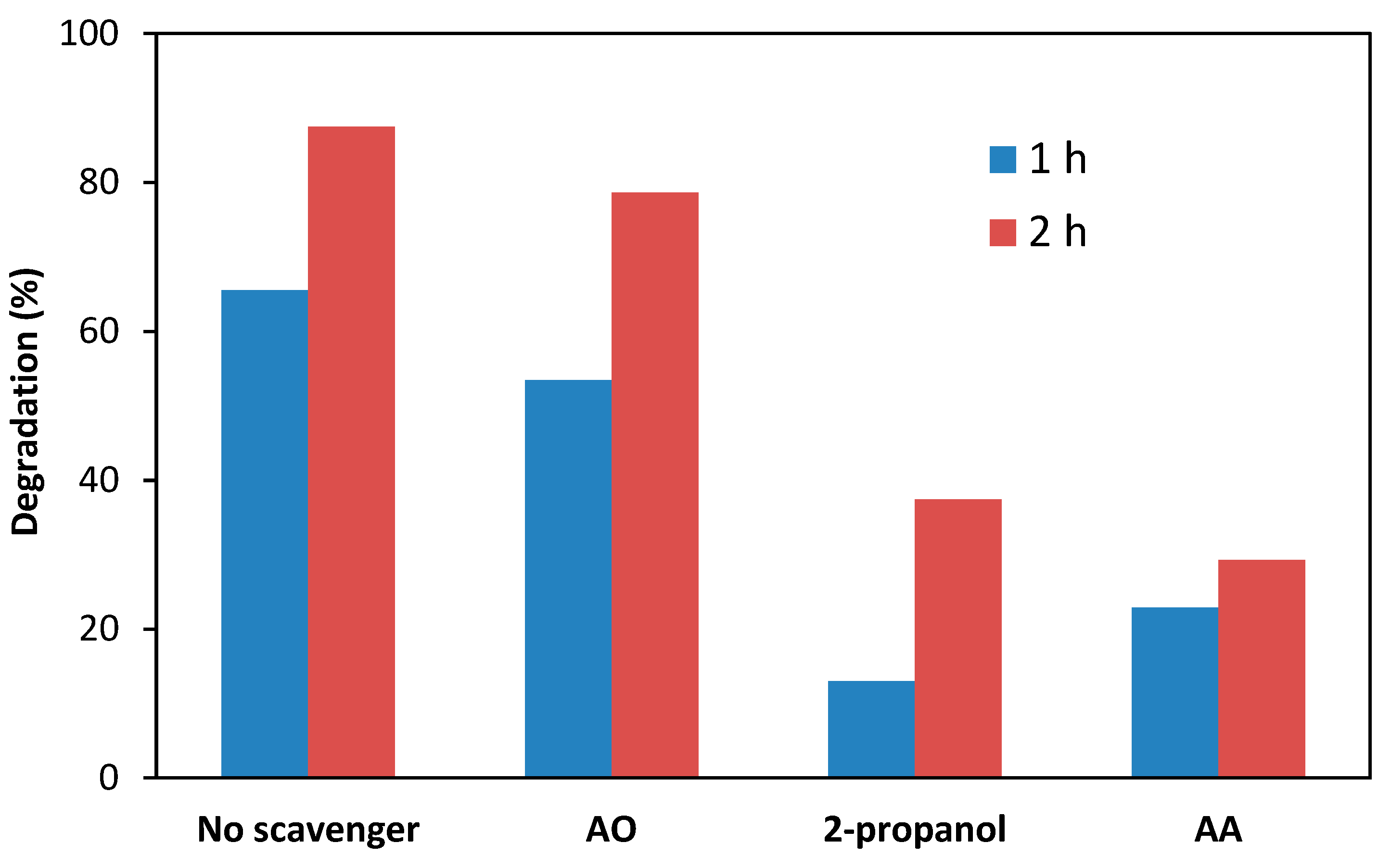
3.7. Role of Reactive Species
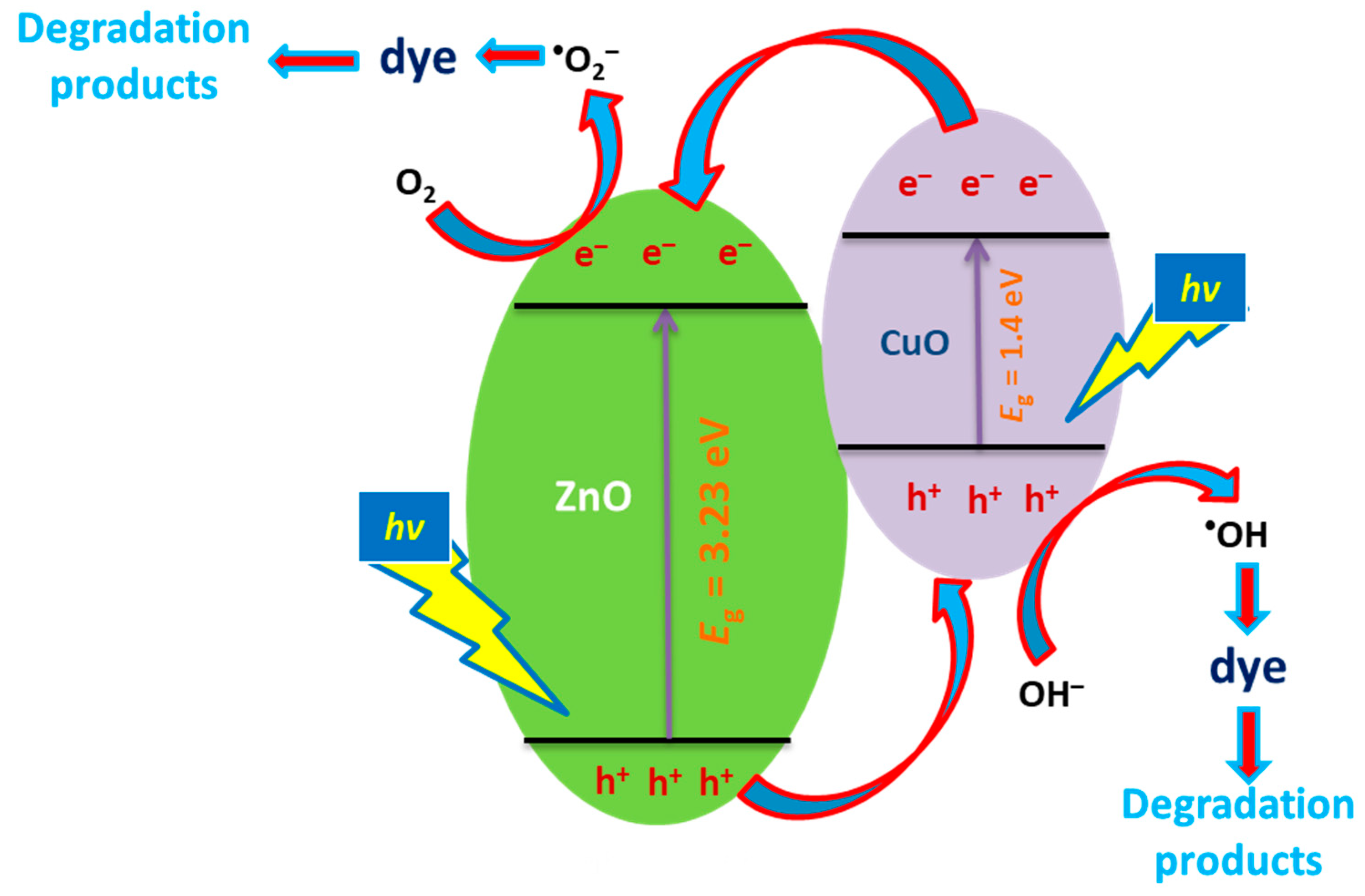
3.8. Degradation Mechanism
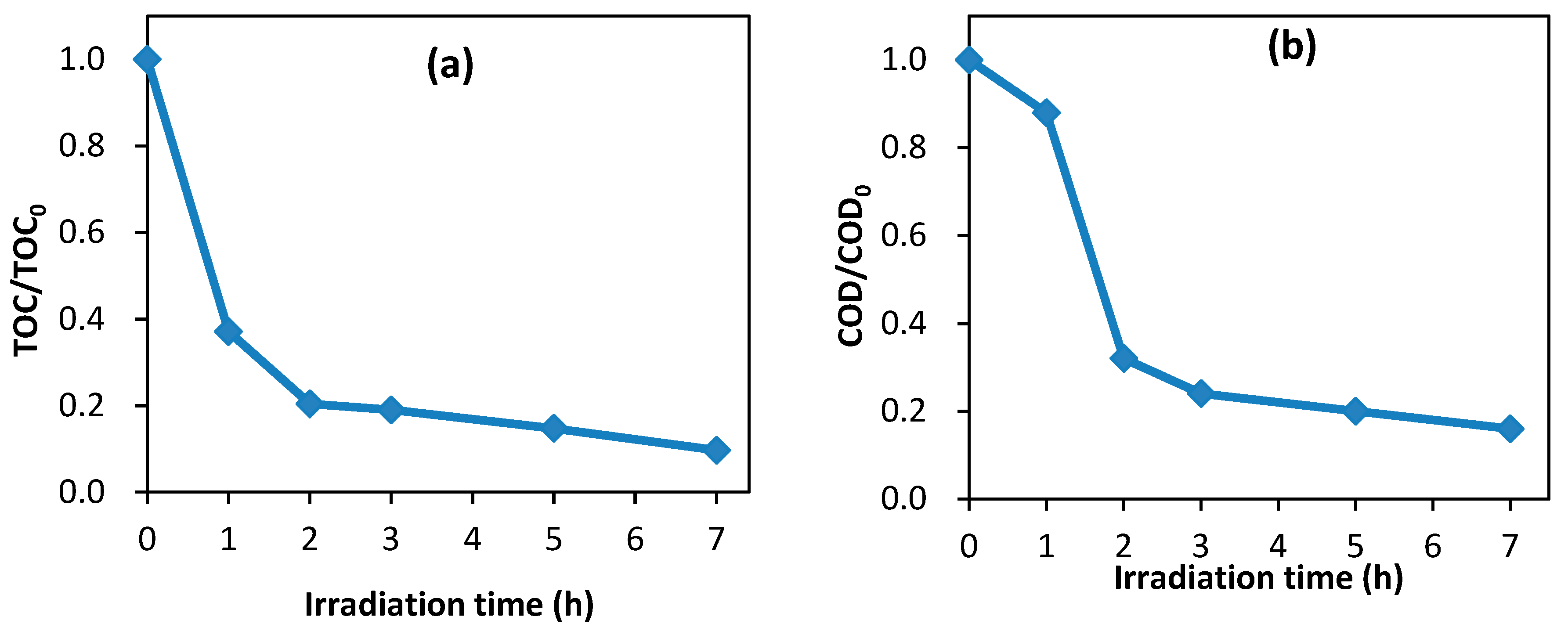
3.9. Mineralization of Dye
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y.C. High photocatalytic activity of ZnO−carbon nanofiber heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.A.I. Development of Advanced Technology for Photocatalytic Degradation of Organic Pollutants in Wastewater. Ph.D. Thesis, Mie University, Tsu, Japan, 2018. [Google Scholar]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Yulizar, Y.; Bakri, R.; Apriandanu, D.O.B.; Hidayat, T. ZnO/CuO nanocomposite prepared in one-pot green synthesis using seed bark extract of Theobroma cacao. Nano Struct. Nano Objects 2018, 16, 300–305. [Google Scholar] [CrossRef]
- Vidic, J.; Stankic, S.; Haque, F.; Ciric, D.; Le Goffic, R.; Vidy, A.; Jupille, J.; Delmas, B. Selective antibacterial effects of mixed ZnMgO nanoparticles. J. Nanopart. Res. 2013, 15, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhou, W.; Li, Y.; Wang, J.; Wu, P. Abnormal room temperature ferromagnetism in CuO/ZnO nanocomposites via hydrothermal method. Appl. Surf. Sci. 2017, 399, 396–402. [Google Scholar] [CrossRef]
- Dorneanu, P.P.; Airinei, A.; Olaru, N.; Homocianu, M.; Nica, V.; Doroftei, F. Preparation and characterization of NiO, ZnO and NiO–ZnO composite nanofibers by electrospinning method. Mater. Chem. Phys. 2014, 148, 1029–1035. [Google Scholar] [CrossRef]
- Hassanpour, M.; Hojaghan, H.S.; Niasari, M.S. Degradation of methylene blue and Rhodamine B as water pollutants via green synthesized Co3O4/ZnO nanocomposite. J. Mol. Liq. 2017, 229, 293–299. [Google Scholar] [CrossRef]
- Hassanpour, M.; Salavati-Niasari, M.; Mousavi, S.A.; Safardoust-Hojaghan, H.; Hamadanian, M. CeO2/ZnO Ceramic nanocomposites, synthesized via microwave method and used for decolorization of dye. J. Nanostruct. 2018, 8, 97–106. [Google Scholar]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef]
- Saravanan, R.; Gupta, V.K.; Mosquera, E.; Gracia, F. Preparation and characterization of V2O5/ZnO nanocomposite system for photocatalytic application. J. Mol. Liq. 2014, 198, 409–412. [Google Scholar] [CrossRef]
- Saravanan, R.; Gupta, V.K.; Narayanan, V.; Stephen, A. Visible light degradation of textile effluent using novel catalyst ZnO/γ-Mn2O3. J. Taiwan Inst. Chem. Eng. 2014, 45, 1910–1917. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y. Facile synthesis and photocatalytic activity of ZnO–CuO nanocomposite. Superlattice Microst. 2010, 47, 615–623. [Google Scholar] [CrossRef]
- Kuriakose, S.; Avasthi, D.K.; Mohapatra, S. Effects of swift heavy ion irradiation on structural, optical and photocatalytic properties of ZnO–CuO nanocomposites prepared by carbothermal evaporation method. Beilstein J. Nanotechnol. 2015, 6, 928–937. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Z.; Zheng, Z.; Xu, H.; Wang, H.; Hu, K.; Yan, K. Remarkably enhanced sulfate radical-based photo-Fenton-like degradation of levofloxacin using the reduced mesoporous MnO@MnOx microspheres. Chem. Eng. J. 2019, 379, 122340. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Saravanan, R.; Qin, J.; Yola, M.L.; Atar, N.; Gracia, F. Nanosized Fe3O4 incorporated on a TiO2 surface for the enhanced photocatalytic degradation of organic pollutants. J. Mol. Liq. 2019, 287, 110967. [Google Scholar] [CrossRef]
- Saravanan, R.; Agarwal, S.; Gupta, V.K.; Khan, M.M.; Gracia, F.; Mosquera, E.; Narayanan, V.; Stephen, A. Line defect Ce3+ induced Ag/CeO2/ZnO nanostructure for visible-light photocatalytic activity. J. Photochem. Photobiol. A Chem. 2018, 353, 499–506. [Google Scholar] [CrossRef]
- Jebaranjitham, J.N.; Mageshwari, C.; Saravanan, R.; Mu, N. Fabrication of amine functionalized graphene oxide–AgNPs nanocomposite with improved dispersibility for reduction of 4-nitrophenol. Compos. Part B 2019, 171, 302–309. [Google Scholar] [CrossRef]
- Qin, J.; Yang, C.; Cao, M.; Zhang, X.; Saravanan, R.; Limpanart, S.; Ma, M.; Liu, R. Two dimensional porous sheet-like carbon-doped ZnO/g-C3N4 nanocomposite with high visible-light photocatalytic performance. Mater. Lett. 2017, 189, 156–159. [Google Scholar] [CrossRef]
- Saravanan, R.; Khan, M.M.; Gracia, F.; Qin, J.; Gupta, V.K.; Arumainathan, S. Ce3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci. Rep. 2016, 6, 31641. [Google Scholar] [CrossRef]
- Yang, C.; Xue, Z.; Qin, J.; Sawangphruk, M.; Saravanan, R.; Zhang, X.; Liu, R. Visible-light Driven Photocatalytic H2 Generation and Mechanism Insights on Bi2O2CO3/G-C3N4 Z-scheme Photocatalyst. Phys. Chem. C 2019, 123, 4795–4804. [Google Scholar] [CrossRef]
- Kanade, K.G.; Kale, B.B.; Baeg, J.O.; Lee, S.M.; Lee, C.W.; Moon, S.J.; Chang, H. Self-assembled aligned Cu doped ZnO nanoparticles for photocatalytic hydrogen production under visible light irradiation. Mater. Chem. Phys. 2007, 102, 98–104. [Google Scholar] [CrossRef]
- Rooydell, R.; Brahma, S.; Wang, R.C.; Modaberi, M.R.; Ebrahimzadeh, F.; Liu, C.P. Cu doped ZnO nanorods with controllable Cu content by using single metal organicprecursors and their photocatalytic and luminescence properties. J. Alloys Compd. 2017, 691, 936–945. [Google Scholar] [CrossRef]
- Hsieh, S.-H.; Ting, J.-M. Characterization and photocatalytic performance of ternary Cu-doped ZnO/Graphene materials. Appl. Surf. Sci. 2018, 427, 465–475. [Google Scholar] [CrossRef]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W.; Pecharapa, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef]
- Jun, S.T.; Choi, G.M. Composition dependence of the electrical conductivity of ZnO(n)–CuO(p) ceramic composite. J. Am. Ceram. Soc. 1998, 81, 695–699. [Google Scholar] [CrossRef]
- Zhang, D. Synthesis and characterization of ZnO-doped cupric oxides and evaluation of their photocatalytic performance under visible light. Transit. Met. Chem. 2010, 35, 689–694. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Studies of effects of calcination temperature on the crystallinity and optical properties of Ag–doped ZnO nanocomposites. J. Compos. Sci. 2019, 3, 18. [Google Scholar] [CrossRef]
- Benavente, E.; Duran, F.; Sotomayor-Torres, C.; Gonzalez, G. Heterostructured layered hybrid ZnO/MoS2 nanosheets with enhanced visible light photocatalytic activity. J. Phys. Chem. Solids 2018, 113, 119–124. [Google Scholar] [CrossRef]
- Su, J.; Zhu, L.; Geng, P.; Chen, G. Self-assembly graphitic carbon nitride quantum dots anchored on TiO2 nanotube arrays: An efficient heterojunction for pollutants degradation under solar light. J. Hazard. Mater. 2016, 316, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Sb2O3–ZnO nanospindles: A potential material for photocatalytic and sensing applications. Ceram. Int. 2015, 41, 5429–5438. [Google Scholar] [CrossRef]
- Khana, S.A.; Noreen, F.; Kanwal, S.; Iqbal, A.; Hussain, G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater. Sci. Eng. C 2018, 82, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Saravanakkumar, D.; Sivaranjani, S.; Kaviyarasu, K.; Ayeshamariam, A.; Ravikumar, B.; Pandiarajan, S.; Veeralakshmi, C.; Jayachandran, M.; Maaza, M. Synthesis and characterization of ZnO–CuO nanocomposites powder by modified perfume spray pyrolysis method and its antimicrobial investigation. J. Semicond. 2018, 39, 033001. [Google Scholar] [CrossRef]
- Ma, G.; Liang, X.; Li, L.; Qiao, R.; Jiang, D.; Ding, Y.; Chen, H. Cu-doped zinc oxide and its polythiophene composites: Preparation and antibacterial properties. Chemosphere 2014, 100, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Fabrication of Ag-doped ZnO by mechanochemical combustion method and their application into photocatalytic Famotidine degradation. J. Environ. Sci. Health Part A 2019, 54, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Kaneco, S.; Li, N.; Itoh, K.-K.; Katsumata, H.; Suzuki, T.; Ohta, K. Titanium dioxide mediated solar photocatalytic degradation of thiram in aqueous solution: Kinetics and mineralization. Chem. Eng. J. 2009, 148, 50–56. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Ahsan, S.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Degradation, Kinetics, and Mineralization in the solar photocatalytic treatment of aqueous amitrole solution with titanium dioxide. Environ. Eng. Sci. 2018, 35, 401–407. [Google Scholar] [CrossRef]
- Wang, A.; Wang, H.; Deng, H.; Wang, S.; Shi, W.; Yi, Z.; Qiu, R.; Yan, K. Controllable synthesis of mesoporous manganese oxide microsphere efficient for photo-Fenton-like removal of fluoroquinolone antibiotics. Appl. Catal. B Environ. 2019, 248, 298–308. [Google Scholar] [CrossRef]
- Chabri, S.; Dhara, A.; Show, B.; Adak, D.; Sinha, A.; Mukherjee, N. Mesoporous CuO-ZnO p-n heterojunction based nanocomposites with high specific surface area for enhanced photocatalysis and electrochemical sensing. Catal. Sci. Technol. 2016, 6, 3238–3252. [Google Scholar] [CrossRef]
- Yang, L.; Xie, C.S.; Zhang, G.Z.; Zhao, J.W.; Yu, X.L.; Zeng, D.W.; Zhang, S.P. Enhanced response to NO2 with CuO/ZnO laminated heterostructured configuration. Sens. Actuator B 2014, 195, 500–508. [Google Scholar] [CrossRef]
- Alsabahi, J.; Bora, T.; Alabri, M.; Dutta, J. Efficient visible light photocatalysis of benzene, toluene, ethylbenzene and xylene (BTEX) in aqueous solutions using supported zinc oxide nanorods. PLoS ONE 2017, 12, e0189276. [Google Scholar]
- Hansen, B.J.; Kouklin, N.; Lu, G.H.; Lin, I.; Chen, J.H.; Zhang, X. Transport, analyte detection, and opto-electronic response of p-Type CuO nanowires. J. Phys. Chem. C 2010, 114, 2440–2447. [Google Scholar] [CrossRef]
- Zhang, J.N.; Chen, T.H.; Yu, J.H.; Liu, C.; Yang, Z.B.; Lu, H.B.; Yin, F.; Gao, J.Z.; Liu, Q.R.; Zhang, X.W.; et al. Enhanced photocatalytic activity of flowerlike CuO-ZnO nanocomposites synthesized by one-step hydrothermal method. J. Mater. Sci. 2016, 27, 10667–10672. [Google Scholar] [CrossRef]
- Harish, S.; Archana, J.; Sabarinathan, M.; Navaneethan, M.; Nisha, K.D.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Aswal, D.K.; Hayakawa, Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2017, 418, 103–112. [Google Scholar] [CrossRef]
- Wu, F.S.; Wang, X.H.; Hu, S.Z.; Hao, C.; Gao, H.W.; Zhou, S.S. Solid-state preparation of CuO/ZnO nanocomposites for functional supercapacitor electrodes and photocatalysts with enhanced photocatalytic properties. Int. J. Hydrogen Energy 2017, 42, 30098–30108. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhao, J.; Zhu, H.; Orthman, J. Photodegradation of dye pollutants on TiO2 nanoparticles dispersed in silicat;e UV-Vis irradiation. Appl. Catal. B Environ. 2002, 37, 331–338. [Google Scholar] [CrossRef]
- Jain, R.; Shirkarwar, S. Removal of hazardous dye Congo-red removal from waste material. J. Hazard. Mater. 2008, 152, 942–948. [Google Scholar] [CrossRef]




| Methylene blue | 30 mL, 5–20 mg/L |
| Photocatalyst | 20 mg |
| Temperature | ~30 °C |
| pH | Natural |
| Light source | Sunlight |
| Irradiation time | 0–150 min |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakib, A.A.M.; Masum, S.M.; Hoinkis, J.; Islam, R.; Molla, M.A.I. Synthesis of CuO/ZnO Nanocomposites and Their Application in Photodegradation of Toxic Textile Dye. J. Compos. Sci. 2019, 3, 91. https://doi.org/10.3390/jcs3030091
Sakib AAM, Masum SM, Hoinkis J, Islam R, Molla MAI. Synthesis of CuO/ZnO Nanocomposites and Their Application in Photodegradation of Toxic Textile Dye. Journal of Composites Science. 2019; 3(3):91. https://doi.org/10.3390/jcs3030091
Chicago/Turabian StyleSakib, Abdullah Al Mamun, Shah Md. Masum, Jan Hoinkis, Rafiqul Islam, and Md. Ashraful Islam Molla. 2019. "Synthesis of CuO/ZnO Nanocomposites and Their Application in Photodegradation of Toxic Textile Dye" Journal of Composites Science 3, no. 3: 91. https://doi.org/10.3390/jcs3030091





